Postpartum Uterine Involution in Martina Franca Jennies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Postpartum Uterine Involution, Endometrial Biopsies, and Foal Heat
2.4. Endometrial Histology and Cytology
2.5. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Ultrasonography
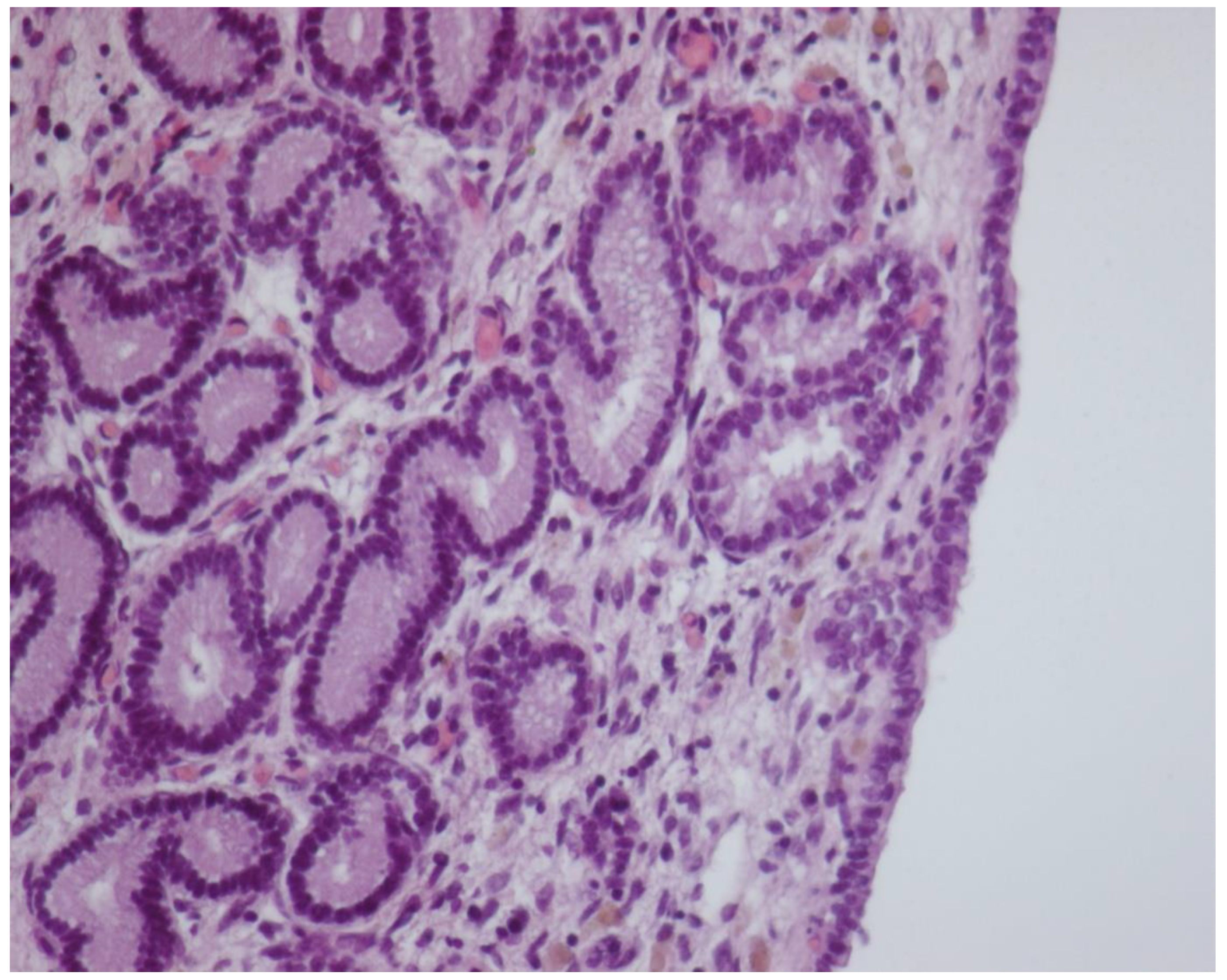
3.3. Histology
3.4. Cytology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nishikawa, Y.; Yamazaki, Y. Studies on reproduction in asses. I. Breeding season, oestrous cycle and length of heat. Jpn. J. Zootech. Sci. 1949, 19, 119–124. [Google Scholar]
- McKinnon, A.O. Reproductive Ultrasonography. In Equine Diagnostic Ultrasonography; Rantanen, N.R., McKinnon, A.O., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 79–102. [Google Scholar]
- Blanchard, T.L.; Varner, D.D.; Brinsko, S.P.; Meyers, S.A.; Johnson, L. Effects of postparturient uterine lavage on uterine involution in the mare. Theriogenology 1989, 32, 527–535. [Google Scholar] [CrossRef]
- Krohn, J.; Eilenberg, R.D.; Gajewski, Z.; Failing, K.; Wehrend, A. Lochial and endometrial cytological changes during the first 10 days post-partum with special reference to the nature of foaling and puerperium in equine. Theriogenology 2019, 139, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Meira, E.B., Jr.; Henriques, L.C.; Sá, L.R.; Gregory, L. Comparison of ultrasonography and histopathology for the diagnosis of endometritis in Holstein-Friesian cows. J. Dairy Sci. 2012, 95, 6969–6973. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.R. Practical control of anestrus in Thoroughbred broodmares. In Endocrine Causes of Seasonal and Lactational Anestrus in Farm Animals; Ellendorff, F., Elsaesser, F., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1985; pp. 98–107. [Google Scholar]
- Noakes, D. The puerperium. In Veterinary Reproduction and Obstetrics; Noakes, D.E., Parkinson, T.J., England, G.C.W., Arthur, G.H., Eds.; Saunders Ltd.: Edinburgh, Scotland, 2009. [Google Scholar]
- Stanton, M.E. Uterine involution. In Equine Reproduction; Mc Kinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley and Blackwell: Oxford, UK, 2011; pp. 2292–2293. [Google Scholar]
- Gygax, A.P.; Ganjam, V.K.; Kenney, R.M. Clinical, microbiological and histological changes associated with uterine involution in the mare. J. Reprod. Fertil. 1979, 27, 571–578. [Google Scholar]
- Jischa, S.; Walter, I.; Nowotny, N.; Palm, F.; Budik, S.; Kolodziejek, J.; Aurich, C. Uterine involution and endometrial function in postpartum pony mares. Am. J. Vet. Res. 2008, 69, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Dadarwal, D.; Tandon, S.N.; Purohit, G.N.; Pareek, P.K. Ultrasonographic evaluation of uterine involution and postpartum follicular dynamics in French jennies (Equus asinus). Theriogenology 2004, 62, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Tosi, U.; Bernabò, N.; Verni, F.; Valbonetti, L.; Muttini, A.; Mattioli, M.; Barboni, B. Postpartum reproductive activities and gestation length in Martina Franca jennies, an endangered Italian donkey breed. Theriogenology 2013, 80, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, A.; Gloria, A.; Robbe, D.; Veronesi, M.C.; De Amicis, I.; Cairoli, F.; Contri, A. Reproductive characteristics of foal heat in female donkeys. Animal 2017, 11, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.C.; Dall’Ara, P.; Gloria, A.; Servida, F.; Sala, E.; Robbe, D. IgG, IgA, and lysozyme in Martina Franca donkey jennies and their foals. Theriogenology 2014, 8, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, A.; Gloria, A.; Veronesi, M.C.; De Amicis, I.; Noto, F.; Contri, A. Factors affecting pregnancy length and phases of parturition in Martina Franca jennies. Theriogenology 2015, 84, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Contri, A.; Tosi, U.; De Amicis, I.; Veronesi, M.C.; Panzani, S.; Carluccio, A. Ultrasonographic evaluation of sexual glands before and after ejaculation in the jackass. Vet. Res. Commun. 2008, 32 (Suppl. 1), S135–S137. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.C.; De Amicis, I.; Panzani, S.; Kindahl, H.; Govoni, N.; Probo, M.; Carluccio, A. PGF(2α), LH, testosterone, oestrone sulphate, and cortisol plasma concentrations around sexual stimulation in jackass. Theriogenology 2011, 75, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Panzani, S.; Carluccio, A.; Probo, M.; Faustini, M.; Kindahl, H.; Veronesi, M.C. Comparative study on 15-ketodihydro-PGF(2α) plasma concentrations in newborn horses, donkeys and calves. Reprod. Domest. Anim. 2012, 47, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Panzani, S.; Carluccio, A.; Faustini, M.; Prandi, A.; Probo, M.; Veronesi, M.C. Comparative study on Insulin-Like Growth Factor I (IGF-I) plasma concentrations in new-born horse foals, donkey foals and calves. Fetal Neonatal Dev. Med. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, M.C.; Gloria, A.; Panzani, S.; Sfirro, M.P.; Carluccio, A.; Contri, A. Blood analysis in newborn donkeys: Hematology, biochemistry, and blood gases analysis. Theriogenology 2014, 82, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Romies, B. Färben der Schnitte. In Microskopicshe Technik; Böck, P., Ed.; Urban & Schwarzenberg: Munich, Germany, 1989; pp. 179–249. [Google Scholar]
- Arrott, C.; Macpherson, M.; Blanchard, T.; Varner, D.; Thompson, J.; Simpson, B.; Bruemmer, J.; Vogelsang, S.; Fernandez, M.; Fleet, T.; et al. Biodegradable estradiol microspheres do not affect uterine involution or characteristics of postpartum estrus in mares. Theriogenology 1994, 42, 371–384. [Google Scholar] [CrossRef]
- Katila, T. Histology of the post partum equine uterus as determined by endometrial biopsies. Acta Vet. Scand. 1988, 29, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.V.; Bristol, F.M. Uterine involution in the mare after induced parturition. Am. J. Vet. Res. 1983, 44, 793–797. [Google Scholar] [PubMed]
- Caballeros, J.E.; Camacho, C.; Cazales, N.; Estradé, M.J.; Fiala-Rechsteiner, S.; Jobim, M.I.M.; Mattos, R.C. Ultrastructural and histological characteristics of the equine endometrium at day 5 post ovulation. Theriogenology 2019, 132, 106–112. [Google Scholar] [CrossRef] [PubMed]





| Cross-Sectional Diameter (mm) Non-Post-Pregnant Horn | Cross-Sectional Diameter (mm) Post-Pregnant Horn | |||||
|---|---|---|---|---|---|---|
| PP Day | Tip | Middle | Corpora-Cornual Junction | Tip | Middle | Corpora-Cornual Junction |
| 1 | 41.8 ± 1.2 | 59.1 ± 0.8 A | 86.0 ± 1.2 | 44.0 ± 1.3 | 76.0 ± 0.8 B | Not assessable |
| 3 | 38.3 ± 1.8 | 56.7 ± 1.1 A | 84.5 ± 2.0 | 41.0 ± 0.8 | 71.6 ± 0.7 B | Not assessable |
| 7 | 35.5 ± 0.9 | 54.0 ± 1.4 A | 77.8 ± 1.4 a | 37.3 ± 0.8 | 62.6 ± 0.7 B | 96.8 ± 1.5 b |
| 14 | 29.0 ± 1.3 | 46.8 ± 1.5 | 67.8 ± 1.6 | 30.5 ± 1.1 | 47.1 ± 2.2 | 72.3 ± 2.4 |
| 21 | 26.6 ± 0.6 | 43.3 ± 1.3 | 64.6 ± 1.0 | 25.7 ± 1.0 | 45.2 ± 1.3 | 64.3 ± 1.4 |
| 28 | 24.5 ± 0.6 | 43.3 ± 0.8 | 58.1 ± 1.1 | 24.8 ± 0.7 | 42.0 ± 1.2 | 59.6 ± 1.4 |
| PP Day | Number | Perimeter (mm) | Area (mm2) | Epithelium Thickness (mm) |
|---|---|---|---|---|
| 1 | 129 ± 110.8 ♦§♣ | 8.8 ± 3.4 A | 5.3 ± 4.2 AA | 1.0 ± 0.3 A |
| 3 | 173 ± 124.6 ♠• | 8.6 ± 3.6 A | 5.2 ± 4.2 AA | 1.3 ± 0.4 B |
| 7 | 240 ± 127.0 ♥# | 7.4 ± 3.2 B | 3.9 ± 3.1 BB | 1.3 ± 0.4 B |
| 14 | 396 ± 320.8 ♦ | 7.2 ± 2.9 B | 3.6 ± 2.5 BB | 1.4 ± 0.4 C |
| 21 | 519 ± 220.0 ♥§♠ | 6.5 ± 2.1 C | 2.8 ± 2.0 CC | 1.2 ± 0.3 B |
| 28 | 546 ± 240.5 #♣• | 6.2 ± 2.9 C | 2.5 ± 2.2 CC | 1.2 ± 0.3 B |
| PP Day | ||||||
|---|---|---|---|---|---|---|
| PP Day 1 | PP Day 3 | PP Day 7 | PP Day 14 | PP Day 21 | PP Day 28 | |
| Eosinophil Score | 0.51 ± 0.42 §# | 0.73 ± 0.49 ♥♣ | 1.94 ± 0.82 §♥♦ | 1.57 ± 0.82 #♣ | 0.96 ± 0.87 ♦ | 0.94 ± 1.20 |
| Neutrophil Score | 1.16 ± 0.46 §#♥♦ | 1.51 ± 0.69 ♣♠AB | 0.51 ± 0.30 §♣C | 0.52 ± 0.34 #♠D | 0.37 ± 0.13 ♥AE | 0.17 ± 0.16 ♦BCDE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Amicis, I.; Fusi, J.; Marruchella, G.; Zedda, M.T.; Mazzatenta, A.; Robbe, D.; Carluccio, A. Postpartum Uterine Involution in Martina Franca Jennies. Animals 2021, 11, 2762. https://doi.org/10.3390/ani11102762
De Amicis I, Fusi J, Marruchella G, Zedda MT, Mazzatenta A, Robbe D, Carluccio A. Postpartum Uterine Involution in Martina Franca Jennies. Animals. 2021; 11(10):2762. https://doi.org/10.3390/ani11102762
Chicago/Turabian StyleDe Amicis, Ippolito, Jasmine Fusi, Giuseppe Marruchella, Maria T. Zedda, Andrea Mazzatenta, Domenico Robbe, and Augusto Carluccio. 2021. "Postpartum Uterine Involution in Martina Franca Jennies" Animals 11, no. 10: 2762. https://doi.org/10.3390/ani11102762






