Simple Summary
There are many parasites that may be transmitted to humans via food, and meat is a major source of such infections. Trichinella spp. is one of the most important meat-transmitted parasites, while Alaria spp. may be considered an emerging pathogen, albeit to date rarely reported in humans. Raw and undercooked wild boar meat has been proven as a major source of human infection by both parasites. In the present study, an investigation of the presence of these parasites in wild boar meat was conducted for the first time in Greece. Classical parasitological methods and molecular techniques were implemented for the examination of samples collected from 128 hunted wild boars, and none of them were found positive for Trichinella spp. or Alaria spp. For the detection of Alaria spp., a novel molecular method was developed, offering a powerful complementary diagnostic tool that may be useful for the epizootiological surveillance of the parasite. The epizootiology/epidemiology, clinical implications, and importance of monitoring of these parasitic infections are briefly discussed.
Abstract
Foodborne parasitic diseases represent a major threat to public health. Trichinellosis, caused by the nematode parasite Trichinella spp., is one of the most important foodborne diseases, while alariosis, caused by the trematode parasite Alaria spp., is less common in humans, and rare cases have been reported only in the USA and Canada. Both parasites can infect humans via the consumption of raw or undercooked wild boar meat. In order to investigate the prevalence of these parasites in wild boar meat in Greece, samples from the diaphragm pillars and the region of the mandibular angle from 128 wild boars, hunted in Greece, were collected. The samples were examined by classical parasitological (compression, artificial digestion, and Alaria spp. migration) and by molecular (real-time PCR) methods. For Trichinella spp. an existent real-time PCR detecting all species likely to be present in Greece was applied, while for Alaria spp. a real-time PCR was developed, employing an LNA TaqMan probe targeting the large subunit ribosomal RNA gene. All examined wild boar samples from Greece resulted negative for Trichinella and Alaria species, indicating a low prevalence of infection in the examined population. The novel real-time PCR for Alaria spp. has 81.5% amplification efficiency and is able to detect 0.12 larvae per 50 g of tissue and could be utilized as a complementary to AMT diagnostic tool in surveillance.
1. Introduction
Foodborne diseases (FBDs) are an important public health problem worldwide, with economic and social implications that involve a wide range of pathogens, i.e., viruses, bacteria, and parasites [1,2]. Foodborne parasitic diseases (FBPDs), many of which are developed after the consumption of infected meat (e.g., toxoplasmosis, sarcocystosis, taeniosis, cysticercosis), represent a major part of FBDs but have not been adequately studied or monitored due to their complex epidemiology/epizootiology and their usually non-acute but rather chronic clinical implications [3,4]. Among food transmitted parasites, Trichinella spp., the agent of trichinellosis, and Alaria spp., the agent of alariosis, are of particular interest due to their potentially severe or even fatal outcome in human infections. Trichinellosis and alariosis share some common features in terms of transmission, diagnosis, and prevention. For example, they are exclusively foodborne, transmitted by the consumption of raw or undercooked infected meat, and domestic pig and wild boar meat are a common source of human infection. Consequently, suitable food inspection methods and thermal treatment of meat may prevent human infection by both parasites [5,6].
Trichinella spp. are nematode parasites, affecting mainly mammals but also birds and some reptile species. Their life cycle is completed in the same host: adult parasites inhabit the intestine, and females lay larvae in the intestinal mucosa. These larvae migrate via the lymphatic and blood vessels to the muscles, especially the highly oxygenated ones, which represent their predilection sites. A new host is infected by consuming raw or undercooked, infected meat. The genus Trichinella is divided into two clades (encapsulated or non-encapsulated), depending on the presence or absence of a capsule, enclosing the parasite in the hosting muscle cell (“nurse cell”) [7]. The encapsulated species are Trichinella spiralis, Trichinella britovi, Trichinella nativa Trichinella murrelli, Trichinella nelsoni, Trichinella patagoniensis, Trichinella chanchalensis, and the genotypes T6, T8, and T9, while the non-encapsulated species are Trichinella pseudospiralis, Trichinella papuae, and Trichinella zimbabwensis [8]. Humans are susceptible to different Trichinella species; however, T. spiralis is the most commonly identified in human cases [7]. The World Health Organization (WHO) ranks trichinellosis as one of the most important FBD due to its impact on human health [3,9].
The trematode Alaria spp. has an indirect, three-host life cycle. The adult parasite inhabits the intestine of carnivores of the families Canidae, Felidae, and Mustelidae (definitive hosts), and the larval stages develop in water snails (first intermediate host) and amphibians (second intermediate host), where the parasite develops into mesocercariae, also known as Distomum musculorum suis (DMS), which is the infective stage for definitive hosts. Mesocercariae are an interjectional stage and change inside the definitive host to metacercariae and then to adult parasites [6]. Paratenic hosts play an important role in the life cycle of Alaria spp. by maintaining and accumulating mesocercariae in their tissues, spreading the infection in more animal species, thus facilitating the infection of the definitive hosts [6]. The migration of the mesocercarial stage is the cause of alariosis that affects both humans and animals. Humans become infected by eating raw or undercooked meat from infected second intermediate and paratenic hosts, such as wild boars [6]. The species Alaria americana is incriminated for most human cases, and it is present in the Americas where the species Alaria mustelae, Alaria intermedia, Alaria marcianae, Alaria arisaemoides, Alaria canis, and Alaria taxideae have also been identified [6,10,11]. Alaria alata is the species found in Europe, and it is closely related to the zoonotic A. americana, as it has a similar life cycle with various wild animals as paratenic hosts that harbor mesocercariae. Furthermore, it has been shown that A. alata mesocercariae are infectious for primates (the Rhesus monkey Macaca mulatta) [12], and thus, it is most likely infectious for humans as well. As a possible cause of FBPD, A. alata is included in the list of zoonotic agents in some countries, e.g., Switzerland and Germany [6,11].
In Greece, human trichinellosis was reported for the first time in 1946 [13]. Since then, the infection has been sporadically reported in humans and animals [14]. Epizootiological studies on trichinellosis in Greece are limited [15,16], leaving a significant gap in our knowledge on the frequency of parasitism in animals, as well as the species of parasites that are enzootic in the country. On the other hand, Alaria spp. has been reported as an adult in several species of wild and domestic carnivores [17,18,19,20] but never in the form of DMS in meat or other tissue.
Both Trichinella spp. and Alaria spp. can be detected in meat by classical parasitological methods, i.e., tissue compression and artificial digestion (AD), while Alaria spp. can be also detected by Alaria spp. migration technique (AMT) [11]. At the food inspection level and according to the EU legislation [21], examination for Trichinella is obligatory for all slaughtered pigs and wild boars, as well as for meat from other game animals, and it is performed by magnetic stirrer artificial digestion before releasing to the consumption. Molecular methods have been developed mainly for the detection and identification of Trichinella spp. at the experimental/investigational level [22,23,24], while for the molecular detection of Alaria spp. only one conventional PCR method has been published so far [25].
The development of novel, sensitive, fast, and cheap methods for the detection of these important food-transmitted parasites should always be a priority for the scientific community. This is particularly true for alariosis, as it is considered an emerging and potentially severe disease for humans [6,26], and to date, no such modern methods have been developed.
In this context, the aims of the present study were (a) to evaluate the prevalence of Trichinella spp. and A. alata in wild boars (Sus scrofa) in Greece, by classical parasitological and by molecular methods, and (b) to develop and apply a highly sensitive and specific real-time PCR protocol for the detection of A. alata in tissues.
2. Materials and Methods
2.1. Samples
2.1.1. Field Samples
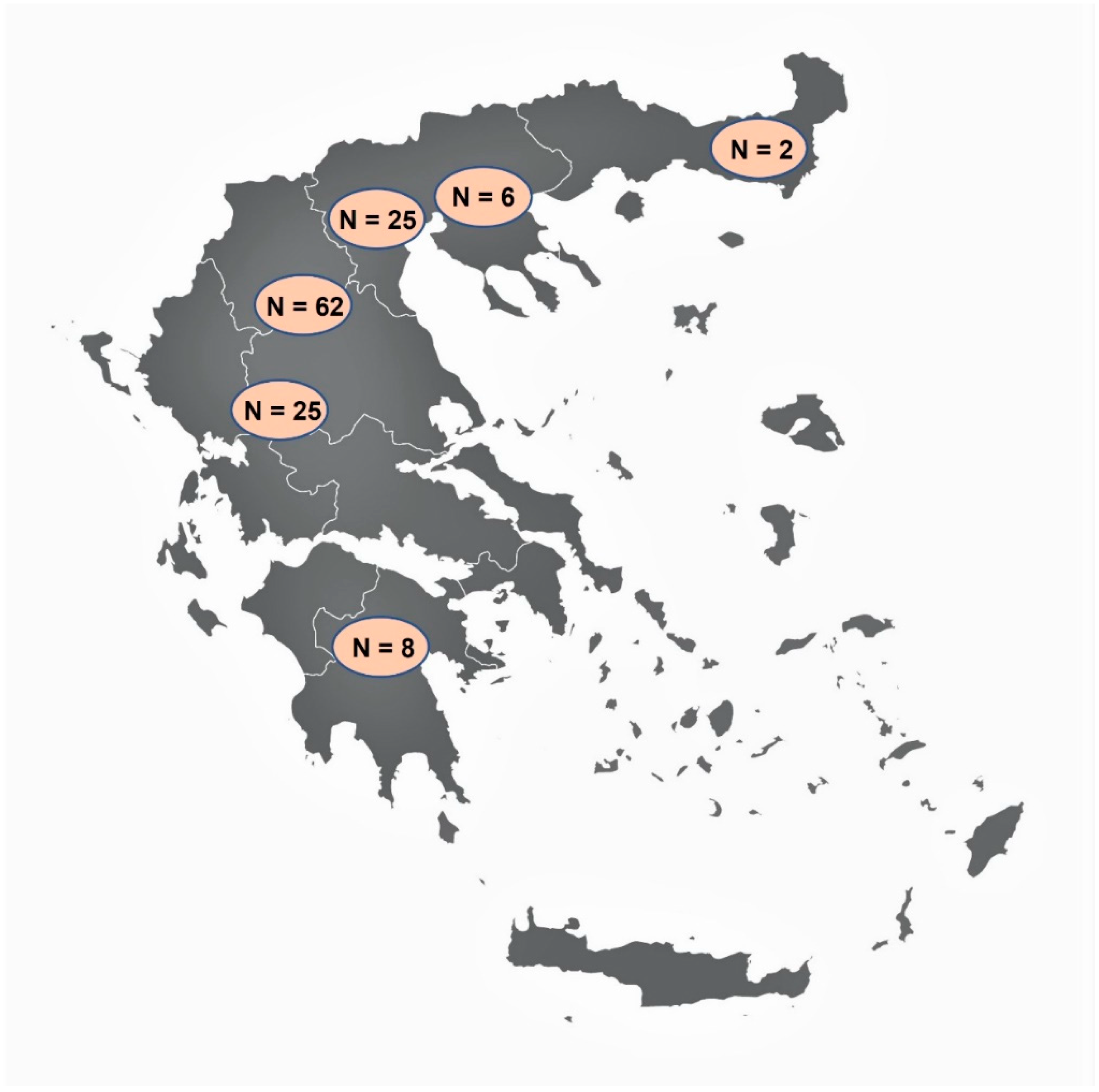
Tissue samples from 128 wild boars were collected during the hunting season 2019–2020 (i.e., October 2019 to January 2020). Sampling was performed voluntarily by hunters, mostly in areas of northern Greece (Figure 1), after a brief training regarding the procedure. For Trichinella spp. detection, a minimum of 15 g muscle tissue from the pillars of the diaphragm (diaphragm pillars, DP) was collected, as this is one of the predilection sites of the parasite [27]. For Alaria spp. detection, a minimum of 110 g of tissue from the region of the mandibular angle including, connective, fat, glandular, lymphatic, and muscle tissues (mandibular area tissues, MT) were sampled, as it has been proven that this is one of the best sites for Alaria spp. detection [28]. All samples were stored in separate containers at 4–7 °C and sent to the Laboratory of Parasitology and Parasitic Diseases, School of Veterinary Medicine, Aristotle University of Thessaloniki, at the latest 48 h after collection.
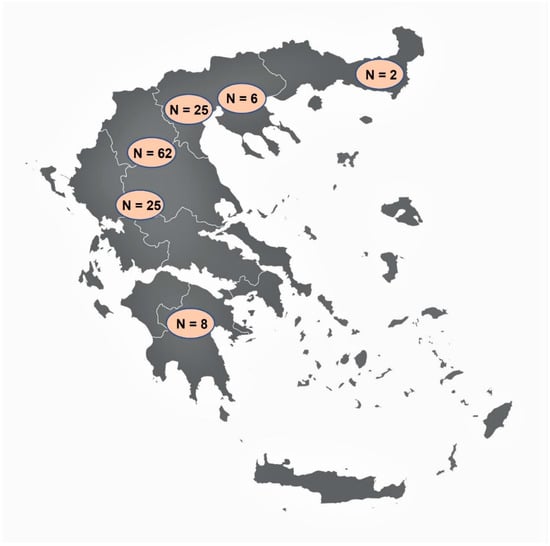
Figure 1.
The map of Greece showing the areas where the examined wild boars originated from, and the number of animals (N) examined from each area (vector map source: https://freevectormaps.com/greece/GR-EPS-01-0002?ref=atr, accessed on 21 September 2021).
2.1.2. Control Samples
Trichinella spp. of all species that are present or could be present in Greece, i.e., T. spiralis, T. britovi, and T. pseudospiralis, conserved in 90–95% ethyl alcohol, were kindly provided by Dr. Edoardo Pozio, Istituto Superiore di Sanità, Rome, and were used as reference DNA and positive controls.
Alaria alata parasites originated from the collection of the Institute of Food Safety, Animal Health and Environment “BIOR”, Latvia. More precisely, 25 mesocercariae isolated by AMT from wild boar muscle tissue were used for reference DNA. Furthermore, six A. alata infected wild boar meat samples from Latvia were used as positive controls. The parasitic load in these six control samples was determined by AMT and it was 7 (in 3 of the samples), 1, 3, and 76 mesocercariae per 50 g of meat tissue.
2.2. Examination Methods
2.2.1. Tissue Compression
From each animal, tissue samples of 1g each from DP and MT were examined by the tissue compression method [21,29]. Briefly, the samples were cut to smaller pieces (rice grain size), compressed between two glass plates (compressorium), and examined under the stereomicroscope (20–40× magnification) and the optical microscope (100× magnification).
2.2.2. Processing of DP Samples and Magnetic Stirrer Artificial Digestion
All the DP tissue samples were examined by the magnetic stirrer AD performed according to the standard protocol and the latest directives of the European Union for the control of trichinellosis [21,30,31]. Initially, 11 g of muscle tissue were minced with 11 mL of saline (NaCl 0.9%) in a meat mincer at max speed for 2–3 s, then the blender was opened, and any muscle piece at the walls of the bowl was transferred to the bottom and blending continued with 2–3 s bursts until no visible pieces of meat remained [30]. An aliquot of 1 mL of the homogenized material was stored at −80 °C, prior to processing for DNA extraction and further analysis by PCR. The rest of the material was mixed with the artificial digestion fluid [1.6 mL 25% HCl and 1 ± 0.02 g of pepsin (1:10,000 NF, 1:12,500 BP, 2000 FIP) to 200 mL of tap water at 46–48 °C]. The fluid was stirred vigorously for less than 60 min to ensure adequate digestion. Once the digestion was performed, the digestion fluid was poured through a 180 µm sieve into a sedimentation glass funnel. After 30 min, the supernatant was discarded, and at least 80 mL of the sediment were examined under a stereomicroscope at 20–40× magnification.
2.2.3. Processing of MT Samples and Alaria spp. Migration Technique
All the MT samples were examined by AMT according to the classical protocol of larval migration [32], implementing the modifications proposed by Riehn et al. [28]. In brief, 50 g were chopped into small pieces (approximately 0.5 cm edge length), transferred to a sieve, and immersed over a glass funnel filled with warm tap water (46–48 °C, cooling to room temperature) for 90–120 min. At least 40 mL of the sediment were examined under a stereomicroscope at 20–40× magnification.
For DNA extraction, an additional quantity of 50 g of tissues, were minced with 25 mL of PBS to ensure complete homogenization. An aliquot of 1 mL of the homogenized material was stored at −80 °C, prior to processing for DNA extraction and further analysis by PCR.
2.2.4. Molecular Based Methods
All DP and all MT samples (a total of 256 samples) were examined separately for Trichinella spp. and for Alaria spp. detection, i.e., each animal was examined by molecular-based methods twice for each parasite (DP and MT).
DNA was extracted from the tissue samples according to a previously described protocol [33], with some modifications. Specifically, 450 μL of the DP and MT homogenized material was transferred to 1950 μL of lysis buffer [4.2 M guanidinium iso-thiocyanate, 1.2 Μ guanidinium hydrochloride, 50 mM Tris-HCl (pH 7.5), 25 mM EDTA, 1% N-lauroylsarcosine, 2% Triton X-100, pH 7.0] and vortexed thoroughly. Then, 250 μL of lysate was transferred to a 2 mL microcentrifuge tube and mixed with 850 μL of lysis buffer, followed by the addition of 600 μL phenol (equilibrated, stabilized, pH 7.8–8.2) and 200 μL of chloroform–isoamyl alcohol (24:1). The remaining steps of the protocol were performed as described previously [33], and 60 μL of preheated (75 °C) elution buffer (5 mM Tris-HCl, pH 8.5) was used for DNA recovery.
DNA purification from ethyl alcohol stored reference larvae was also performed using the aforementioned published protocol [33], with some modifications. Specifically, following centrifugation (500× g, 10 min), the supernatant was discarded, and the resulting pellets were resuspended in 250 μL of phosphate-buffered saline (PBS). The pellet suspensions (250 μL) were mixed with 850 μL of Lysis buffer. The remaining steps of the protocol were performed as described previously (2)l and 60 μL of preheated (75 °C) elution buffer (5 mM Tris-HCl, pH 8.5) was used for DNA recovery.
For the detection of Trichinella spp. a real-time PCR was performed according to a published protocol [24] able to detect all known Trichinella species in muscle samples from various wild animals.
For the molecular detection of A. alata DNA, a real-time PCR methodology was developed, employing an LNA TaqMan probe and targeting the large subunit ribosomal RNA gene (lsrDNA), as previously suggested for a respective conventional PCR protocol [25]. The oligonucleotide selection was based on homologous lsrDNA gene regions of A. alata, A. americana, A. marcianae, and A. mustelae (N = 27) and closely related Diplostomum spp. (N = 14) available in GenBank. All sequences were imported in MEGA 7 software [34] and were aligned by MUSCLE [35]. Primers and probes for the specific detection of all Alaria species were designed from conserved genomic regions and were further evaluated for their melting temperature (Tm) and self- or hetero- dimer formation using the IDT OligoAnalyzer 3.1 software (https://eu.idtdna.com/calc/analyzer, accessed on 15 June 2021) [36]. Selected primers AlarDME1F2 and AlarDME1R2 amplify a 309 bp region (Table 1).

Table 1.
Primers, TaqMan probe, and amplification conditions used in the developed assay.
A highly conserved short region located 115 nucleotides downstream of the AlarDME1F2 3′-end was identified as suitable for the design of a probe containing locked nucleic acid (LNA) modifications. For efficient mismatch discrimination with the closely related Diplostomum spp. sequences presenting two specific A/G mutations in the homologous targeted probe region, a triplet of LNA modifications with the central base of the triplet at each mismatch site, was incorporated [37,38].
The optimized real-time PCR amplification was performed in a final volume of 20 μL. Reactions comprised 1 × PCR buffer [(Tris·Cl, KCl, (NH4)2SO4, 15 mM MgCl2; pH 8.7 (20 °C), (Qiagen, Hilden, Germany)] and 5 U of HotStarTaq DNA polymerase (Qiagen, Hilden, Germany), 3 mM MgCl2, 0.2 mM of each dNTP, the primers AlarDME1F2 and AlarDME1R2 (Integrated DNA Technologies; IDT, Coralville, IA, USA), the LNA TaqMan probe AlAlprobe (IDT) at concentrations shown in Table 1, nuclease-free water, and 2 μL of DNA extract. The assay was performed using a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA), with the cycling conditions shown in Table 1. Fluorescence was measured at the end of each cycle and analysis of fluorescence data was performed using CFX Maestro Software (v4.1, Bio-Rad Laboratories).
The PCR efficiency for Alaria spp. detection was determined by amplification of 10-fold serial dilutions of pure A. alata genomic DNA prepared in siliconized tubes with nuclease-free water containing 50 ng of carrier RNA (QIAGEN, Hilden, Germany) per µL and were stored at −80 °C. Dilution series contained 2.5 × 10−2–2.5 × 10−6 larvae per PCR assay in 4 replicates. Specificity was assessed by testing purified genomic DNA derived from negative samples spiked with Trichinella spiralis, Trichinella pseudospiralis, and Trichinella britovi genomic DNA. In order to evaluate the presence of PCR inhibitors in muscle samples, PCR assays of genomic DNA of AMT negative muscle tissue (n = 3) were spiked with A. alata genomic DNA (2.5 × 10−2 larvae) and was tested in parallel with A. alata genomic DNA samples (2.5 × 10−2 larvae per PCR assay).
3. Results
3.1. Field Samples’ Results
All classical parasitological methods, i.e., compression, AD, and AMT, of all examined samples were negative for Trichinella spp. larvae and for Alaria spp. mesocercariae. The results of the classical parasitological methods were confirmed by the molecular methods as real-time PCRs of all DP and MT were negative for Trichinella spp. and Alaria spp. infection.
3.2. Performance of Real-Time PCR for Alaria spp. Detection
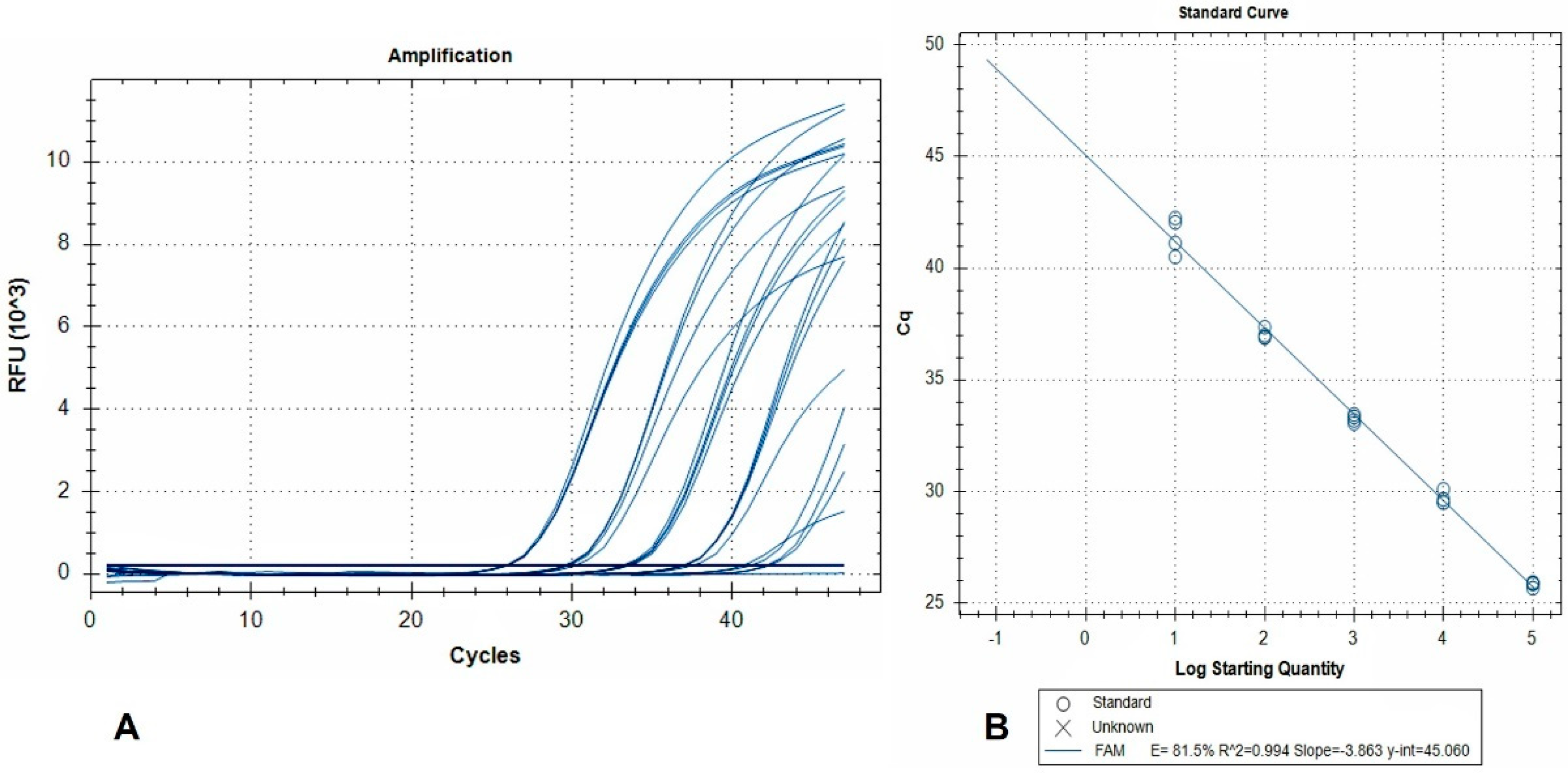
The standard curve and linear regression analysis revealed a linear range of quantification extending over a 5-log10 range (2.5 × 10−2–2.5 × 10−6 larvae per reaction), and amplification efficiency of 81.5% (Figure 2).
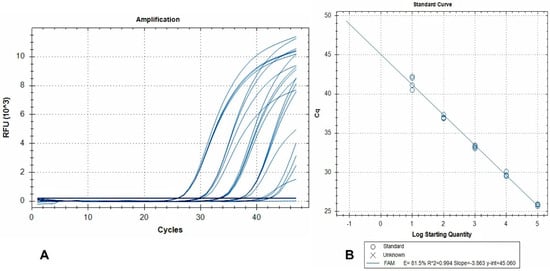
Figure 2.
(A) Amplification plots (FAM fluorescence signals) generated by a dilution series of pure A. alata genomic DNA. Curves represent 10-fold DNA serial dilutions, from 2.5 × 10−2 (left) to 2.5 × 10−6 (right) larvae per reaction, tested in 4 replicates. Non-template controls are also included; (B) the corresponding standard curve. RFU: relative fluorescence units.
This indicates that detection of 2.5 × 10−6 larvae per reaction is achievable, and when accounting for the dilutions due to DNA extraction from 50 g AMF samples, this level of sensitivity corresponds to 0.12 larvae per 50 g.
The developed real-time PCR assay successfully tested positive all naturally infected tissue samples (i.e., positive controls). In contrast, the assay delivered a negative result when testing purified genomic DNA derived from negative samples spiked with T. spiralis, T. pseudospiralis, and T. britovi genomic DNA. The observed Ct shift (0.073) between the A. alata spiked DNA extracts and inhibition controls was negligible, indicating no inhibition of the real-time PCR.
4. Discussion
Food transmitted parasites and related diseases are often neglected, at the level of food safety control systems and etiological diagnosis. The apparent reasons are that infected animals usually do not show any clinical signs, associated monetary losses are not easy to determine, and FBPDs often remain subclinical in humans [39]. However, occasionally, FBPDs have significant health implications and may be fatal.
Trichinellosis is transmitted to humans by the consumption of raw or inadequately cooked meat and raw cured meat products. Humans trichinellosis may remain asymptomatic; however, clinical manifestations often include an initial phase of nonspecific symptoms, e.g., headache, malaise, fever, and gastrointestinal disorders (epigastric pain, diarrhea, nausea, and vomiting) and a subsequent phase characterized by myalgia, arthralgia, dyspnoea, as well as some characteristic, but not always present, lesions, i.e., periorbital or facial edema and subungual petechiae. Severe complications such as myocarditis and encephalitis can also occur and may lead to death [7,40]. Despite the reduction of cases numbers in the past 10 years, human trichinellosis is still reported in Europe. During the period 2013–2017, 1022 new cases have been reported in the EU [41], while according to the latest report, 96 cases of human trichinellosis were confirmed in 2019, i.e., 0.02 cases per 100,000 population [42].
On the other hand, to date, human alariosis has not been confirmed in Europe [43]. In their review, Möhl et al. [6] cite seven human cases, as attributed to Alaria spp., albeit the etiological diagnosis was not always confirmed. All these cases were reported between 1969 and 1993 in the USA and Canada and in those for which the investigation was fruitful, indicated that the source of human infection was game meat (paratenic host) or frog legs (second intermediate host). Ocular (unilateral decreased vision, diffuse unilateral subacute neuroretinitis), skin (intradermal swellings), and generalized parasitism have been described in human alariosis [44,45,46,47]. The case of generalized infection had a fatal outcome, as thousands of mesocercariae migrated to vital organs of the patient, who died just 9 days from the onset of symptoms, due to respiratory insufficiency caused by extensive pulmonary hemorrhage [45]. The various paratenic hosts of the parasite that include wild mammals and birds may have an important role in the transmission of this zoonosis, especially given the growing popularity and need for game and organic meat [10].
Trichinella spp. and Alaria spp. have been found in wild boar meat (in single and mixed infection), and therefore, this kind of meat has been reasonably characterized as a potentially important source for human infection by both parasites [6]. According to a quantitative microbial risk assessment applied by Franssen et al. [5], wild boar meat is incriminated for 55% of modeled cases of human trichinellosis. The same model suggests that wild boars have a 4100 times higher prevalence of Trichinella infection than pigs raised in non-controlled farming conditions [5].
Nevertheless, the prevalence found in wild boars in Europe may be characterized as low, as it has been found less than 1% in all cases. In some of the most recent, wide-range epizootiological surveys in Europe where AD methods were implemented, hundreds or thousands of wild boars were examined (in most cases these surveys were analysis of national authorities’ records); the prevalence of infection was 0.1% in Portugal [48], 0.17% in Croatia [49], 0.04% in Slovakia [50], 0% in Denmark [51], and 0.51% in Poland [52]. In a survey similar to the present study, conducted in Italy, diaphragm muscle samples of 100 wild boars hunted in two hunting seasons were examined, and none (0%) was found positive to Trichinella larvae [53]. Despite the generally low prevalence of infection, wild boar meat is to date the second, after pork, the most frequent source of human trichinellosis [54], presumably because it is the most popular game meat in most parts of Europe.
Interestingly, larvae of T. britovi, i.e., the only species identified in Greece so far in free-ranging domestic pigs and in humans [14,15], and presumably, the dominant species in the area, show significant differences in abundance and life span in pig muscles, compared to T. spiralis [55]. It was shown that 2 months post infection (p.i.), there is a remarkably lower number of T. britovi LPG in pig meat, i.e., 70 times lower than T. spiralis, and that these larvae had a shorter life span, as only a few survived for 6 months (none was detected 12 months p.i.), while T. spiralis were alive at least 2 years p.i. [55]. This short life span most probably results in a minimum accumulation of larvae in the muscles of wild boars and could explain the absence of larvae in the examined animals in Greece. In contrast to the short life span of larvae, antibodies can be detected for longer time periods (at least two years for both T. britovi and T. spiralis) and thus serosurveys investigating Trichinella infection in wild boars usually result in higher prevalences [55]. For example, in a serosurvey for Trichinella antibodies in wild boars in Greece, the seropositivity was 6.4% [16]. Similarly, antibodies were detected in 32 out of the 1462 wild boars in Italy, while in only one of them (1/1462), T. britovi larvae were found by AD [56].
Alaria alata mesocercariae can be found by the AD method applied obligatorily according to the current European regulations to all wild boars entering commercial circuits. As a result of the systematic Trichinella inspection, A. alata has been detected as an incidental finding in many European countries [6,57]. However, the samples for Trichinella inspection have to be free from fat, while Alaria shows a particular affiliation to adipose tissue [6]. Furthermore, it has been shown that apart from the diaphragm, the “cheek”, i.e., the various tissues included in the caudoventral region of the head (muscle, connective, fat, glandular, and lymphatic tissues) [28], as well as the tongue, muscle around the larynx, and intercostal muscles [58], are suitable spots for Alaria detection. It is thus reasonable to assume that the prevalence of Alaria found in the obligatory meat inspection for Trichinella in slaughterhouses is significantly underestimated. In some of the most recent surveys, conducted especially for the detection of Alaria mesocercariae, thus implementing the most sensitive direct method of detection, i.e., AMT [59], the prevalence found in European countries was 44.3% in northeastern Poland [26], 43.9% in Latvia [58], 11.5% in Germany [60], 10.3% in Serbia [61], 6.8% in the Czech Republic [62], 6% in Austria [63], 4.2% in Poland [64], 1.6% in Hungary [65], and 1% in northern regions of Italy [53]. In the most recent survey in Germany, the prevalence of infection in wild boar meat was 28.3%, i.e., the highest ever recorded in the country [66]. Similarly, Portier et al. [57] observed a clear and steady increase of A. alata detection in wild boar meat in the eastern areas of France (a rise in prevalence from 1.5% in 2007 to 6.6% in 2011), suggesting that this should be considered a true emergence of the parasite.
While wetlands are significant for the frequency of Alaria in wild boar meat, as the life cycle of the parasite includes two water-dependent organisms (water snails and amphibians), elevation also seems to play a role [57,58]. The average elevation of Greece is 247 m, however, most of the samples were collected from areas with an elevation of more than 300 m and this may have affected the results. Even though some of the examined wild boars were hunted close to wetlands, no infection with Alaria was detected in any of these samples. Finally, there is evidence that A. alata prevalence in wild boards is higher in the summer season, which is attributed to increased activity of the second intermediate host and to an adapted wild boar diet [58]. In the present study, most of the samples were collected during the winter months, i.e., the wild boar hunting season in Greece, which is generally between mid-September and mid-January. Mesocercariae of A. alata have never been found in Greece in any animal species. However, the parasite (eggs or adult trematodes) has been found in some species of final hosts in the country, e.g., in wolves (0.7%) [19], foxes (1.6%), jackals (20%) [17], wildcats (17.4%) [20], and dogs (2.5%) [18].
Sensitive and practical methods of Alaria mesocercariae detection are necessary for monitoring the parasite’s geographic distribution, prevalence, and range of paratenic hosts, in order to assess the parasite’s epizootiological trends and any risk for human infection. In this context, it has been repeatedly shown that even though the parasite may be found by the compression method and by AD, the most effective is the AMT, probably because mesocercariae are sensitive to the AD procedure and many are destroyed [28,58]. In a recent comparative study, Strokowska et al. [11] showed that of the 43 mesocercariae positive samples found by AMT, only 20 were positive by the magnetic stirrer AD method, and 25 by AD using Pancreatin® bile and pancreatic enzymes, while the less sensitive method was compression. Even though AMT is the most effective method for mesocercariae detection, a combination of AD and AMT would reveal more Alaria positive meat samples than any one of these methods alone [58]. In the present study, each animal was examined by both methods, i.e., DP sample by AD and the MT by AMT, while additionally, compression method was applied to both samples, in an effort to increase the overall sensitivity of inspection. The negative result of these classical methods was confirmed by the very sensitive, newly developed real-time PCR.
Molecular diagnosis and identification of A. alata are based on previously developed conventional PCR [25], which uses primers targeting the large subunit ribosomal RNA gene. Furthermore, a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) method has been recently tested for the identification of Alaria mesocercariae isolated from various hosts, with promising results [67]. In the present study, the previously designed primers [25] were modified to accommodate for real-time PCR conditions, and an LNA modified TaqMan probe was further designed for the specific detection and quantification of Alaria spp. genomic DNA, thus creating a tool for the confirmation of Alaria spp. infection in suspected samples. The developed methodology proved to be specific and in complete agreement with AMT. The developed real-time PCR showed 81.5% amplification efficiency, and this can be attributed to the large size of the amplicon (309 bp). However, it was proved to be sensitive (0.12 larvae per 50 g) and was able to test positive, animals with parasitic load from 1 up to 76 mesocercariae per 50 g of meat tissue. Importantly, the real-time PCR format exhibits minimum laboratory contamination risk and minimizes the chances of false-positive results, compared to gel-based assays. This assay is the first real-time A. alata detection method aiming at rapid diagnosis in clinical specimens. This method could be applied as a complementary to AMT diagnostic tool in future surveillance programs.
5. Conclusions
Trichinellosis is one of the most significant FBPD worldwide. Even though the modern systems of pig farming have diminished the infection in pig meat, game meat remains an important source of human infection. The increase of game meat consumption evidenced in recent years [10] renders the continuous vigilance and monitoring of this infection, at the level of meat inspection and at the level of surveillance surveys, an imperative. On the other hand, A. alata, as an agent of a potentially emerging disease and a possible threat to human health, should also be monitored in a more systematic way. It is thus important to increase awareness about this FBPD in the scientific community, food industry, and general public.
Toward this end, new, effective, and sensitive methods of detection that may be applied at the meat inspection level, as well as at the laboratory level, are needed. Indeed, the WHO supports the development and use of standardized methodologies for the assessment of FBD diseases in different countries, as a tool for reliable monitoring and effective, harmonized food safety policy [1]. Hopefully, the results of the present study contribute toward this end by describing a sensitive and specific real-time PCR that may be applied in various investigation settings of alariosis.
Author Contributions
Conceptualization, D.D. and A.D.; methodology, D.D., T.C., C.I.D., Z.O. and A.D.; software, T.C. and C.I.D.; validation, A.D. and C.I.D.; investigation, D.D., A.D. and T.C.; resources, D.D. and A.D.; data curation, D.D. and T.C.; writing—original draft preparation, D.D. and A.D.; writing—review and editing, D.D., T.C., C.I.D., Z.O. and A.D.; supervision, A.D.; project administration, A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research is co-financed by Greece and the European Union (European Social Fund-ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning 2014–2020» in the context of the project “Foodborne parasitic zoonoses: epizootiological investigation of wild boars as reservoir of the parasites Trichinella spp. and Alaria spp. and development of modern diagnostic methods” (MIS 5047896).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data produced in this study are provided herein.
Acknowledgments
The Authors would like to express their acknowledgments to Edoardo Pozio for kindly providing the reference Trichinella samples and for all his help and support. Many thanks are also due to the Hunting Organization of Macedonia and Thrace and all the hunters that provided the samples for this survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pires, S.M.; Desta, B.N.; Mughini-Gras, L.; Mmbaga, B.T.; Fayemi, O.E.; Salvador, E.M.; Gobena, T.; Majowicz, S.E.; Hald, T.; Hoejskov, P.S.; et al. Burden of foodborne diseases: Think global, act local. Curr. Opin. Food Sci. 2021, 39, 152–159. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015; ISBN 9241565160. [Google Scholar]
- van der Giessen, J.; Deksne, G.; Gómez-Morales, M.A.; Troell, K.; Gomes, J.; Sotiraki, S.; Rozycki, M.; Kucsera, I.; Djurković-Djaković, O.; Robertson, L.J. Surveillance of foodborne parasitic diseases in Europe in a One Health approach. Parasite Epidemiol. Control 2021, 13, e00205. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J. Parasites in Food: From a Neglected Position to an Emerging Issue. Adv. Food Nutr. Res. 2018, 86, 71–113. [Google Scholar] [CrossRef] [PubMed]
- Franssen, F.; Swart, A.; van der Giessen, J.; Havelaar, A.; Takumi, K. Parasite to patient: A quantitative risk model for Trichinella spp. in pork and wild boar meat. Int. J. Food Microbiol. 2017, 241, 262–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Möhl, K.; Große, K.; Hamedy, A.; Wüste, T.; Kabelitz, P.; Lücker, E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae—A review. Parasitol. Res. 2009, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Zarlenga, D.; Thompson, P.; Pozio, E. Trichinella species and genotypes. Res. Vet. Sci. 2020, 133, 289–296. [Google Scholar] [CrossRef]
- Murrell, K.D.; Pozio, E. Worldwide Occurrence and Impact of Human Trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar] [CrossRef]
- Korpysa-Dzirba, W.; Różycki, M.; Bilska-Zając, E.; Karamon, J.; Sroka, J.; Bełcik, A.; Wasiak, M.; Cencek, T. Alaria alata in Terms of Risks to Consumers’ Health. Foods 2021, 10, 1614. [Google Scholar] [CrossRef]
- Strokowska, N.; Nowicki, M.; Klich, D.; Didkowska, A.; Filip-Hutsch, K.; Wiśniewski, J.; Bełkot, Z.; Anusz, K. A comparison of detection methods of Alaria alata mesocercariae in wild boar (Sus scrofa) meat. Int. J. Parasitol. Parasites Wildl. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Odening, K. Der “Dunckersche Muskelegel” kann experimentell auf den Affen übertragen werden. Monatsh. Veterinarmed. 1961, 16, 395–399. [Google Scholar]
- Livieratos, S.; Danopoulos, E.; Logothetopoulos, I. The first outbreak of trichinellosis in Greece: Study of the disease. Hell. Iatriki 1946, 17, 681–710. [Google Scholar]
- Dimzas, D.; Diakou, A.; Koutras, C.; Gómez Morales, M.A.; Psalla, D.; Keryttopoulos, P.; Deligianni, D.; Kontotasios, K.; Pozio, E. Human trichinellosis caused by Trichinella britovi in Greece, and literature review. J. Helminthol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Boutsini, S.; Papatsiros, V.G.; Stougiou, D.; Marucci, G.; Liandris, E.; Athanasiou, L.V.; Papadoudis, A.; Karagiozopoulos, E.; Bisias, A.; Pozio, E. Emerging Trichinella britovi infections in free ranging pigs of Greece. Vet. Parasitol. 2014, 199, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Touloudi, A.; Valiakos, G.; Athanasiou, L.V.; Birtsas, P.; Giannakopoulos, A.; Papaspyropoulos, K.; Kalaitzis, C.; Sokos, C.; Tsokana, C.N.; Spyrou, V.; et al. A serosurvey for selected pathogens in Greek European wild boar. Vet. Rec. Open 2015, 2, e000077. [Google Scholar] [CrossRef]
- Papadopoulos, H.; Himonas, C.; Papazahariadou, M.; Antoniadou-Sotiriadou, K. Helminths of foxes and other wild carnivores from rural areas in Greece. J. Helminthol. 1997, 71, 227–231. [Google Scholar] [CrossRef]
- Papazahariadou, M.; Founta, A.; Papadopoulos, E.; Chliounakis, S.; Antoniadou-Sotiriadou, K.; Theodorides, Y. Gastrointestinal parasites of shepherd and hunting dogs in the Serres Prefecture, Northern Greece. Vet. Parasitol. 2007, 148, 170–173. [Google Scholar] [CrossRef]
- Diakou, A.; Karaiosif, R.; Petridou, M.; Iliopoulos, Y. Endoparasites of the wolf (Canis lupus) in central Greece. In Proceedings of the 11th European Wildlife Diseases Association Conference, Edinburgh, UK, 26–29 August 2014. [Google Scholar]
- Diakou, A.; Migli, D.; Dimzas, D.; Morelli, S.; Di Cesare, A.; Youlatos, D.; Lymberakis, P.; Traversa, D. Endoparasites of european wildcats (Felis silvestris) in Greece. Pathogens 2021, 10, 594. [Google Scholar] [CrossRef]
- European-Commission. Commission regulation (EC) no 2015/1375 of 10 August 2015 laying down specific rules for Trichinella in meat. Off. J. Eur. Union 2015, 212, 7–34. [Google Scholar]
- Zarlenga, D.S.; Chute, M.B.; Martin, A.; Kapel, C.M.O. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int. J. Parasitol. 1999, 29, 1859–1867. [Google Scholar] [CrossRef]
- Pozio, E.; La Rosa, G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003, 216, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Cuttell, L.; Corley, S.W.; Gray, C.P.; Vanderlinde, P.B.; Jackson, L.A.; Traub, R.J. Real-time PCR as a surveillance tool for the detection of Trichinella infection in muscle samples from wildlife. Vet. Parasitol. 2012, 188, 285–293. [Google Scholar] [CrossRef]
- Riehn, K.; Hamedy, A.; Alter, T.; Lücker, E. Development of a PCR approach for differentiation of Alaria spp. mesocercariae. Parasitol. Res. 2011, 108, 1327–1332. [Google Scholar] [CrossRef]
- Strokowska, N.; Nowicki, M.; Klich, D.; Bełkot, Z.; Wiśniewski, J.; Didkowska, A.; Chyla, P.; Anusz, K. The occurrence of Alaria alata mesocercariae in wild boars (Sus scrofa) in north-eastern Poland. Int. J. Parasitol. Parasites Wildl. 2020, 12, 25–28. [Google Scholar] [CrossRef]
- Nöckler, K.; Kapel, C.M.O. Detection and surveillance for Trichinella: Meat inspection and hygiene, and legislation. In FAO/WHO/OIE Guidelines for the Surveillance, Management, Prevention and Control of Trichinellosis; Dupouy-Camet, J., Murrell, K.D., Eds.; World Organization for Animal Health (OIE): Paris, France, 2007; pp. 69–98. [Google Scholar]
- Riehn, K.; Hamedy, A.; Große, K.; Zeitler, L.; Lücker, E. A novel detection method for Alaria alata mesocercariae in meat. Parasitol. Res. 2010, 107, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.B.; Parker, S.; Scandrett, W.B. Comparison of a modified digestion assay with trichinoscopy for the detection of Trichinella larvae in pork. J. Food Prot. 2003, 66, 1043–1046. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Pozio, E.; Gayda, J.; Thaben, N.; Bahn, P.; Nöckler, K. Magnetic stirrer method for the detection of Trichinella larvae in muscle samples. J. Vis. Exp. 2017, 2017, 55354. [Google Scholar] [CrossRef]
- Dupouy-Camet, J.; Murrell, K.D. (Eds.) FAO/WHO/OIE Guidelines for the Surveillance, Management, Prevention and Control of Trichinellosis; Food and Agriculture Organization of the United Nations (FAO): Roma, Italy; World Health Organization (WHO): Geneva, Switzerland; World Organisation for Animal Health (OIE): Paris, France, 2007; ISBN 9290447044. [Google Scholar]
- Ministry of Agriculture Fisheries and Food (MAFF). Manual of Veterinary Parasitological Techniques, 3rd ed.; Her Majesty’s Stationery Office: London, UK, 1986.
- Chassalevris, T.; Chaintoutis, S.C.; Apostolidi, E.D.; Giadinis, N.D.; Vlemmas, I.; Brellou, G.D.; Dovas, C.I. A highly sensitive semi-nested real-time PCR utilizing oligospermine-conjugated degenerate primers for the detection of diverse strains of small ruminant lentiviruses. Mol. Cell. Probes 2020, 51, 101528. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Owczarzy, R.; Tataurov, A.V.; Wu, Y.; Manthey, J.A.; McQuisten, K.A.; Almabrazi, H.G.; Pedersen, K.F.; Lin, Y.; Garretson, J.; McEntaggart, N.O.; et al. IDT SciTools: A suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 2008, 36, W163–W169. [Google Scholar] [CrossRef]
- Owczarzy, R.; You, Y.; Groth, C.L.; Tataurov, A.V. Stability and mismatch discrimination of locked nucleic acid-DNA duplexes. Biochemistry 2011, 50, 9352–9367. [Google Scholar] [CrossRef]
- You, Y.; Moreira, B.G.; Behlke, M.A.; Owczarzy, R. Design of LNA probes that improve mismatch discrimination. Nucleic Acids Res. 2006, 34, e60. [Google Scholar] [CrossRef]
- FAO. Parasites in Foods: An Invisible Threat; Food Safety Technical Toolkit for Asia and the Pacific No. 7; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2021. [Google Scholar]
- Lupu, M.; Pavel, R.; Lazureanu, V.; Popovici, E.; Olariu, T. Trichinellosis in hospitalized patients from Western Romania: A retrospective study. Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Robertson, L.J.; Sprong, H.; Ortega, Y.R.; van der Giessen, J.W.B.; Fayer, R. Impacts of globalisation on foodborne parasites. Trends Parasitol. 2014, 30, 37–52. [Google Scholar] [CrossRef]
- Freeman, R.S.; Stuart, P.F.; Cullen, J.B.; Ritchie, A.C.; Mildon, A.; Fernandes, B.J.; Bonin, R. Fatal human infection with mesocercariae of the trematode Alaria americana. Am. J. Trop. Med. Hyg. 1976, 25, 803–807. [Google Scholar] [CrossRef]
- Fernandes, B.J.; Cooper, J.D.; Cullen, J.B.; Freeman, R.S.; Ritchie, A.C.; Scott, A.A.; Stuart, P.F. Systemic infection with Alaria americana (Trematoda). Can. Med. Assoc. J. 1976, 115, 1111–1114. [Google Scholar]
- Beaver, P.C.; Little, M.D.; Tucker, C.F.; Reed, R.J. Mesocercaria in the skin of man in Louisiana. Am. J. Trop. Med. Hyg. 1977, 26, 422–426. [Google Scholar] [CrossRef]
- McDonald, H.R.; Kazacos, K.R.; Schatz, H.; Johnson, R.N. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am. J. Ophthalmol. 1994, 117, 447–455. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Fernandes, A.R.G.; Santos, M.H.; Marucci, G. Trichinella britovi infection in wild boar in Portugal. Zoonoses Public Health 2021, 68, 103–109. [Google Scholar] [CrossRef]
- Balić, D.; Marucci, G.; Agičić, M.; Benić, M.; Krovina, Z.; Miškić, T.; Aladić, K.; Škrivanko, M. Trichinella spp. in wild boar (Sus scrofa) populations in Croatia during an eight-year study (2010–2017). One Health 2020, 11, 100172. [Google Scholar] [CrossRef]
- Antolová, D.; Fecková, M.; Valentová, D.; Hurníková, Z.; Miklisová, D.; Avdičová, M.; Halánová, M. Trichinellosis in Slovakia–epidemiological situation in humans and animals (2009–2018). Ann. Agric. Environ. Med. 2020, 27, 361–367. [Google Scholar] [CrossRef]
- Petersen, H.H.; Takeuchi-Storm, N.; Enemark, H.L.; Nielsen, S.T.; Larsen, G.; Chriél, M. Surveillance of important bacterial and parasitic infections in Danish wild boars (Sus scrofa). Acta Vet. Scand. 2020, 62, 41. [Google Scholar] [CrossRef]
- Flis, M.; Grela, E.R.; Gugała, D. Epizootic and epidemiological situation of Trichinella sp. infection in Poland in 2006–2015 in view of wild boar population dynamics. J. Vet. Res. 2017, 61, 181–187. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Villa, L.; Riehn, K.; Hamedy, A.; Minazzi, S.; Olivieri, E.; Zanzani, S.A.; Manfredi, M.T. Occurrence of selected zoonotic food-borne parasites and first molecular identification of Alaria alata in wild boars (Sus scrofa) in Italy. Parasitol. Res. 2018, 117, 2207–2215. [Google Scholar] [CrossRef]
- Rostami, A.; Gamble, H.R.; Dupouy-Camet, J.; Khazan, H.; Bruschi, F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017, 64, 65–71. [Google Scholar] [CrossRef]
- Pozio, E.; Merialdi, G.; Licata, E.; Della Casa, G.; Fabiani, M.; Amati, M.; Cherchi, S.; Ramini, M.; Faeti, V.; Interisano, M.; et al. Differences in larval survival and IgG response patterns in long-lasting infections by Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis in pigs. Parasites Vectors 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Bandino, E.; Capelli, G.; Corrias, F.; Gelmini, L.; Nardi, A.; Sacchi, C.; Cherchi, S.; et al. Indirect versus direct detection methods of Trichinella spp. infection in wild boar (Sus scrofa). Parasites Vectors 2014, 7, 171. [Google Scholar] [CrossRef]
- Portier, J.; Vallée, I.; Lacour, S.A.; Martin-Schaller, R.; Ferté, H.; Durand, B. Increasing circulation of Alaria alata mesocercaria in wild boar populations of the Rhine valley, France, 2007–2011. Vet. Parasitol. 2014, 199, 153–159. [Google Scholar] [CrossRef]
- Ozoliņa, Z.; Mateusa, M.; Šuksta, L.; Liepiņa, L.; Deksne, G. The wild boar (Sus scrofa, Linnaeus, 1758) as an important reservoir host for Alaria alata in the Baltic region and potential risk of infection in humans. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100485. [Google Scholar] [CrossRef] [PubMed]
- Ozoliņa, Z.; Deksne, G. Effectiveness of two methods for mesocercariae of Alaria alata detection in wild boars (Sus scrofa). Environ. Exp. Biol. 2017, 15, 25–28. [Google Scholar] [CrossRef]
- Riehn, K.; Hamedy, A.; Große, K.; Wüste, T.; Lücker, E. Alaria alata in wild boars (Sus scrofa, linnaeus, 1758) in the eastern parts of Germany. Parasitol. Res. 2012, 111, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Malesevic, M.; Smulders, F.J.M.; Petrovic, J.; Eta, J.; Paulsen, P. Alaria alata mesocercariae in wild boars (Sus scrofa) in northern Serbia after the flood disaster of 2014. Wien. Tierarztl. Monatsschr. 2016, 103, 345–349. [Google Scholar]
- Paulsen, P.; Forejtek, P.; Hutarova, Z.; Vodnansky, M. Alaria alata mesocercariae in wild boar (Sus scrofa, Linnaeus, 1758) in south regions of the Czech Republic. Vet. Parasitol. 2013, 197, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, P.; Ehebruster, J.; Irschik, I.; Lücker, E.; Riehn, K.; Winkelmayer, R.; Smulders, F.J.M. Findings of Alaria alata mesocercariae in wild boars (Sus scrofa) in eastern Austria. Eur. J. Wildl. Res. 2012, 58, 991–995. [Google Scholar] [CrossRef]
- Bilska-Zając, E.; Marucci, G.; Piróg-Komorowska, A.; Cichocka, M.; Różycki, M.; Karamon, J.; Sroka, J.; Bełcik, A.; Mizak, I.; Cencek, T. Occurrence of Alaria alata in wild boars (Sus scrofa) in Poland and detection of genetic variability between isolates. Parasitol. Res. 2021, 120, 83–91. [Google Scholar] [CrossRef]
- Berger, E.M.; Paulsen, P. Findings of Alaria alata mesocercariae in wild boars (Sus scrofa, Linnaeus, 1758) in west Hungary (Transdanubia regions). Wien. Tierarztl. Monatsschr. 2014, 101, 120–123. [Google Scholar]
- Kästner, C.; Bier, N.S.; Mayer-Scholl, A.; Nöckler, K.; Richter, M.H.; Johne, A. Prevalence of Alaria alata mesocercariae in wild boars from Brandenburg, Germany. Parasitol. Res. 2021, 120, 2103–2108. [Google Scholar] [CrossRef]
- Kästner, C.; Bahn, P.; Schönfelder, R.; Ozoliņa, Z.; Alksne, L.; Richter, M.H.; Deksne, G.; Mayer-Scholl, A.; Johne, A. Development of a Novel Method for Identification of Alaria alata Mesocercariae by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Microorganisms 2021, 9, 1664. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).