Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diet and Chitin Determination
2.2. Mucin Histochemical Staining and Quantification
2.3. Real Time Quantitative PCR (rt-qPCR)
2.4. Caecal Microbiota Characterization
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. Birds, Diet and Chitin Determination
3.2. Mucin Histochemical Evaluation
3.3. Real Time Quantitative PCR (rt-qPCR)
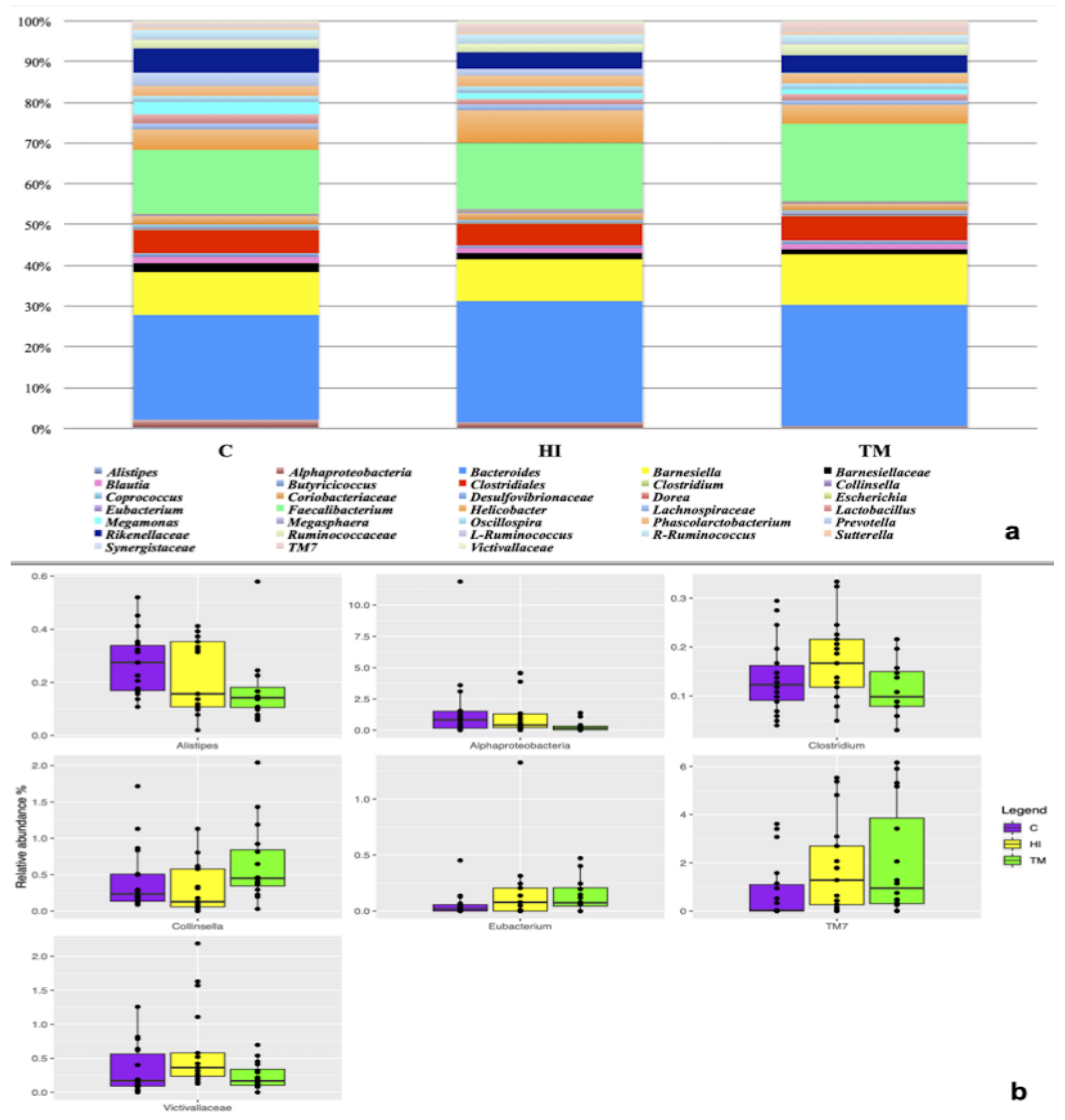
3.4. Caecal Microbiota Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, S.Y.; Macharia, J.; Diiro, G.M.; Kassie, M.; Ekesi, S.; van Loon, J.J.A.; Dicke, M.; Tanga, C.M. Smallholder farmers’ knowledge and willingness to pay for insect-based feeds in Kenya. PLoS ONE 2020, 15, e0230552. [Google Scholar] [CrossRef]
- Schmitt, E.; Belghit, I.; Johansen, J.; Leushuis, R.; Lock, E.J.; Melsen, D.; Ramasamy Shanmugam, R.K.; Van Loon, J.; Paul, A. Growth and safety assessment of feed streams for black soldier fly larvae: A case study with aquaculture sludge. Animals 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Ipema, A.F.; Gerrits, W.J.J.; Bokkers, E.A.M.; Kemp, B.; Bolhuis, J.E. Provisioning of live black soldier fly larvae (Hermetia illucens) benefits broiler activity and leg health in a frequency- and dose-dependent manner. Appl. Anim. Behav. Sci. 2020, 230, 105082. [Google Scholar] [CrossRef]
- Ipema, A.F.; Bokkers, E.A.M.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Long-term access to live black soldier fly larvae (Hermetia illucens) stimulates activity and reduces fearfulness of broilers, without affecting health. Sci. Rep. 2020, 10, 17428. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C.S. Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Yang, C.J. Efficacy of mealworm and super mealworm larvae probiotics as an alternative to antibiotics challenged orally with Salmonella and E. coli infection in broiler chicks. Poult. Sci. 2017, 96, 27–34. [Google Scholar] [CrossRef]
- Bailey, R.A. Gut Health in Poultry-The World Within: Update. Aviagen. 2019, pp. 2–10. Available online: https://eu.aviagen.com/assets/Tech_Center/Ross_Tech_Articles/RossNote-GutHealth-2019-EN.pdf (accessed on 9 July 2021). [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 71, 374. [Google Scholar] [CrossRef] [Green Version]
- Biasato, I.; Ferrocino, I.; Dabbou, S.; Evangelista, R.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Black soldier fly and gut health in broiler chickens: Insights into the relationship between cecal microbiota and intestinal mucin composition. J. Anim. Sci. Biotechnol. 2020, 11, 11–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitargue, F.M.; Kim, J.H.; Goo, D.; Delos Reyes, J.B.; Kil, D.Y. Effect of Vitamin E sources and inclusion levels in diets on growth performance, meat quality, alpha-tocopherol retention, and intestinal inflammatory cytokine expression in broiler chickens. Poult. Sci. 2019. [Google Scholar] [CrossRef]
- Murai, A.; Kitahara, K.; Terada, H.; Ueno, A.; Ohmori, Y.; Kobayashi, M.; Horio, F. Ingestion of paddy rice increases intestinal mucin secretion and goblet cell number and prevents dextran sodium sulfate-induced intestinal barrier defect in chickens. Poult. Sci. 2018, 97, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Gariglio, M.; Dabbou, S.; Crispo, M.; Biasato, I.; Gai, F.; Gasco, L.; Piacente, F.; Odetti, P.; Bergagna, S.; Plachà, I.; et al. Effects of the dietary inclusion of partially defatted black soldier fly (Hermetia illucens) meal on the blood chemistry and tissue (spleen, liver, thymus, and bursa of fabricius) histology of muscovy ducks (cairina moschata domestica). Animals 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Caimi, C.; Biasato, I.; Chemello, G.; Oddon, S.B.; Lussiana, C.; Malfatto, V.M.; Capucchio, M.T.; Colombino, E.; Schiavone, A.; Gai, F.; et al. Dietary inclusion of a partially defatted black soldier fly (Hermetia illucens) larva meal in low fishmeal-based diets for rainbow trout (Oncorhynchus mykiss). J. Anim. Sci. Biotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut microbiota and mucin composition in female broiler chickens fed diets including yellow mealworm (Tenebrio molitor, L.). Animals 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: Effects on growth performance, gut morphology, and histological findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef]
- Bellezza-Oddon, S.; Biasato, I.; Imarisio, A.; Pipan, M.; Dekleva, D.; Capucchio, M.T.; Meneguz, M.; Stefania, B.; Barbero, R.; Gariglio, M.; et al. Black Soldier Fly and Yellow Mealworm live larvae for broiler chickens: Effects on bird performance and health status. J. Anim. Physiol. Anim. Nutr. 2021, 1–9. [Google Scholar] [CrossRef]
- Woods, M.J.; Goosen, N.J.; Hoffman, L.C.; Pieterse, E. A simple and rapid protocol for measuring the chitin content of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae. J. Insects Food Feed 2020, 6, 285–290. [Google Scholar] [CrossRef]
- Forder, R.E.A.; Howarth, G.S.; Tivey, D.R.; Hughes, R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult. Sci. 2007, 86, 2396–2403. [Google Scholar] [CrossRef]
- Culling, C. Handbook of Histopathological and Histochemical Technique; Butterworth: London, UK, 1974. [Google Scholar]
- Biasato, I.; Ferrocino, I.; Colombino, E.; Gai, F.; Schiavone, A.; Cocolin, L.; Vincenti, V.; Capucchio, M.T.; Gasco, L. Effects of dietary Hermetia illucens meal inclusion on cecal microbiota and small intestinal mucin dynamics and infiltration with immune cells of weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Rebrikov, D.V.; Trofimov, D.Y. Real-time PCR: A review of approaches to data analysis. Appl. Biochem. Microbiol. 2006, 42, 455–463. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.; Li, M.; Wang, G.; Wang, K.; Shang, R.; Wang, Z.; Li, L. Evaluation of the Low Inclusion of Full-Fatted Hermetia illucens Larvae Meal for Layer Chickens: Growth Performance, Nutrient Digestibility, and Gut Health. Front. Vet. Sci. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Eicher, S.D.; Applegate, T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015, 94, 172–180. [Google Scholar] [CrossRef]
- Elahi, U.; Wang, J.; Ma, Y.B.; Wu, S.G.; Wu, J.; Qi, G.H.; Zhang, H.J. Evaluation of yellow mealworm meal as a protein feedstuff in the diet of broiler chicks. Animals 2020, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Yellow mealworm inclusion in diets for heavy-size broiler chickens: Implications for intestinal microbiota and mucin dynamics. Animals 2020, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Ramirez, N.; Woytschak, J.; Boyman, O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015, 36, 763–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- D’Hondt, E.; Soetemans, L.; Bastiaens, L.; Maesen, M.; Jespers, V.; Van den Bosch, B.; Voorspoels, S.; Elst, K. Simplified determination of the content and average degree of acetylation of chitin in crude black soldier fly larvae samples. Carbohydr. Res. 2020, 488, 107899. [Google Scholar] [CrossRef] [PubMed]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-S.; Kim, M.-W.; Moon, C.; Seo, D.-J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.-K.; Kim, S.-A.; Kim, Y.W.; et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Shi, S.; Tu, J.; Li, S. Comparative metagenomic sequencing analysis of cecum microbiotal diversity and function in broilers and layers. 3 Biotech 2019, 9, 316. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353. [Google Scholar] [CrossRef]
- Stanley, D.; Keyburn, A.L.; Denman, S.E.; Moore, R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012, 159, 155–162. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of Antimicrobial Host Defense Peptide Gene Expression by Free Fatty Acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef] [Green Version]
- Zoetendal, E.G.; Plugge, C.M.; Akkermans, A.D.L.; de Vos, W.M. Victivallis vadensis gen. nov., sp. nov., a sugar-fermenting anaerobe from human faeces. Int. J. Syst. Evol. Microbiol. 2003, 53, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, X.; Sun, S.; Chen, L.; Jin, J.; Liu, S.; Song, X.; Wu, C.; Lu, L. Protective role of dryland rearing on netting floors against mortality through gut microbiota-associated immune performance in Shaoxing ducks. Poult. Sci. 2019, 98, 4530–4538. [Google Scholar] [CrossRef] [PubMed]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering Endogenous Butyrate Production in the Intestinal Tract of Broilers as a Tool to Improve Gut Health. Front. Vet. Sci. 2015, 2, 75. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, X.; Wang, Y.; Mahmood, T.; Shahid, M.; Yin, D.; Yuan, J. Effect of 2-hydroxy-4-(methylthio) butanoic acid and acidifier on the performance, chyme pH, and microbiota of broilers. Anim. Sci. J. 2020, 91, e13409. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2019, 99, 196–206. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; De Filippis, F.; Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The intestinal microbiota of Hermetia illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Appl. Environ. Microbiol. 2019, 85, e01864-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przemieniecki, S.W.; Kosewska, A.; Ciesielski, S.; Kosewska, O. Changes in the gut microbiome and enzymatic profile of Tenebrio molitor larvae biodegrading cellulose, polyethylene and polystyrene waste. Environ. Pollut. 2020, 256, 113265. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the Microbiota of Black Soldier Fly Larvae (Hermetia illucens) Reared on Organic Waste Streams on Four Different Locations at Laboratory and Large Scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef]
| Starter (4–11 Day) | Grower-Finisher (12–38 Day) | |
|---|---|---|
| Crude protein (%) | 22.40 | 20.00 |
| Ether extract (%) | 4.90 | 5.90 |
| Crude fiber (%) | 2.75 | 2.65 |
| Ash (%) | 5.00 | 3.90 |
| Methionine (%) | 0.50 | 0.47 |
| Lysine (%) | 1.20 | 1.05 |
| Calcium (%) | 0.70 | 0.45 |
| Phosphorus (%) | 0.64 | 0.48 |
| Sodium (%) | 0.10 | 0.10 |
| Type | RNA Target | Primer Sequence | GenBank Accession No. |
|---|---|---|---|
| Reference gene | B-actina | F:5′-GAGAAATTGTGCGTGACATCA-3′ R:5′-CCTGAACCTCTCATTGCCA-3′ | L08165.1 |
| GAPDH | F:5′-GGTGGTGCTAAGCGTGTTAT-3′ R:5′-ACCTCTGTCATCTCTCCACA-3′ | K01458 | |
| Target gene | TNF-α | F:5′-CCCATCTGCACCACCTTCAT-3′ R:5′-CATCTGAACTGGGCGGTCAT-3′ | AY765397.1 |
| IL-6 | F:5′-CAAGGTGACGGAGGAGGAC-3′ R:5′-GGTAGGTCTGAAAGGCGAACA-3′ | AJ309540 | |
| INF-γ | F:5′-AGCTGACGGTGGACCTATTATT-3′ R:5′-GGCTTTGCGCTGGATTC-3′ | Y07922.1 | |
| IL-2 | F:5′-TCTGGGACCACTGTATGCTCT-3′ R:5′-ACACCAGTGGGAAACAGTATCA-3′ | AF000631 | |
| IL-4 | F:5′-CTTCCTCAACATGCGTCAGC-3′ R:5′-TGAAGTAGTGTTGCCTGCTGC-3′ | AJ621735 | |
| MUC-2 | F: 5′-ACTCCTCCTTTGTATGCGTGA-3′ R: 5′-GTTAACGCTGCATTCAACCTT-3′ | NM.001318434.1 |
| Diet (D) | Intestinal Segment (I) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | HI | TM | DU | JE | I | D | I | D × I | |
| Neutral mucins, mean (SD) | 3.154 (1.18) | 3.155 (1.07) | 3.563 (1.39) | 2.162 a (0.63) | 3.634 b (0.88) | 4.077 b (1.16) | 0.194 | <0.001 | 0.439 |
| Sialomucins, mean (SD) | 2.752 (0.85) | 2.975 (1.43) | 3.345 (2.28) | 1.957 a (0.60) | 3.392 b (1.20) | 3.722 b (2.13) | 0.659 | <0.001 | 0.922 |
| Sulfomucins, mean (SD) | 3.481 (1.58) | 3.302 (1.30) | 3.897 (2.04) | 2.234 a (0.91) | 4.078 b (1.79) | 4.367 b (1.32) | 0.544 | <0.001 | 0.293 |
| Total mucins, mean (SD) | 9.387 (3.19) | 9.432 (3.12) | 10.805 (4.42) | 6.353 a (1.49) | 11.105 b (2.65) | 12.166 b (3.44) | 0.217 | <0.001 | 0.697 |
| Diet | p-Value | |||
|---|---|---|---|---|
| C | HI | TM | ||
| IL-2, mean (SD) | 1.879 a (0.02) | 2.417 a (0.16) | 0.568 b (0.17) | 0.044 |
| IL-4, mean (SD) | 2.215 (0.28) | 1.199 (0.43) | 1.805 (0.004) | 0.961 |
| INF-γ, mean (SD) | 1.259 (0.40) | 0.953 (0.45) | 1.477 (0.21) | 0.860 |
| TNF-α, mean (SD) | 1.057 (0.06) | 0.639 (0.51) | 1.039 (0.66) | 0.125 |
| IL-6, mean (SD) | 1.865 (0.44) | 0.420 (0.11) | 1.208 (0.45) | 0.146 |
| MUC-2, mean (SD) | 1.221 (0.33) | 1.518 (0.52) | 1.577 (0.37) | 0.444 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombino, E.; Biasato, I.; Ferrocino, I.; Bellezza Oddon, S.; Caimi, C.; Gariglio, M.; Dabbou, S.; Caramori, M.; Battisti, E.; Zanet, S.; et al. Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation. Animals 2021, 11, 2819. https://doi.org/10.3390/ani11102819
Colombino E, Biasato I, Ferrocino I, Bellezza Oddon S, Caimi C, Gariglio M, Dabbou S, Caramori M, Battisti E, Zanet S, et al. Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation. Animals. 2021; 11(10):2819. https://doi.org/10.3390/ani11102819
Chicago/Turabian StyleColombino, Elena, Ilaria Biasato, Ilario Ferrocino, Sara Bellezza Oddon, Christian Caimi, Marta Gariglio, Sihem Dabbou, Marta Caramori, Elena Battisti, Stefania Zanet, and et al. 2021. "Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation" Animals 11, no. 10: 2819. https://doi.org/10.3390/ani11102819








