Impact of Nano-Bromocriptine on Egg Production Performance and Prolactin Expression in Layers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Alginate-Bromocriptine Nano-Composite
2.2. Experimental Assessment of the Efficacy of Bromocriptine and Alginate-Nano-Bromocriptine
2.2.1. Ethical Approval
2.2.2. Experimental Design
2.2.3. Egg Production Performance
2.2.4. Prolactin Gene Expression in Pituitary Gland Using qRT-PCR
2.3. Statistical Analysis
3. Results
3.1. Analysis of Alginate-Bromocriptine
3.2. Assessment of the Efficacy of Bromocriptine and Nano-Bromocriptine on Egg Production Performance
3.2.1. Pause Days, Egg Production Percentage, and Feed per Dozen Egg
3.2.2. Feed Consumption per Dozen Egg, and Haugh Unit
3.2.3. Ovarian Follicles, and Prolactin Gene Expression in the Pituitary Gland Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/ (accessed on 15 July 2021).
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Reddy, I.; David, C.; Sarma, P.; Singh, K. Modulation of prolactin hormone and intersequence pause days in domestic chickens. Vet. Rec. 2001, 149, 590. [Google Scholar] [CrossRef]
- Ohkubo, T.; Tanaka, M.; Nakashima, K.; Talbot, R.; Sharp, P. Prolactin receptor gene expression in the brain and peripheral tissues in broody and nonbroody breeds of domestic hen. Gen. Comp. Endocrinol. 1998, 109, 60–68. [Google Scholar] [CrossRef]
- Hrabia, A.; Paczoska-Eliasiewicz, H.; Rząsa, J. Effect of prolactin on estradiol and progesterone secretion by isolated chicken ovarian follicles. Folia Biol. 2004, 52, 197–203, (In Kraków). [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.J.; Dawson, A.; Lea, R.W. Control of luteinizing hormone and prolactin secretion in birds. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 119, 275–282. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Yu, Y.; Huang, Y.; Liang, S.; Shi, Z. Prolactin plays a stimulatory role in ovarian follicular development and egg laying in chicken hens. Domest. Anim. Endocrinol. 2011, 41, 57–66. [Google Scholar] [CrossRef]
- Sharp, P.; Sterling, R.; Talbot, R.; Huskisson, N. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: Observations using passive immunization, radioimmunoassay and immunohistochemistry. J. Endocrinol. 1989, 122, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Badakhshan, Y.; Mazhari, M. Effect of epinephrine and bromocriptine on ovary folliculogenesis and productive ratits of laying hens. Iran. J. Appl. Anim. Sci. 2014, 4, 421–424. [Google Scholar]
- Reddy, I.; David, C.; Raju, S. Chemical control of prolactin secretion and it’s effects on pause days, egg production and steroid hormone concentration in Girirani birds. Int. J. Poult. Sci. 2006, 5, 685–692. [Google Scholar]
- Parvez, M.M.I.R.; Rashid, B.; Hasan, M.; Mobarak, H.; Roy, K.K.; Haque, M.A. Effect of serum from laying hen and antiprolactin drug on egg production of indigenous chicken in Bangladesh. Asian Australas. J. Biosci. Biotechnol. 2017, 2, 51–54. [Google Scholar]
- Siddique, Y.H.; Khan, W.; Fatima, A.; Jyoti, S.; Khanam, S.; Naz, F.; Ali, F.; Singh, B.R.; Naqvi, A.H. Effect of bromocriptine alginate nanocomposite (BANC) on a transgenic Drosophila model of Parkinson’s disease. Dis. Models Mech. 2016, 9, 63–68. [Google Scholar]
- El-Sissi, A.F.; Mohamed, F.H.; Danial, N.M.; Gaballah, A.Q.; Ali, K.A. Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Hashem, N.M.; El-Azrak, K.E.-D.M.; Gonzalez-Bulnes, A.; Hassan, G.A.; Salem, M.H. Efficiency of GnRH–loaded chitosan nanoparticles for inducing LH secretion and fertile ovulations in protocols for artificial insemination in rabbit does. Animals 2021, 11, 440. [Google Scholar] [CrossRef]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, X.; Jiang, W.; Lu, W.; Su, L.; Shi, Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int. J. Pharm. 2004, 275, 85–96. [Google Scholar] [CrossRef]
- Reddy, I.; David, C.; Raju, S. Effect of suppression of plasma prolactin on luteinizing hormone concentration, intersequence pause days and egg production in domestic hen. Domest. Anim. Endocrinol. 2007, 33, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Haugh, R. The Haugh unit for measuring egg quality. U. S. Egg Poult. Mag. 1937, 43, 522–555. [Google Scholar]
- Renema, R.; Robinson, F.; Melnychuk, V.; Hardin, R.; Bagley, L.; Emmerson, D.; Blackman, J. The use of feed restriction for improving reproductive traits in male-line large white Turkey hens.: 2. Ovary morphology and laying traits. Poult. Sci. 1995, 74, 102–120. [Google Scholar] [CrossRef]
- Jiang, R.-S.; Xu, G.-Y.; Zhang, X.-Q.; Yang, N. Association of polymorphisms for prolactin and prolactin receptor genes with broody traits in chickens. Poult. Sci. 2005, 84, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Chatterjee, R.; Sharma, R.; Niranjan, M.; Rajkumar, U. Associations between novel polymorphisms at the 5′-UTR region of the prolactin gene and egg production and quality in chickens. Theriogenology 2011, 75, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Molnár, S.; Szőllősi, L. Sustainability and quality aspects of different table egg production systems: A literature review. Sustainability 2020, 12, 7884. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate nanoformulation: Influence of process and selected variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine 2010, 5, 287–306. [Google Scholar] [CrossRef] [Green Version]
- Reddy, I.; David, C.; Sarma, P.; Singh, K. The possible role of prolactin in laying performance and steroid hormone secretion in domestic hen (Gallus domesticus). Gen. Comp. Endocrinol. 2002, 127, 249–255. [Google Scholar] [CrossRef]
- Banu, M.N.; Rashid, M.B.; Hasan, M.M.; Aziz, F.B.; Islam, M.R.; Haque, M.A. Effect of anti-prolactin drug and peppermint on broodiness, laying performance and egg quality in indigenous hens. Asian J. Med Biol. Res. 2016, 2, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Crisostomo, S.; Guémené, D.; Garreau-Mills, M.; Morvan, C.; Zadworny, D. Prevention of incubation behavior expression in turkey hens by active immunization against prolactin. Theriogenology 1998, 50, 675–690. [Google Scholar] [CrossRef]
- Dajee, M.; Fey, G.H.; Richards, J.S. Stat 5b and the orphan nuclear receptors regulate expression of the α2-macroglobulin (α2M) gene in rat ovarian granulosa cells. Mol. Endocrinol. 1998, 12, 1393–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef] [PubMed]
- Vautier, S.; Lacomblez, L.; Chacun, H.; Picard, V.; Gimenez, F.; Farinotti, R.; Fernandez, C. Interactions between the dopamine agonist, bromocriptine and the efflux protein, P-glycoprotein at the blood–brain barrier in the mouse. Eur. J. Pharm. Sci. 2006, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupanŏiŏ, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Havel, H.A. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar] [CrossRef]
- Ciccarelli, E.; Grottoli, S.; Miola, C.; Avataneo, T.; Lancranjan, I.; Camanni, F. Double blind randomized study using oral or injectable bromocriptine in patients with hyperprolactinaemia. Clin. Endocrinol. 1994, 40, 193–198. [Google Scholar] [CrossRef] [PubMed]
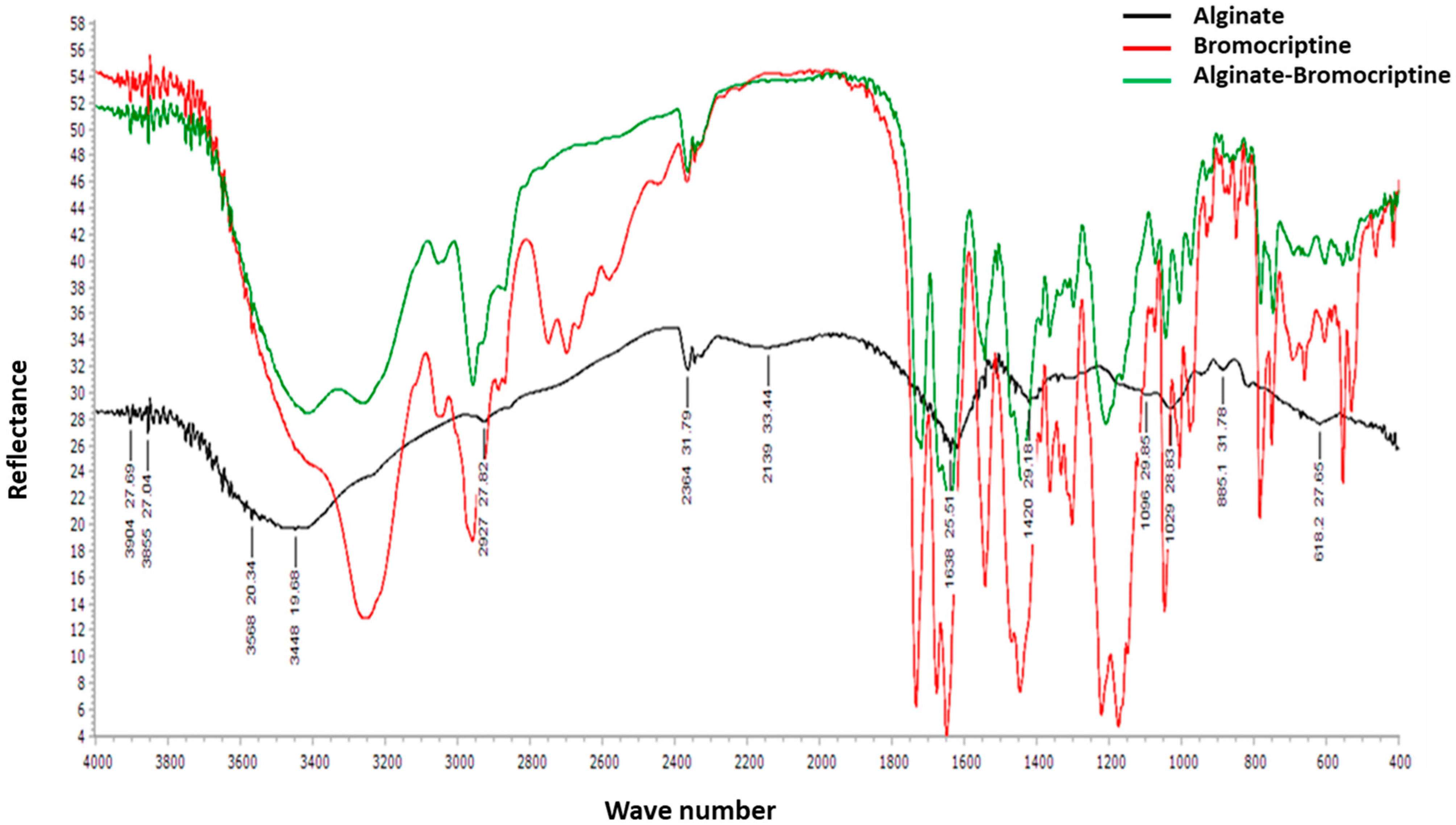
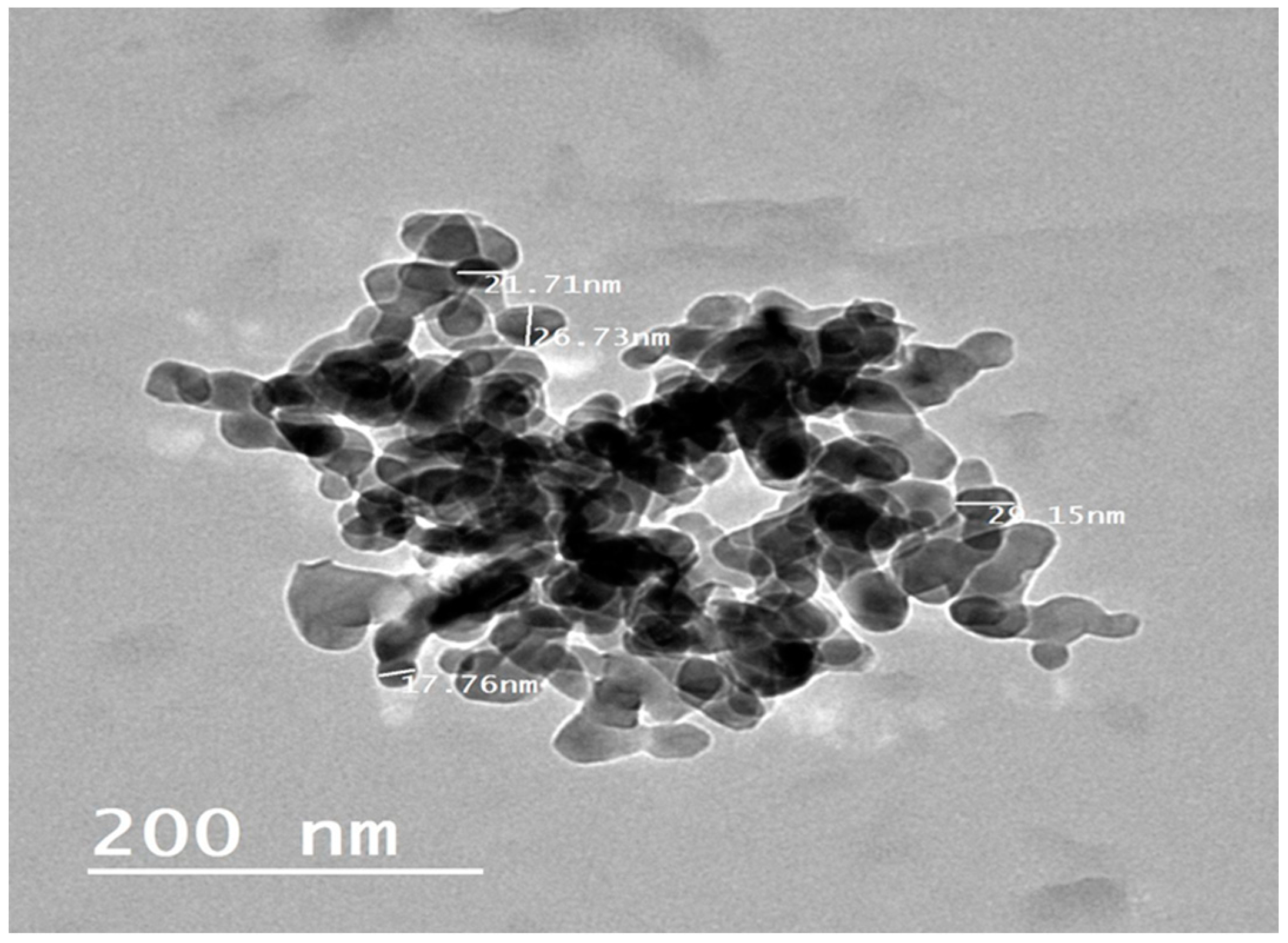


| Parameter | Pause Days (Day) | Egg/Hen/D (%) | |||||
|---|---|---|---|---|---|---|---|
| 76th Week | 80th Week | 84th Week | 76th Week | 80th Week | 84th Week | ||
| Treatment | Control (n = 50) | 11.07 ± 0.26 a | 10.00 ± 0.25 a | 11.56 ± 0.34 a | 61.42 ± 0.95 c | 61.90 ± 0.78 c | 51.16 ± 1.42 b |
| Bromocriptine (n = 50) | 7.05 ± 0.32 b | 6.89 ± 0.28 b | 8.20 ± 0.35 b | 71.55 ± 1.25 b | 71.11 ± 1.01 b | 65.54 ± 1.71 a | |
| Nano-bromocriptine (n = 50) | 5.38 ± 0.33 c | 5.93 ± 0.26 c | 7.27 ± 0.30 c | 78.33 ± 1.26 a | 74.54 ± 1.16 a | 68.13 ± 1.45 a | |
| Administration | Orally (n = 75) | 7.14 ± 0.39 b | 7.15 ± 0.32 b | 8.94 ± 0.36 | 73.37 ± 1.26 a | 71.04 ± 1.12 a | 62.41 ± 1.62 a |
| Injection (n = 75) | 8.52 ± 0.32 a | 8.06 ± 0.24 a | 9.08 ± 0.31 | 67.49 ± 1.04 b | 67.33 ± 0.78 b | 60.82 ± 1.44 a | |
| Treatment χ administration | Control (oral, n = 25) | 11.11 ± 0.37 a | 10.07 ± 0.37 a | 11.50 ± 0.48 a | 61.15 ± 1.34 d | 61.56 ± 1.12 c | 51.65 ± 1.99 c |
| Control (injection, n = 25) | 11.04 ± 0.38 a | 9.93 ± 0.35 a | 11.63 ± 0.48 a | 61.69 ± 1.37 d | 62.25 ± 1.09 c | 50.67 ± 2.05 c | |
| Bromocriptine (oral, n = 25) | 6.39 ± 0.37 c | 6.11 ± 0.44 c | 8.86 ± 0.53 b | 74.71 ± 1.50 b | 72.75 ± 1.81 b | 63.04 ± 2.62 b | |
| Bromocriptine (injection, n = 25) | 7.71 ± 0.49 b | 7.68 ± 0.28 b | 7.54 ± 0.42 c,d | 68.39 ± 1.87 c | 69.46 ± 0.84 b | 68.05 ± 2.12 a,b | |
| Nano-bromocriptine (oral, n = 25) | 3.93 ± 0.35 d | 5.29 ± 0.35 c | 6.46 ± 0.44 d | 84.26 ± 1.28 a | 78.80 ± 1.34 a | 72.52 ± 1.92 a | |
| Nano-bromocriptine (injection, n = 25) | 6.82 ± 0.41 c | 6.57 ± 0.36 c | 8.07 ± 0.34 b,c | 72.40 ± 1.58 b,c | 70.29 ± 1.55 b | 63.75 ± 1.78 b | |
| p-value | Treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Administration | <0.0001 | <0.0026 | <0.7091 | <0.0001 | <0.0007 | <0.3572 | |
| Interaction | <0.0014 | <0.0425 | <0.0062 | <0.0003 | <0.0032 | <0.0052 | |
| Parameter | Feed/Dozen Egg (kg) | Haugh Unit | |||||
|---|---|---|---|---|---|---|---|
| 76th Week | 80th Week | 84th Week | 76th Week | 80th Week | 84th Week | ||
| Treatment | Control (n = 50) | 2.38 ± 0.04 a | 2.35 ± 0.03 a | 2.94 ± 0.11 a | 76.70 ± 0.53 | 77.01 ± 1.19 | 83.06 ± 1.16 a |
| Bromocriptine (n = 50) | 2.06 ± 0.05 b | 2.05 ± 0.03 b | 2.29 ± 0.08 b | 75.63 ± 1.74 | 77.29 ± 1.39 | 78.99 ± 1.16 b | |
| Nano-bromocriptine (n = 50) | 1.87 ± 0.03 c | 1.96 ± 0.03 c | 2.16 ± 0.05 b | 76.24 ± 1.60 | 75.20 ± 0.84 | 76.82 ± 1.08 b | |
| Administration | Orally (n = 75) | 2.02 ± 0.04 b | 2.07 ± 0.03 b | 2.44 ± 0.08 a | 75.12 ± 0.58 | 76.70 ± 0.90 | 79.21 ± 0.92 |
| Injection (n = 75) | 2.18 ± 0.04 a | 2.16 ± 0.03 a | 2.48 ± 0.07 a | 77.26 ± 1.52 | 76.30 ± 1.03 | 80.04 ± 0.93 | |
| Treatment χ administration | Control (oral, n = 25) | 2.39 ± 0.05 a | 2.36 ± 0.04 a | 2.91 ± 0.15 a | 76.83 ± 0.72 | 77.99 ± 2.07 | 83.29 ± 1.58 a |
| Control (injection, n = 25) | 2.37 ± 0.05 a | 2.33 ± 0.04 a | 2.96 ± 0.16 a | 76.58 ± 0.88 | 76.02 ± 0.80 | 82.83 ± 1.70 a,b | |
| Bromocriptine (oral, n = 25) | 1.95 ± 0.04 b,c | 2.02 ± 0.05 b | 2.41 ± 0.14 b | 74.50 ± 0.85 | 75.75 ± 1.63 | 75.28 ± 1.77 b,c | |
| Bromocriptine (injection, n = 25) | 2.17 ± 0.08 b | 2.08 ± 0.03 b | 2.17 ± 0.08 b,c | 76.76 ± 3.49 | 78.83 ± 2.19 | 82.71 ± 1.49 a | |
| Nano-bromocriptine (oral, n = 25) | 1.72 ± 0.03 c | 1.84 ± 0.03 c | 2.02 ± 0.06 c | 74.04 ± 1.08 | 76.35 ± 0.92 | 79.06 ± 1.41 b,c | |
| Nano-bromocriptine (injection, n = 25) | 2.02 ± 0.05 b | 2.08 ± 0.05 b | 2.30 ± 0.07 b,c | 78.44 ± 2.81 | 74.05 ± 1.28 | 74.58 ± 1.64 c | |
| p-value | Treatment | <0.0001 | <0.0001 | <0.0001 | <0.8726 | <0.3834 | <0.0007 |
| Administration | <0.0002 | <0.0079 | <0.7266 | <0.2117 | <0.7665 | <0.5290 | |
| Interaction | <0.0083 | <0.0066 | <0.0473 | <0.5373 | <0.1923 | <0.0013 | |
| Parameter | Ovarian Follicles | |||
|---|---|---|---|---|
| LYF | SYF | LWF | ||
| Treatment | Control (n = 50) | 5.33 ± 0.20 b | 5.85 ± 0.63 | 8.93 ± 1.20 |
| Bromocriptine (n = 50) | 5.90 ± 0.19 a | 6.40 ± 1.37 | 6.80 ± 0.81 | |
| Nano-bromocriptine (n = 50) | 6.30 ± 0.15 a | 8.10 ± 0.95 | 6.80 ± 0.88 | |
| Administration | Orally (n = 75) | 5.87 ± 0.13 | 6.73 ± 1.02 | 7.33 ± 0.87 |
| Injection (n = 75) | 5.82 ± 0.19 | 6.83 ± 0.68 | 7.68 ± 0.75 | |
| Treatment χ administration | Control (oral, n = 25) | 5.40 ± 0.27 | 6.20 ± 0.97 | 8.60 ± 1.66 |
| Control (injection, n = 25) | 5.25 ± 0.31 | 5.50 ± 0.87 | 9.25 ± 1.97 | |
| Bromocriptine (oral, n = 25) | 6.00 ± 0.21 | 6.60 ± 2.64 | 6.60 ± 1.60 | |
| Bromocriptine (injection, n = 25) | 5.80 ± 0.33 | 6.20 ± 1.20 | 7.00 ± 0.63 | |
| Nano-bromocriptine (oral, n = 25) | 6.20 ± 0.13 | 7.40 ± 1.69 | 6.80 ± 1.39 | |
| Nano-bromocriptine (injection, n = 25) | 6.40 ± 0.27 | 8.80 ± 0.97 | 6.80 ± 1.24 | |
| p-value | Treatment | <0.0021 | <0.3429 | <0.2705 |
| Administration | <0.8141 | <0.9385 | <0.7695 | |
| Interaction | <0.6965 | <0.7694 | <0.9749 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawod, A.; Osman, N.; Heikal, H.S.; Ali, K.A.; Kandil, O.M.; Shehata, A.A.; Hafez, H.M.; Mahboub, H. Impact of Nano-Bromocriptine on Egg Production Performance and Prolactin Expression in Layers. Animals 2021, 11, 2842. https://doi.org/10.3390/ani11102842
Dawod A, Osman N, Heikal HS, Ali KA, Kandil OM, Shehata AA, Hafez HM, Mahboub H. Impact of Nano-Bromocriptine on Egg Production Performance and Prolactin Expression in Layers. Animals. 2021; 11(10):2842. https://doi.org/10.3390/ani11102842
Chicago/Turabian StyleDawod, Ahmed, Noha Osman, Hanim S. Heikal, Korany A. Ali, Omaima M. Kandil, Awad A. Shehata, Hafez M. Hafez, and Hamada Mahboub. 2021. "Impact of Nano-Bromocriptine on Egg Production Performance and Prolactin Expression in Layers" Animals 11, no. 10: 2842. https://doi.org/10.3390/ani11102842
APA StyleDawod, A., Osman, N., Heikal, H. S., Ali, K. A., Kandil, O. M., Shehata, A. A., Hafez, H. M., & Mahboub, H. (2021). Impact of Nano-Bromocriptine on Egg Production Performance and Prolactin Expression in Layers. Animals, 11(10), 2842. https://doi.org/10.3390/ani11102842






