Evaluating the Protein Value of Fresh Tropical Forage Grasses and Forage Legumes Using In Vitro and Chemical Fractionation Methods
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Proximate Nutrient and Fiber Analysis
2.2. Reference Post-Ruminal Protein Estimation
2.3. Modified Hohenheim Gas Test
2.4. Chemical Crude Protein Fractionation
2.5. Statistical Analyses
3. Results
3.1. Nutritional Characteristics of Forages
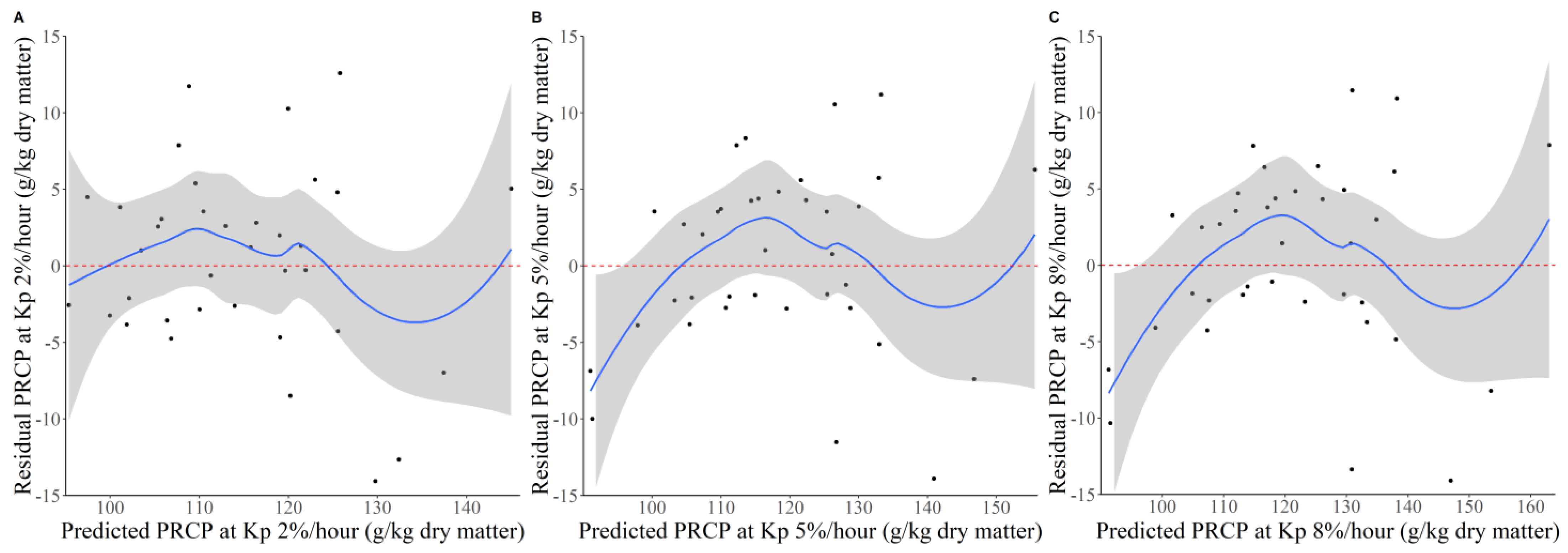
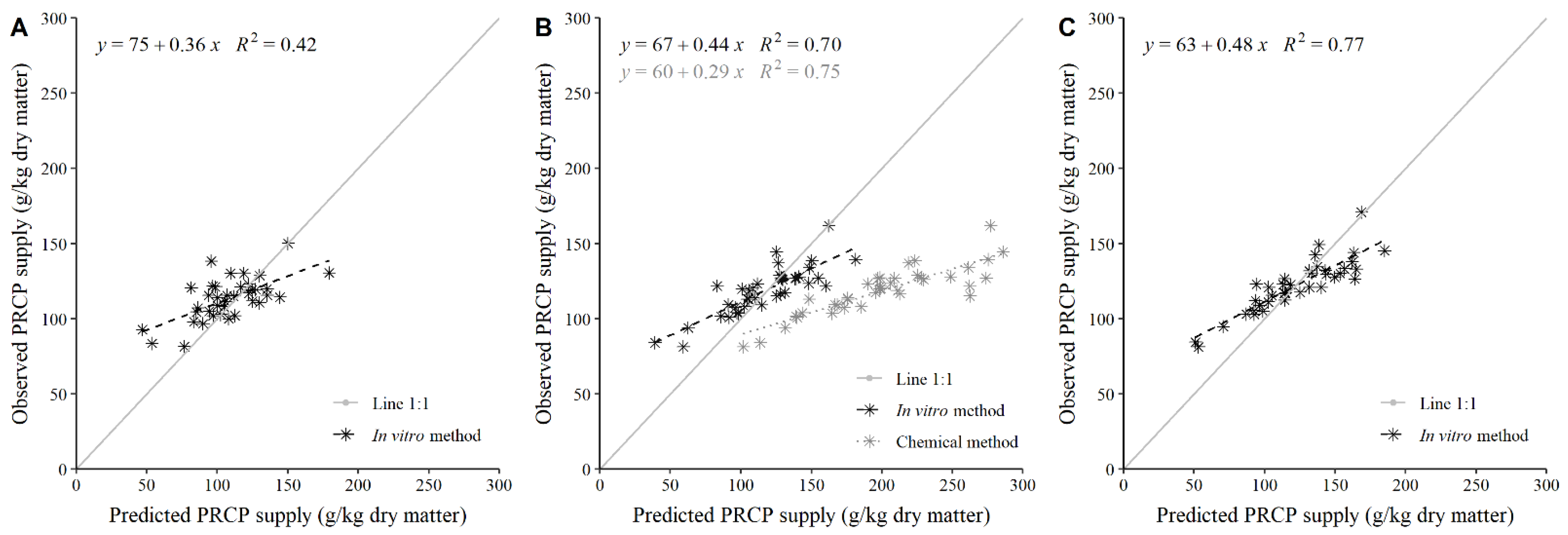
3.2. Adequacy of the In Vitro Method to Predict PRCP Supply
3.3. Adequacy of the Chemical Method to Predict PRCP Supply and Its Comparison with the In Vitro Method
3.4. Multivariate Regressions to Predict PRCP Supply in Tropical Forages
4. Discussion
4.1. Experimental Design and Methods
4.2. Nutritional Characteristics of Forages
4.3. Adequacy of the Estimates of Post-Ruminal Crude Protein Supply Using the In Vitro Method
4.4. Adequacy of the Estimates of Post-Ruminal Crude Protein Supply Using the Chemical Method
4.5. Prediction of Post-Ruminal Crude Protein Supply of Tropical Forages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Forage Samples | Origin a | Season b | Crude Protein Fractions c | Post-Ruminal Crude Protein d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B1 | B2 | B3 | C | Kp 2%/h | Kp 5%/h | Kp 8%/h | |||
| Fresh tropical forage grasses | ||||||||||
| Andropogon gayanus Kunth | ES | RS | 20.3 | 4.3 | 13.4 | 34.0 | 8.6 | 97 | 101 | 103 |
| Andropogon gayanus Kunth | KY | DS | 22.2 | 1.7 | 11.3 | 32.5 | 17.4 | 98 | 102 | 103 |
| Brachiaria brizantha (Hochst. ex A. Rich.) Stapf | PE | RS | 47.9 | 4.1 | 27.1 | 18.3 | 12.6 | 100 | 104 | 105 |
| Brachiaria brizantha (Hochst. ex A. Rich.) Stapf x Brachiaria ruziziensis R. Germ. and C.M. Evrard | KY | DS | 15.7 | 3.3 | 15.6 | 6.1 | 5.4 | 82 | 81 | 81 |
| Cenchrus ciliaris L. | KY | DS | 39.8 | 4.1 | 35.9 | 35.4 | 24.7 | 119 | 127 | 130 |
| Chloris gayana Kunth | KY | DS | 41.0 | 3.9 | 25.4 | 41.8 | 20.8 | 116 | 123 | 127 |
| Cynodon dactylon (L.) Pers. | KY | DS | 54.7 | 4.9 | 22.9 | 45.9 | 11.1 | 114 | 120 | 123 |
| Cynodon nlemfuensis Vanderyst | KY | DS | 77.8 | 0.5 | 21.3 | 34.2 | 15.7 | 116 | 120 | 123 |
| Digitaria decumbens Stent | PE | RS | 29.2 | 4.4 | 21.0 | 12.5 | 8.2 | 93 | 94 | 95 |
| Digitaria eriantha Steud. | KY | DS | 44.1 | 4.5 | 13.6 | 36.1 | 12.9 | 104 | 109 | 112 |
| Eragrostis echinochloidea Stapf | KY | DS | 57.6 | 1.4 | 19.1 | 31.7 | 10.0 | 105 | 107 | 109 |
| Hyparrhenia rufa (Nees) Stapf | ET | RS | 34.9 | 0.8 | 29.2 | 39.9 | 11.4 | 107 | 113 | 117 |
| Hyparrhenia rufa (Nees) Stapf | KY | DS | 19.7 | 2.0 | 18.8 | 28.2 | 27.0 | 108 | 113 | 116 |
| Melinis minutiflora P. Beauv. | KY | DS | 29.1 | 4.7 | 16.9 | 39.5 | 22.3 | 102 | 109 | 113 |
| Panicum coloratum L. | ET | RS | 69.2 | 8.9 | 31.8 | 33.7 | 6.6 | 115 | 119 | 121 |
| Panicum coloratum L. | KY | DS | 56.7 | 1.2 | 23.1 | 44.6 | 14.4 | 111 | 117 | 121 |
| Panicum maximum Jacq. | PE | RS | 26.9 | 3.0 | 13.3 | 9.8 | 7.1 | 84 | 84 | 84 |
| Pennisetum clandestinum Hochst. ex Chiov. | ET | RS | 93.6 | 9.2 | 37.1 | 51.8 | 8.8 | 112 | 115 | 118 |
| Pennisetum clandestinum Hochst. ex Chiov. | KY | DS | 40.2 | 2.5 | 40.4 | 42.1 | 21.1 | 114 | 124 | 128 |
| Pennisetum pedicellatum Trin. | ET | RS | 50.4 | 5.4 | 27.8 | 34.0 | 7.8 | 103 | 108 | 111 |
| Pennisetum purpureum Schumach. | ET | RS | 51.4 | 0.3 | 27.5 | 32.2 | 8.1 | 109 | 114 | 117 |
| Pennisetum purpureum Schumach. | KY | DS | 38.3 | 1.9 | 23.7 | 12.1 | 15.7 | 102 | 104 | 105 |
| Tripsacum andersonii J. R. Gray | KY | DS | 43.7 | 1.8 | 33.5 | 39.7 | 23.8 | 117 | 127 | 132 |
| Fresh tropical forage legumes | ||||||||||
| Arachis glabrata Benth. | ES | RS | 24.4 | 12.9 | 48.7 | 47.8 | 18.1 | 130 | 139 | 144 |
| Arachis pintoi Krapov. and W. C. Greg. | BR | DS | 66.2 | 2.2 | 53.8 | 23.0 | 28.9 | 123 | 127 | 130 |
| Calopogonium mucunoides Desv. | BR | DS | 57.4 | 4.3 | 81.6 | 33.9 | 16.6 | 116 | 122 | 126 |
| Canavalia ensiformis (L.) DC. | ES | RS | 61.0 | 1.8 | 65.2 | 40.4 | 16.4 | 121 | 128 | 133 |
| Centrosema sp. (DC.) Benth. | BR | DS | 35.7 | 8.6 | 61.4 | 69.9 | 27.9 | 130 | 139 | 145 |
| Crotalaria longirostrata Hook. and Arn. | ES | RS | 42.5 | 1.9 | 74.6 | 6.2 | 9.6 | 121 | 122 | 123 |
| Dolichos lablab L. | ES | RS | 38.6 | 1.7 | 59.6 | 38.0 | 16.2 | 130 | 137 | 142 |
| Glycine max (L.) Merr. | ES | RS | 75.4 | 17.2 | 95.7 | 12.1 | 10.8 | 138 | 144 | 149 |
| Giricidia sepium (Jacq.) Kunth | ES | RS | 47.6 | 0.2 | 67.4 | 65.5 | 31.1 | 150 | 162 | 171 |
| Macroptilium atropurpureum (DC.) Urb. | ES | RS | 42.3 | 11.0 | 71.1 | 57.8 | 15.6 | 120 | 127 | 133 |
| Mucuna pruriens (L.) DC. | ES | RS | 30.4 | 5.1 | 24.2 | 49.9 | 47.0 | 119 | 127 | 132 |
| Pueraria phaseoloides (Roxb.) Benth. | PE | RS | 65.4 | 6.1 | 85.2 | 21.5 | 15.9 | 129 | 134 | 138 |
| Stylosanthes guianensis (Aubl.) Sw. | ES | RS | 37.7 | 12.2 | 46.1 | 42.5 | 19.1 | 121 | 129 | 135 |
| Stylosanthes guianensis (Aubl.) Sw. | BR | DS | 42.2 | 9.2 | 33.1 | 39.5 | 30.4 | 111 | 117 | 121 |
| Vigna sinensis (L.) Savi ex Hassk. | PE | RS | 51.1 | 2.3 | 66.8 | 30.5 | 14.4 | 122 | 126 | 130 |
Appendix B
References
- GfE (Gesellschaft Für Ernährungsphysiologie). Empfehlungen zur Energie-und Nährstoffversorgung der Milchkühe und Aufzuchtrinder; DLG-Verlag: Frankfurt, Germany, 2001; ISBN 978-3-7690-0591-2. [Google Scholar]
- Stern, M.D.; Bach, A.; Calsamiglia, S. Alternative techniques for measuring nutrient digestion in ruminants. J. Anim. Sci. 1997, 75, 2256–2276. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, B.; Südekum, K.-H.; Spiekers, H.; Schuster, M.; Schwarz, F.J. Estimating utilisable crude protein at the duodenum, a precursor to metabolisable protein for ruminants, from forages using a modified gas test. Anim. Feed Sci. Technol. 2012, 175, 106–113. [Google Scholar] [CrossRef]
- Steingaß, H.; Nibbe, D.; Südekum, K.-H.; Lebzien, P.; Spiekers, H. Schätzung Des NXP-Gehaltes Mit Hilfe Des Modifizierten Hohenheimer Futterwerttests Und Dessen Anwendung Zur Bewertung von Raps- und Sojaextraktionsschroten; VDLUFA-Verl: Darmstadt, Germany, 2001; p. 114. [Google Scholar]
- Zhao, G.Y.; Cao, J.E. Relationship between the in vitro-estimated utilizable crude protein and the cornell net carbohydrate and protein system crude protein fractions in feeds for ruminants. J. Anim. Physiol. Anim. Nutr. 2004, 88, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Lebzien, P. Development of an in vitro incubation technique for the estimation of the utilizable crude protein (uCP) in feeds for cattle. Arch. Anim. Nutr. 2000, 53, 293–302. [Google Scholar] [CrossRef]
- Westreicher-Kristen, E.; Steingass, H.; Rodehutscord, M. Estimation of utilisable crude protein at the duodenum of dried distillers’ grains with solubles using a modified gas test. Arch. Anim. Nutr. 2015, 69, 351–365. [Google Scholar] [CrossRef]
- Gidlund, H.; Vaga, M.; Ahvenjarvi, S.; Rinne, M.; Ramin, M.; Huhtanen, P. predicting omasal flow of nonammonia n and milk protein yield from in vitro-determined utilizable crude protein at the duodenum. J. Dairy Sci. 2018, 101, 1164–1176. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Minson, D.J. Forage in Ruminant Nutrition; Academic Press: San Diego, California, CA, USA, 1990. [Google Scholar]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik: (Methodenbuch III). Die chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Lebzien, P.; Voigt, J.; Gabel, M.; Gädeken, D. Zur schätzung der menge an nutzbarem rohprotein am duodenum von milchkühen (Estimation of utilizable crude protein at the duodenum of dairy cattle). J. Anim. Physiol. Anim. Nutr. 1996, 76, 218–223. [Google Scholar] [CrossRef]
- Madsen, J.; Hvelplund, T. Prediction of in situ protein degradability in the rumen: Results of a European ringtest. Livest. Prod. Sci. 1994, 39, 201–212. [Google Scholar] [CrossRef]
- Weisbjerg, M.R.; Bhargava, P.K.; Hvelplund, T.; Madsen, J. Use of degradation curves in feed evaluation. Beret. Statens Husdyrbr. 1990, 679, 33. [Google Scholar]
- Krawielitzki, K.; Schmidt, T.; Voigt, J.; Kowalczyk, J.; Gabel, M. Dynamics of microbial contamination of protein during ruminal in situ incubation of feedstuffs. J. Anim. Feed Sci. 2006, 15, 313–328. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; France, J.; López, S.; Dijkstra, J.; Lister, S.J.; Davies, D.R.; Bannink, A. Correcting the calculation of extent of degradation to account for particulate matter loss at zero time when applying the polyester bag method. J. Anim. Sci. 1999, 77, 3385–3391. [Google Scholar] [CrossRef][Green Version]
- Leberl, P.; Gruber, L.; Steingass, H.; Schenkel, H. Comparison of the methods modified Hohenheimer Futter-Werttest (moHFT) and cornell system for determination of nXP-content of concentrates. In Proceedings of the 16th International Science Symposium on Nutrition of Domestic Animals, Murska Sobota, Slovenia, 8–9 November 2007; pp. 171–176. [Google Scholar]
- Greenberg, N.A.; Shipe, W.F. Comparison of the abilities of trichloroacetic, picric, sulfosalicylic, and tungstic acids to precipitate protein hydrolysates and proteins. J. Food Sci. 1979, 44, 735–737. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.V.; Sniffen, C.J.; Van Soest, P.J. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- van Soest, P.J.; Mason, V.C. The influence of the maillard reaction upon the nutritive value of fibrous feeds. Anim. Feed Sci. Technol. 1991, 32, 45–53. [Google Scholar] [CrossRef]
- Grubbs, F.E. Procedures for detecting outlying observations in samples. Technometrics 1969, 11, 1–21. [Google Scholar] [CrossRef]
- Lin, L.I.-K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- McBride, G.B. A Proposal for Strength-Of-Agreement Criteria for Lin’s Concordance Correlation Coefficient; NIWA: Hamilton, New Zealand, 2005. [Google Scholar]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Pacific Grove, CA, USA, 1996; ISBN 978-0-534-23100-2. [Google Scholar]
- Hair, J.F.; Black, W.C.; Black, B.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Pearson Education Limited: London, UK, 2013; ISBN 978-1-292-02190-4. [Google Scholar]
- Salazar-Cubillas, K.; Dickhoefer, U. Evaluating the Accuracy of Conceptual Models for Predicting the Nitrogen Excretion of Cattle in Tropical Environments. In Proceedings of the Advances in Animal Biosciences; Cambridge University Press: Leipzig, Germany, 2019; p. 487. [Google Scholar]
- INRA (Institut National de La Recherche Agronomique). INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; ISBN 978-90-8686-292-4. [Google Scholar]
- Nogueira Filho, J.C.M.; Fondevila, M.; Urdaneta, A.B.; Ronquillo, M.G. In vitro microbial fermentation of tropical grasses at an advanced maturity stage. Anim. Feed Sci. Technol. 2000, 83, 145–157. [Google Scholar] [CrossRef]
- Tedeschi, L.O. Assessment of the adequacy of mathematical models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- Fondevila, M.; Nogueira-Filho, J.C.M.; Barrios-Urdaneta, A. In vitro microbial fermentation and protein utilisation of tropical forage legumes grown during the dry season. Anim. Feed Sci. Technol. 2002, 95, 1–14. [Google Scholar] [CrossRef]
- Osuga, I.M.; Abdulrazak, S.A.; Ichinohe, T.; Fujihara, T. Rumen degradation and in vitro gas production parameters in some browse forages, grasses and maize stover from Kenya. J. Food Agric. Environ. 2006, 4, 60–64. [Google Scholar]
- Evitayani; Warly, L. ; Fariani, A.; Ichinohe, T.; Fujihara, T. Study on nutritive value of tropical forages in North Sumatra, Indonesia. Asian-Australas. J. Anim. Sci. 2004, 17, 1518–1523. [Google Scholar] [CrossRef]
- Singh, S.; Kushwaha, B.P.; Nag, S.K.; Mishra, A.K.; Singh, A.; Anele, U.Y. In vitro ruminal fermentation, protein and carbohydrate fractionation, methane production and prediction of twelve commonly used Indian green forages. Anim. Feed Sci. Technol. 2012, 178, 2–11. [Google Scholar] [CrossRef]
- Melesse, A.; Steingass, H.; Schollenberger, M.; Rodehutscord, M. Screening of common tropical grass and legume forages in ethiopia for their nutrient composition and methane production profile in vitro. Trop. Grassl.-Forrajes Trop. 2017, 5, 163–175. [Google Scholar] [CrossRef]
- Juárez Lagunes, F.I.; Pell, A.N.; Blake, R.W.; Montero Lagunes, M.; Pinos Rodríguez, J.M. In vitro ruminal degradation of neutral detergent fiber insoluble protein from tropical pastures fertilized with nitrogen. Rev. Mex. Cienc. Pecu. 2018, 9, 588–600. [Google Scholar] [CrossRef]
- Khandaker, Z.H.; Tareque, A.M.M. Studies on protein degradabilities of feedstuffs in bangladesh. Asian-Australas. J. Anim. Sci. 1996, 9, 637–642. [Google Scholar] [CrossRef]
- Khamseekhiew, B.; Liang, J.B.; Wong, C.C.; Jalan, Z.A. Ruminal and intestinal digestibility of some tropical legume forages. Asian-Australas. J. Anim. Sci. 2001, 14, 321–325. [Google Scholar] [CrossRef]
- Ramírez Lozano, R.G.; González Rodríguez, H.; García Dessommes, G. Chemical composition and rumen digestion of forage from kleingrass (Panicum coloratum). Interciencia 2002, 27, 705–709. [Google Scholar]
- Tedeschi, L.O.; Fox, D.G.; Pell, A.N.; Lanna, D.P.D.; Boin, C. Development and evaluation of a tropical feed library for the cornell net carbohydrate and protein system model. Sci. Agric. 2002, 59, 1–18. [Google Scholar] [CrossRef]
- Mupangwa, J.F.; Ngongoni, N.T.; Hamudikuwanda, H. Effects of stage of maturity and method of drying on in situ nitrogen degradability of fresh herbage of Cassia rotundifolia, Lablab purpureus and Macroptilium atropurpureum. Livest. Res. Rural. Dev. 2003, 15, 1–10. [Google Scholar]
- La, O.; Chongo, B.; Delgado, D.; Ruiz, T.; Ruiz, O. Fraccionamiento protéico y digestión ruminal de nutrientes de siratro (Macroptilium Atropurpureum); (Protein fractionation and ruminal nutrient digestion of siratro (Macroptilium Atropurpureum). Rev. Cuba. Cienc. Agríc. 2006, 40, 315–320. [Google Scholar]
- Valarini, M.J.; Possenti, R.A. Research note: Nutritive value of a range of tropical forage legumes. Trop. Grassl. 2006, 40, 183–187. [Google Scholar]
- Ajayi, F.T.; Babayemi, O.J.; Taiwo, A.A. Effects of Stylosanthes guianensis and Aeschynomene histrix on the yield, proximate composition and in situ dry matter and crude protein degradation of Panicum Maximum (Ntchisi). Livest. Res. Rural. Dev. 2007, 19, 1–9. [Google Scholar]
- Bowen, M.K.; Poppi, D.P.; McLennan, S.R. Ruminal protein degradability of a range of tropical pastures. Aust. J. Exp. Agric. 2008, 48, 806–810. [Google Scholar] [CrossRef]
- Wigati, S.; Adiwimarta, K.; Wiyanto, E.; Orskov, E. In sacco degradability of six different tropical feedstuffs. In Proceedings of the 16th AAAP Animal Science Congress, Yogyakarta, Indonesia, 10–14 November 2014; Volume 2, pp. 376–379. [Google Scholar]
- Wallace, R.J.; Lahlou-Kassi, A. Rumen ecology research planning. In Proceedings of the a Workshop held at ILRI (International Livestock Research Institute), Addis Ababa, Ethiopia, 13–18 March 1995; p. 270. [Google Scholar]
- Salgueiro da Silva, M.A.; Seixas, T.M. The role of data range in linear regression. Phys. Teach. 2017, 55, 371–372. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Tamminga, S.; Zemmelink, G. Degradation of tropical roughages and concentrate feeds in the rumen. Anim. Feed Sci. Technol. 1995, 54, 81–92. [Google Scholar] [CrossRef]
- Nurdianti, R.R.; Dickhoefer, U.; Castro-Montoya, J. Evaluation of undigested and potential digestible fiber in tropical grasses and tropical legumes. In Proceedings of the Tropentag, Kassel/Goettingen, Germany, 18–20 September 2019; p. 217. [Google Scholar]
- Fox, D.G.; Tedeschi, L.O.; Tylutki, T.P.; Russell, J.B.; Van Amburgh, M.E.; Chase, L.E.; Pell, A.N.; Overton, T.R. The Cornell Net Carbohydrate and Protein System model for evaluating herd nutrition and nutrient excretion. Anim. Feed Sci. Technol. 2004, 112, 29–78. [Google Scholar] [CrossRef]
- Schroeder, J.W. Forage Nutrition for Ruminants; NDSU Extension Service, North Dakota State University: Fargo, ND, USA, 2004. [Google Scholar]
- Tran, H.; Salgado, P.; Lecomte, P. Species, climate and fertilizer effects on grass fibre and protein in tropical environments. J. Agric. Sci. 2009, 147, 555–568. [Google Scholar] [CrossRef]
- Salazar-Cubillas, K.; Dickhoefer, U. Validation of prediction equations to estimate rumen-undegradable crude protein in tropical feedstuffs using protein fractionation technique. In Proceedings of the Advances in Animal Biosciences; Cambridge University Press: Clermont-Ferrand, France, 2018; p. 526. [Google Scholar]
- Kirchhof, S. Untersuchungen zur Kinetik des Ruminalen In Situ-Nährstoffabbaus von Grünlandaufwüchsen des Alpenraumes Unterschiedlicher Vegetationsstadien Sowie von Maissilagen und Heu—Ein Beitrag zur Weiterentwicklung der Rationsgestaltung für Milchkühe. Ph.D. Thesis, Christian-Albrechts-Universität zu Kiel, Kiel, Germany, 2007. [Google Scholar]
| Tropical Forage Grasses (n = 23) | Tropical Forage Legumes (n = 15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | |
| Proximate nutrient and chemical fiber fractions [g/kg dry matter] | ||||||||||
| Crude ash | 123 | 119 | 29 | 76 | 178 | 74 | 70 | 16 | 45 | 99 |
| Crude protein | 117 | 119 | 34 | 46 | 201 | 177 | 174 | 25 | 135 | 212 |
| Neutral-detergent fiber a | 576 | 573 | 41 | 481 | 654 | 448 | 460 | 69 | 328 | 586 |
| Acid-detergent fiber b | 308 | 304 | 33 | 220 | 359 | 313 | 320 | 59 | 201 | 414 |
| Lignin(sa) c | 33 | 30 | 20 | 6 | 93 | 69 | 69 | 19 | 46 | 125 |
| NDFp d | 677 | 678 | 40 | 592 | 758 | 477 | 459 | 65 | 382 | 585 |
| ADFp e | 357 | 363 | 33 | 278 | 421 | 356 | 340 | 62 | 269 | 486 |
| Crude protein fractions [g/kg dry matter] f | ||||||||||
| A | 43.7 | 41.0 | 18.8 | 15.7 | 93.6 | 47.9 | 42.5 | 14.1 | 24.4 | 75.4 |
| B1 | 3.4 | 3.3 | 2.3 | 0.3 | 9.2 | 6.4 | 5.1 | 5.0 | 0.2 | 17.2 |
| B2 | 23.9 | 23.1 | 8.0 | 11.3 | 40.4 | 62.3 | 65.2 | 18.5 | 24.2 | 95.7 |
| B3 | 32.0 | 34.0 | 12.0 | 6.1 | 51.8 | 38.6 | 39.5 | 17.7 | 6.2 | 69.9 |
| C | 14.0 | 12.6 | 6.4 | 5.4 | 27.0 | 21.2 | 16.6 | 9.6 | 9.6 | 47.0 |
| In vitro fermentation parameters (24 h) g | ||||||||||
| GP [mL/200 mg dry matter] | 29 | 29 | 3 | 24 | 34 | 34 | 33 | 6 | 25 | 43 |
| DOM [g/g dry matter] | 0.48 | 0.48 | 0.03 | 0.43 | 0.53 | 0.55 | 0.55 | 0.05 | 0.49 | 0.64 |
| ME [MJ/kg dry matter] | 6.73 | 6.73 | 0.43 | 5.81 | 7.62 | 8.02 | 7.97 | 0.89 | 6.81 | 10.01 |
| Post-ruminal crude protein [g/kg dry matter] h | ||||||||||
| 2%/h | 105 | 107 | 10 | 82 | 119 | 125 | 122 | 9 | 111 | 150 |
| 5%/h | 110 | 113 | 12 | 81 | 127 | 132 | 128 | 11 | 117 | 162 |
| 8%/h | 113 | 116 | 13 | 81 | 132 | 137 | 133 | 12 | 121 | 171 |
| Error-Index c | Dimensionless d | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Kp a | PRCP Method b | Mean | Mean Bias | RMSE | MAPE | RSR | Concordance Correlation Coefficient | ||
| [%/h] | [g/kg Dry Matter] | [g/kg Dry Matter] | [% Mean Reference PRCP] | [% Mean Reference PRCP] | [From 0 to ∞] | Coefficient [From −1 to 1] | ρ [From −1 to 1] | Cb [From 0 to 1] | |
| Fresh Tropical Forages (n = 38) | |||||||||
| 2 | Reference PRCP | 113 | |||||||
| In vitro PRCP | 108 | 5.17 | 17 | 14 | 1.26 | 0.53 | 0.65 | 0.82 | |
| 5 | Reference PRCP | 119 | |||||||
| In vitro PRCP | 117 | 2.83 | 16 | 13 | 0.99 | 0.69 | 0.84 | 0.83 | |
| Chemical PRCP | 200 | −66.91 | 74 | 67 | 4.68 | 0.14 | 0.87 | 0.16 | |
| 8 | Reference PRCP | 122 | |||||||
| In vitro PRCP | 123 | 1.17 | 15 | 13 | 0.86 | 0.74 | 0.88 | 0.84 | |
| Fresh Tropical Forage Grasses (n = 23) | |||||||||
| 2 | Reference PRCP | 105 | |||||||
| In vitro PRCP | 100 | 5.90 | 16 | 13 | 1.60 | 0.53 | 0.75 | 0.71 | |
| 5 | Reference PRCP | 110 | |||||||
| In vitro PRCP | 102 | 8.61 | 15 | 13 | 1.22 | 0.66 | 0.89 | 0.73 | |
| Chemical PRCP | 173 | −56.04 | 62 | 56 | 5.05 | 0.13 | 0.83 | 0.16 | |
| 8 | Reference PRCP | 113 | |||||||
| In vitro PRCP | 105 | 8.37 | 13 | 12 | 0.98 | 0.73 | 0.93 | 0.78 | |
| Fresh Tropical Forage Legumes (n = 15) | |||||||||
| 2 | Reference PRCP | 125 | |||||||
| In vitro PRCP | 120 | 4.05 | 19 | 14 | 1.93 | 0.20 | 0.30 | 0.65 | |
| 5 | Reference PRCP | 132 | |||||||
| In vitro PRCP | 140 | −6.03 | 16 | 13 | 1.46 | 0.29 | 0.39 | 0.73 | |
| Chemical PRCP | 242 | −83.59 | 85 | 84 | 7.62 | 0.03 | 0.56 | 0.05 | |
| 8 | Reference PRCP | 137 | |||||||
| In vitro PRCP | 150 | −9.87 | 17 | 15 | 1.33 | 0.30 | 0.44 | 0.68 | |
| Dependent Variables a | Intercept and Independent Variables b | Parameters Estimate | SEM | Value | Adjusted R2 c | RMSE d | MAPE e |
|---|---|---|---|---|---|---|---|
| [g/kg Dry Matter] | [g/kg Dry Matter] | [% Mean Reference PRCP] | [% Mean Reference PRCP] | ||||
| PRCP | Intercept | 94.96 | 8.23 | <0.01 | 0.80 | 5.29 | 4.25 |
| Kp 2%/h | B2 + B3 + C | 0.36 | 0.03 | <0.01 | |||
| ADF | −0.05 | 0.02 | 0.05 | ||||
| PRCP | Intercept | 97.45 | 8.66 | <0.01 | 0.82 | 5.31 | 4.37 |
| Kp 5%/h | B2 + B3 + C | 0.42 | 0.03 | <0.01 | |||
| ADF | −0.05 | 0.02 | 0.03 | ||||
| PRCP | Intercept | 97.52 | 9.07 | <0.01 | 0.85 | 5.40 | 4.41 |
| Kp 8%/h | B2 + B3 + C | 0.47 | 0.04 | <0.01 | |||
| ADF | −0.06 | 0.02 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Cubillas, K.C.; Dickhoefer, U. Evaluating the Protein Value of Fresh Tropical Forage Grasses and Forage Legumes Using In Vitro and Chemical Fractionation Methods. Animals 2021, 11, 2853. https://doi.org/10.3390/ani11102853
Salazar-Cubillas KC, Dickhoefer U. Evaluating the Protein Value of Fresh Tropical Forage Grasses and Forage Legumes Using In Vitro and Chemical Fractionation Methods. Animals. 2021; 11(10):2853. https://doi.org/10.3390/ani11102853
Chicago/Turabian StyleSalazar-Cubillas, Khaterine C., and Uta Dickhoefer. 2021. "Evaluating the Protein Value of Fresh Tropical Forage Grasses and Forage Legumes Using In Vitro and Chemical Fractionation Methods" Animals 11, no. 10: 2853. https://doi.org/10.3390/ani11102853
APA StyleSalazar-Cubillas, K. C., & Dickhoefer, U. (2021). Evaluating the Protein Value of Fresh Tropical Forage Grasses and Forage Legumes Using In Vitro and Chemical Fractionation Methods. Animals, 11(10), 2853. https://doi.org/10.3390/ani11102853





