Dietary Fiber and Lysolecithin Supplementation in Growing Ducks: Effect on Performance, Immune Response, Intestinal Morphology and Lipid Metabolism-Regulating Genes
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Duck’s Care and Experimental Design
2.2. Growth Performance
2.3. Serum Lipid Profile and Immune Response
2.4. Intestinal Morphology
2.5. Expression Analysis of Genes Related to Lipid Metabolism
2.6. Statistical Analysis
3. Results
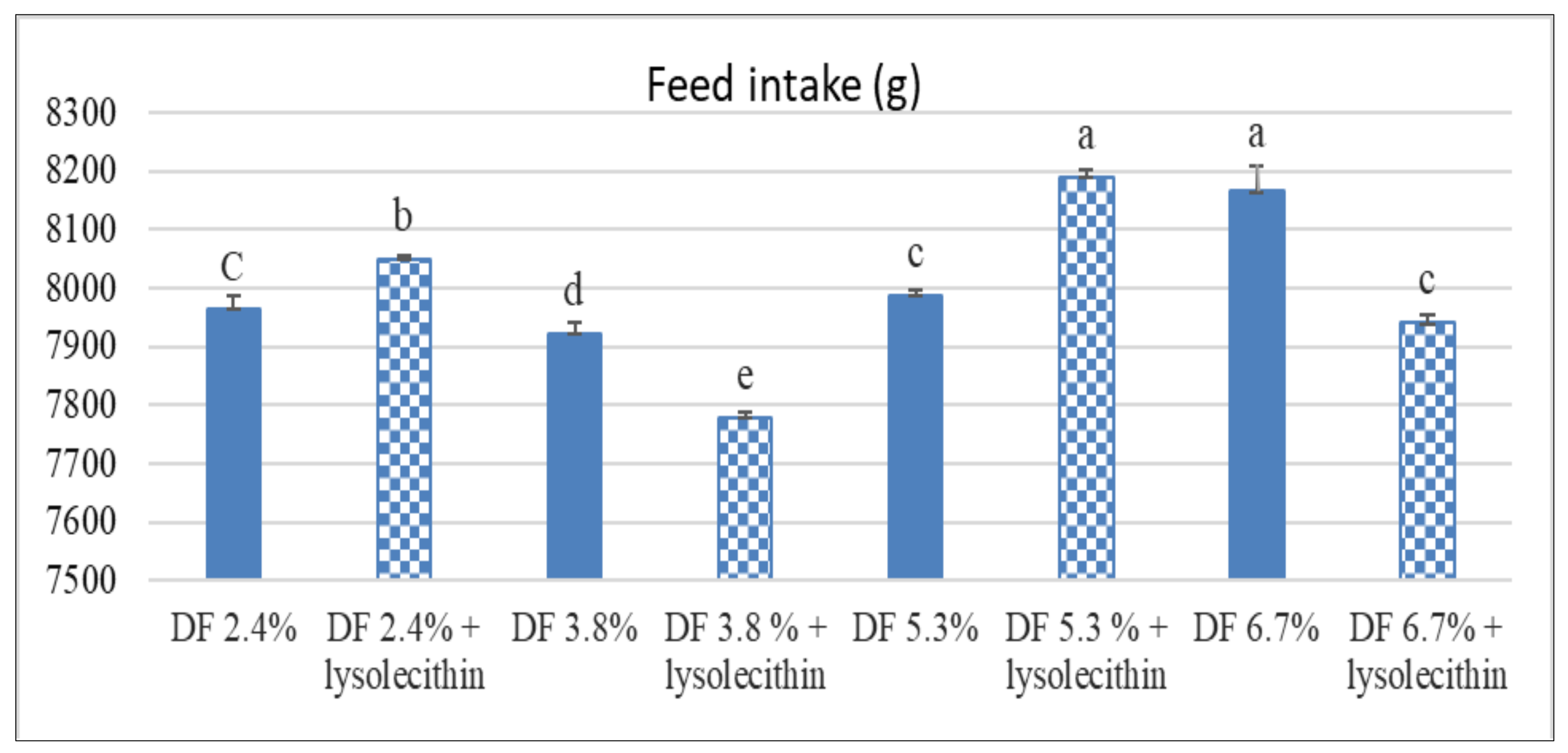
3.1. Performance and Feed Efficiency Parameters
3.2. Immune Response, Antioxidants and Serum Lipid Profile
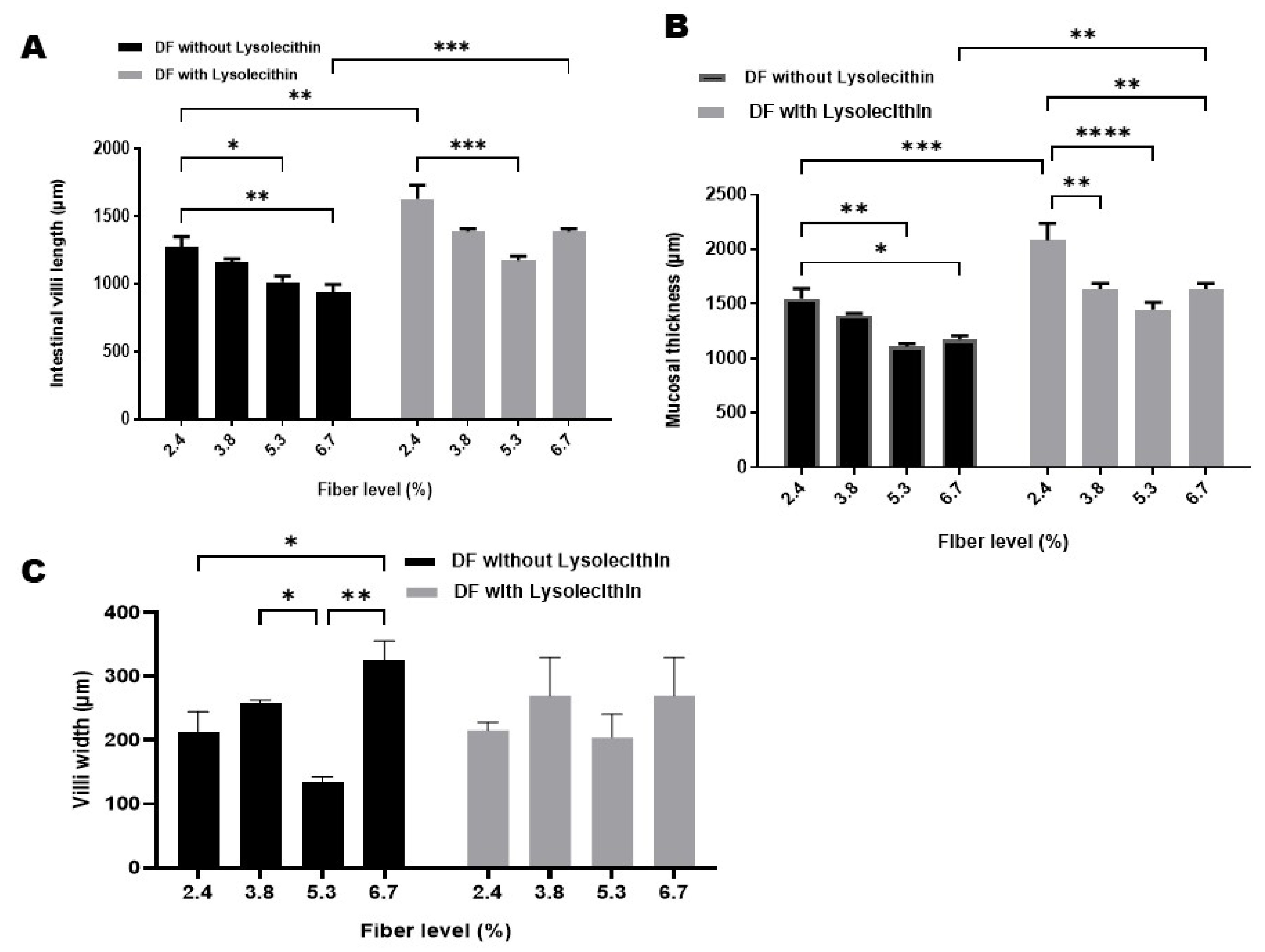
3.3. Intestinal Morphology
3.4. Expression of Some Fat Metabolism-Regulating Genes in Duck Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sklan, D.; Smirnov, A.; Plavnik, I. The effect of dietary fibre on the small intestines and apparent digestion in the turkey. Br. Poult. Sci. 2003, 44, 735–740. [Google Scholar] [CrossRef]
- Leung, H.; Arrazola, A.; Torrey, S.; Kiarie, E. Utilization of soy hulls, oat hulls, and flax meal fiber in adult broiler breeder hens. Poult. Sci. 2018, 97, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alvarado, J.M.; Jimenez-Moreno, E.; Lazaro, R.; Mateos, G.G. Effect of type of cereal, heat processing of the cereal, and inclusion of fiber in the diet on productive performance and digestive traits of broilers. Poult. Sci. 2007, 86, 1705–1715. [Google Scholar] [CrossRef]
- Svihus, B. The gizzard: Function, influence of diet structure and effects on nutrient availability. World’s Poult. Sci. J. 2011, 67, 207–224. [Google Scholar] [CrossRef]
- Gonzalez-Alvarado, J.M.; Jimenez-Moreno, E.; Valencia, D.G.; Lazaro, R.; Mateos, G.G. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci. 2008, 87, 1779–1795. [Google Scholar] [CrossRef] [PubMed]
- Yokhana, J.S.; Parkinson, G.; Frankel, T.L. Effect of insoluble fiber supplementation applied at different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 2016, 95, 550–559. [Google Scholar] [CrossRef]
- Han, H.; Zhang, K.; Ding, X.; Bai, S.; Luo, Y.; Wang, J.; Peng, H.; Zeng, Q. Effects of dietary nanocrystalline cellulose supplementation on growth performance, carcass traits, intestinal development and lipid metabolism of meat ducks. Anim. Nutr. 2016, 2, 192–197. [Google Scholar] [CrossRef]
- Hetland, H.; Svihus, B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br. Poult. Sci. 2001, 42, 633–637. [Google Scholar] [CrossRef]
- Gu, X.; Li, D. Fat nutrition and metabolism in piglets: A review. Anim. Feed Sci. Technol. 2003, 109, 151–170. [Google Scholar] [CrossRef]
- Al-Marzooqi, W.; Leeson, S. Evaluation of dietary supplements of lipase, detergent, and crude porcine pancreas on fat utilization by young broiler chicks. Poult. Sci. 1999, 78, 1561–1566. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Jansen, M.; di Benedetto, M. The addition of lysolecithin to broiler diets improves growth performance across fat levels and sources: A meta analysis of 33 trials. Br. Poult. Sci. 2020, 61, 51–56. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Buyse, J.; Scholey, D.; Van Campenhout, L.; Burton, E.; Di Benedetto, M.; Pritchard, S.; Nuyens, F.; Jansen, M. Lysolecithin, but not lecithin, improves nutrient digestibility and growth rates in young broilers. Br. Poult. Sci. 2020, 61, 414–423. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- Joshi, A.; Paratkar, S.G.; Thorat, B.N. Modification of lecithin by physical, chemical and enzymatic methods. Eur. J. Lipid Sci. Technol. 2006, 108, 363–373. [Google Scholar] [CrossRef]
- Liu, D.; Ma, F. Soybean phospholipids. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; Intech: Rijeka, Croatia, 2011. [Google Scholar]
- Park, J.H.; Nguyen, D.H.; Kim, I.H. Effects of exogenous lysolecithin emulsifier supplementation on the growth performance, nutrient digestibility, and blood lipid profiles of broiler chickens. J. Poult. Sci. 2018, 55, 190–194. [Google Scholar] [CrossRef]
- Boontiam, W.; Jung, B.; Kim, Y.Y. Effects of lysophospholipid supplementation to lower nutrient diets on growth performance, intestinal morphology, and blood metabolites in broiler chickens. Poult. Sci. 2017, 96, 593–601. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Nourmohammadi, R.; Nazarizadeh, H.; Latshaw, J.D. Effects of lysolecithin and xylanase supplementation on the growth performance, nutrient digestibility and lipogenic gene expression in broilers fed low-energy wheat-based diets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1564–1573. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Kawahara, E.; Ueda, T.; Nomura, S. In vitro phagocytic activity of white-spotted shark cells after injection with Aermonas salmonicida extracellular products. Gyobyo Kenkyu Jpn. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; El-Naggar, K.; Abdo, W.; Kirrella, A.A.K.; Nashar, T.O. Ameliorative effect of dietary supplementation of copper oxide nanoparticles on inflammatory and immune responses in commercial broiler under normal and heat-stress housing conditions. J. Therm. Biol. 2018, 78, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Engstad, R.E.; Robertsen, B.; Frivold, E. Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol. 1992, 2, 287–297. [Google Scholar] [CrossRef]
- Rainger, G.E.; Rowley, A.F. Antibacterial activity in the serum and mucus of rainbow trout, Oncorhynchus mykiss, following immunisation with Aeromonas salmonicida. Fish Shellfish Immunol. 1993, 3, 475–482. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C.; Suvarna, S.K. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 151. [Google Scholar]
- Jiang, Y.; Liao, X.D.; Xie, M.; Tang, J.; Qiao, S.Y.; Wen, Z.G.; Hou, S.S. Dietary threonine supplementation improves hepatic lipid metabolism of Pekin ducks. Anim. Prod. Sci. 2019, 59, 673–680. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, O.J.; Kim, W.K. The effects of cellulose and soybean hulls as sources of dietary fiber on the growth performance, organ growth, gut histomorphology, and nutrient digestibility of broiler chickens. Poult. Sci. 2020, 99, 6828–6836. [Google Scholar] [CrossRef]
- Sadeghi, A.; Toghyani, M.; Gheisari, A. Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 2015, 94, 2734–2743. [Google Scholar] [CrossRef]
- Abd El-latif, S.A. Effect of dietary fiber on performance and digestibility of nutrients for growing Pekin ducks. In Proceedings of the 3rd All Africa Conference of Animal and Agriculture. Egyptian Society of Animal Production, Alexandria, Egypt, 6–9 November 2000. [Google Scholar]
- Beshara, M.M.; Rizk, Y.S.; El-Shhat, A.M.; Awad, W.A.; Abdallah, A.G. Effect of feeding different levels of dietary fiber on productive and economical performance in local ducks: 1-During growing period and subsequent laying performance. J. Anim. Poult. Prod. 2017, 8, 425–433. [Google Scholar] [CrossRef]
- Han, H.Y.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Luo, Y.H.; Wang, J.P.; Zeng, Q.F. Effect of dietary fiber levels on performance, gizzard development, intestinal morphology, and nutrient utilization in meat ducks from 1 to 21 days of age. Poult. Sci. 2017, 96, 4333–4341. [Google Scholar] [CrossRef]
- Zosangpuii, A.K.; Samanta, G. Inclusion of an emulsifier to the diets containing different sources of fats on performances of Khaki Campbell ducks. Iran. J. Vet. Res. 2015, 16, 156–160. [Google Scholar]
- Jansen, M.; Nuyens, F.; Buyse, J.; Leleu, S.; Van Campenhout, L. Interaction between fat type and lysolecithin supplementation in broiler feeds. Poult. Sci. 2015, 94, 2506–2515. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Ahmed, I.; Basha, H.; Sadek, K. Influence of feeding dried vegetable fat blend with or without emulsifier and/or yeast culture on productive, economic performances and some biochemical parameters of broiler chickens. Int. J. Curr. Res. Biosci. Plant Biol. 2015, 2, 1–12. [Google Scholar]
- Asaoka, Y.; Oka, M.; Yoshida, K.; Sasaki, Y.; Nishizuka, Y. Role of lysophosphatidylcholine in T-lymphocyte activation: Involvement of phospholipase A2 in signal transduction through protein kinase C. Proc. Natl. Acad. Sci. USA 1992, 89, 6447–6451. [Google Scholar] [CrossRef]
- Ousman, S.S.; David, S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 2000, 30, 92–104. [Google Scholar] [CrossRef]
- Behera, D.P.; Sethi, A.P.S.; Singh, C.; Singh, U.; Wadhwa, M. Effect of citrus waste on blood parameters of broiler birds with and without cocktail of enzymes. Vet. World 2019, 12, 483–488. [Google Scholar] [CrossRef]
- King, M.F.; Boyd, L.C.; Sheldon, B.W. Antioxidant properties of individual phospholipids in a salmon oil model system. J. Am. Oil Chem. Soc. 1992, 69, 545–551. [Google Scholar] [CrossRef]
- El-Katcha, M.; Soltan, M.; El-Naggar, K.; El-Shobokshy, S.; El-Erian, M. Laying performance, fat digestibility and liver condition of laying hens supplmented with vitamin B12 or biotin and/or bile acids in diet. Slov. Vet. Res. 2019, 56, 341–352. [Google Scholar]
- Qin, S.; Han, H.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Zeng, Q. Dietary fibre alleviates hepatic fat deposition via inhibiting lipogenic gene expression in meat ducks. J. Anim. Physiol. Anim. Nutr. 2018, 102, e736–e745. [Google Scholar] [CrossRef]
- Safaa, H.; Jiménez-Moreno, E.; Frikha, M.; Mateos, G. Plasma lipid metabolites and liver lipid components in broilers at 21 days of age in response to dietary different fiber sources. Egypt. J. Anim. Prod. 2014, 51, 115–127. [Google Scholar]
- Bogusławska-Tryk, M.; Piotrowska, A.; Szymeczko, R.; Burlikowska, K.; Głowińska, B. Lipid metabolism indices and fatty acids profile in the blood serum of broiler chickens fed a diet with lignocellulose. Braz. J. Poultry. Sci. 2016, 18, 451–456. [Google Scholar] [CrossRef][Green Version]
- Jung, H.G.; Fahey, G.C.J. Nutritional implications of phenolic monomers and lignin: A review. J. Anim. Sci. 1983, 57, 206–219. [Google Scholar] [CrossRef]
- Huang, J.; Yang, D.; Gao, S.; Wang, T. Effects of soy-lecithin on lipid metabolism and hepatic expression of lipogenic genes in broiler chickens. Livest. Sci. 2008, 118, 53–60. [Google Scholar] [CrossRef]
- Spilburg, C.A.; Goldberg, A.C.; McGill, J.B.; Stenson, W.F.; Racette, S.B.; Bateman, J.; McPherson, T.B.; Ostlund, R.E.J. Fat-free foods supplemented with soy stanol-lecithin powder reduce cholesterol absorption and LDL cholesterol. J. Am. Diet. Assoc. 2003, 103, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Wang, W.B.; Liu, L.; Wang, C.; Feng, W.; Luo, Q.P.; Han, R.; Wang, X.D. Effects of fat type and emulsifier in feed on growth performance, slaughter traits, and lipid metabolism of Cherry Valley ducks. Poult. Sci. 2019, 98, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.; Åman, P. Nutritional aspects of dietary fibres. Anim. Feed Sci. Tech. 1991, 32, 143–158. [Google Scholar] [CrossRef]
- Ling, J.; Gao, Y.-Y.; Ye, H.; Wang, W.-C.; Lin, Z.-P.; Yang, H.-Y.; Huang, S.-B.; Yang, L. Effects of dietary fiber and grit on performance, gastrointestinal tract development, lipometabolism, and grit retention of goslings. J. Integr. Agric. 2014, 13, 2731–2740. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (%) | Starter | Grower-Finisher | ||||||
|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D1 | D2 | D3 | D4 | |
| Soybean hulls | 0 | 4.5 | 9.0 | 13.5 | 0 | 4.5 | 9.0 | 13.5 |
| Corn grain | 58.95 | 53.7 | 48.7 | 44.15 | 68.45 | 63.2 | 58.45 | 53.95 |
| Soybean meal | 30.0 | 30.0 | 30.0 | 30.0 | 23.5 | 23.0 | 22.5 | 22.0 |
| Corn gluten | 7.0 | 6.5 | 6.25 | 5.8 | 4.0 | 4.0 | 4.0 | 4.0 |
| Vegetable oil | 0.5 | 1.75 | 2.5 | 3.0 | 0.5 | 1.75 | 2.5 | 3.0 |
| Dicalcium phosphate 1 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Limestone | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Premix 2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Toxin Binder 3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Anticolostridi 4 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline 5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Lysine 6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Methionine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Chemical composition (%) | ||||||||
| Crude protein | 21.98 | 21.84 | 21.87 | 21.80 | 18.04 | 17.97 | 17.95 | 17.94 |
| Ether extract | 3.27 | 4.35 | 4.96 | 5.33 | 3.48 | 4.57 | 5.18 | 5.56 |
| Crude fiber | 2.40 | 3.85 | 5.31 | 6.78 | 2.36 | 3.79 | 5.24 | 6.69 |
| Calcium | 0.89 | 0.91 | 0.93 | 0.95 | 0.88 | 0.89 | 0.91 | 0.93 |
| Phosphorus | 0.37 | 0.37 | 0.37 | 0.37 | 0.36 | 0.36 | 0.36 | 0.35 |
| Lysine | 1.10 | 1.12 | 1.13 | 1.14 | 0.93 | 0.93 | 0.94 | 0.95 |
| Methionine | 0.49 | 0.48 | 0.47 | 0.46 | 0.43 | 0.42 | 0.41 | 0.40 |
| Threonine | 0.82 | 0.81 | 0.81 | 0.80 | 0.68 | 0.67 | 0.67 | 0.66 |
| Metabolizable Energy, kcal/kg | 2981.7 | 2937.75 | 2913.95 | 2897.0 | 3003.4 | 3006.9 | 2989.6 | 2961.9 |
| NDF * | 10.93 | 13.38 | 15.88 | 18.41 | 10.47 | 12.91 | 15.41 | 17.92 |
| Gene | Primer Sequence | Accession |
|---|---|---|
| FAS 1 | F: GCTGAGAAACGCCAATACC R: GAGCAAGACACCGCAAACT | NM_001310798.1 |
| B-actin | F: GGTATCGGCAGCAGTCTTA R: TTCACAGAGGCGAGTAACTT | NM_00131042.1 |
| LPL 2 | F: AAGAGGGAACCTGATTCAAACG R: CCATCCAGTCAATAAACATAGCG | FJ859348.1 |
| Item | Initial BW (g) | Final BW (g) | Total BW Gain (g) | FI (g) | FCR (g Feed/g Gain) | LER (g Gain/Lipid Intake) | EEU (ME Intake/g Gain) |
|---|---|---|---|---|---|---|---|
| Fiber Effect (%) | |||||||
| 2.4 | 54.500 | 2531.300 | 2476.800 | 8007.325 b | 3.270 ab | 9.097 a | 9.794 ab |
| 3.8 | 54.031 | 2541.837 | 2487.806 | 7850.643 c | 3.187 b | 7.062 b | 9.558 b |
| 5.3 | 54.350 | 2545.000 | 2490.650 | 8088.150 a | 3.286 ab | 6.042 c | 9.796 ab |
| 6.7 | 54.050 | 2460.500 | 2406.450 | 8052.800 a | 3.392 a | 5.461 d | 10.025 a |
| SEM | 0.440 | 27.280 | 26.922 | 14.070 | 0.036 | 0.075 | 0.107 |
| Lysolecithin effect (%) | |||||||
| 0 | 54.343 | 2546.104 | 2491.761 | 8009.973 | 3.248 | 6.987 | 9.688 |
| 0.05 | 54.122 | 2493.214 | 2439.092 | 7989.486 | 3.319 | 6.845 | 9.897 |
| SEM | 0.136 | 19.344 | 19.085 | 9.974 | 0.025 | 0.053 | 0.76 |
| Two-way Anova (p-value) | |||||||
| DF level | 0.893 | 0.09 | 0.09 | <0.001 | 0.001 | <0.001 | 0.025 |
| Lysolecithin | 0.621 | 0.054 | 0.052 | 0.147 | 0.050 | 0.060 | 0.051 |
| Interaction | 0.107 | 0.105 | 0.090 | 0.001 | 0.841 | 0.385 | 0.849 |
| Item | Phagocytic Activity | Phagocytic Index | Lysozyme Activity | Bactericidal Activity | |
|---|---|---|---|---|---|
| Fiber Effect (%) | |||||
| 2.4 | 44.533 c | 1.151 b | 0.405 b | 58.664 | |
| 3.8 | 46.721 b | 1.365 ab | 0.766 a | 62.863 | |
| 5.3 | 47.623 b | 1.329 b | 0.670 a | 65.063 | |
| 6.7 | 49.273 a | 1.579 a | 0.649 a | 63.375 | |
| SEM | 0.483 | 0.067 | 0.051 | 1.809 | |
| Lysolecithin effect (%) | |||||
| 0 | 45.668 | 1.229 | 0.579 | 57.682 | |
| 0.05 | 48.406 | 1.483 | 0.666 | 67.300 | |
| SEM | 0.342 | 0.047 | 0.036 | 1.279 | |
| Two-way Anova (p-value) | |||||
| DF level | <0.001 | 0.002 | <0.001 | 0.107 | |
| Lysolecithin | 0.001 | 0.107 | <0.001 | 0.951 | |
| Interaction | 0.389 | 0.126 | 0.093 | 0.404 | |
| Item | Total Antioxidant Capacity (TAC, u/mL) | Glutathione Peroxidase (GPx, u/mL) | Catalase (u/mL) |
|---|---|---|---|
| Fiber Effect (%) | |||
| 2.4 | 18.369 c | 205.838 | 1.021 |
| 3.8 | 26.088 b | 270.763 | 1.138 |
| 5.3 | 26.513 b | 279.488 | 1.178 |
| 6.7 | 32.063 a | 309.700 | 1.188 |
| SEM | 3.106 | 28.405 | 0.048 |
| Lysolecithin effect (%) | |||
| 0 | 22.153 | 231.638 | 1.090 |
| 0.05 | 29.363 | 301.256 | 1.172 |
| SEM | 2.197 | 20.085 | 0.034 |
| Two-way Anova (p-value) | |||
| DF level | 0.038 | 0.096 | 0.080 |
| Lysolecithin | 0.029 | 0.022 | 0.100 |
| Interaction | 0.305 | 0.985 | 0.738 |
| Item | Triglyceride (mg/dL) | Total Cholesterol (mg/dL) | High-Density Lipoprotein (HDL, mg/dL) | Low-Density Lipoprotein (LDL, mg/dL) | Very Low-Density Lipoprotein (VLDL, mg/dL) |
|---|---|---|---|---|---|
| Fiber Effect (%) | |||||
| 2.4 | 200.288 | 206.526 | 50.436 c | 116.033 | 40.058 |
| 3.8 | 199.505 | 206.076 | 52.778 b | 113.398 | 39.901 |
| 5.3 | 198.960 | 206.639 | 53.420 b | 113.427 | 39.792 |
| 6.7 | 195.958 | 205.749 | 54.566 a | 111.991 | 39.192 |
| SEM | 1.227 | 0.889 | 0.887 | 1.337 | 0.245 |
| Lysolecithin effect (%) | |||||
| 0 | 198.639 | 206.570 | 52.469 | 114.373 | 39.728 |
| 0.05 | 198.716 | 205.925 | 53.131 | 113.051 | 39.743 |
| SEM | 0.868 | 0.629 | 0.627 | 0.945 | 0.174 |
| Two-way Anova (p-value) | |||||
| DF level | 0.095 | 0.885 | 0.022 | 0.218 | 0.095 |
| Lysolecithin | 0.951 | 0.475 | 0.462 | 0.332 | 0.951 |
| Interaction | 0.663 | 0.262 | 0.091 | 0.086 | 0.636 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Katcha, M.I.; Soltan, M.A.; Shewita, R.; Abdo, S.E.; Sanad, A.S.; Tufarelli, V.; Alagawany, M.; El-Naggar, K. Dietary Fiber and Lysolecithin Supplementation in Growing Ducks: Effect on Performance, Immune Response, Intestinal Morphology and Lipid Metabolism-Regulating Genes. Animals 2021, 11, 2873. https://doi.org/10.3390/ani11102873
El-Katcha MI, Soltan MA, Shewita R, Abdo SE, Sanad AS, Tufarelli V, Alagawany M, El-Naggar K. Dietary Fiber and Lysolecithin Supplementation in Growing Ducks: Effect on Performance, Immune Response, Intestinal Morphology and Lipid Metabolism-Regulating Genes. Animals. 2021; 11(10):2873. https://doi.org/10.3390/ani11102873
Chicago/Turabian StyleEl-Katcha, Mohamed I., Mosaad A. Soltan, Ramadan Shewita, Safaa E. Abdo, Amr S. Sanad, Vincenzo Tufarelli, Mahmoud Alagawany, and Karima El-Naggar. 2021. "Dietary Fiber and Lysolecithin Supplementation in Growing Ducks: Effect on Performance, Immune Response, Intestinal Morphology and Lipid Metabolism-Regulating Genes" Animals 11, no. 10: 2873. https://doi.org/10.3390/ani11102873
APA StyleEl-Katcha, M. I., Soltan, M. A., Shewita, R., Abdo, S. E., Sanad, A. S., Tufarelli, V., Alagawany, M., & El-Naggar, K. (2021). Dietary Fiber and Lysolecithin Supplementation in Growing Ducks: Effect on Performance, Immune Response, Intestinal Morphology and Lipid Metabolism-Regulating Genes. Animals, 11(10), 2873. https://doi.org/10.3390/ani11102873








