Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Tissue Specimens
2.2. Serum Collection
2.3. Immunohistochemical Analysis
2.4. Immunoblotting Analysis
2.5. Preparation of Recombinant Canine AGR2 Proteins
2.6. Establishment of a Competitive Enzyme-Linked Immunosorbent Assay (ELISA) for Serum eAGR2 Detection
2.7. Precision Evaluation of the Established Competitive ELISA
2.8. Cell Culture
2.9. Immunofluorescence Staining
2.10. Collection of Conditioned Media and Cell Lysates of Canine MMT Cells
2.11. Follow-Up of the MT Patients
2.12. Statistical Analysis
3. Results
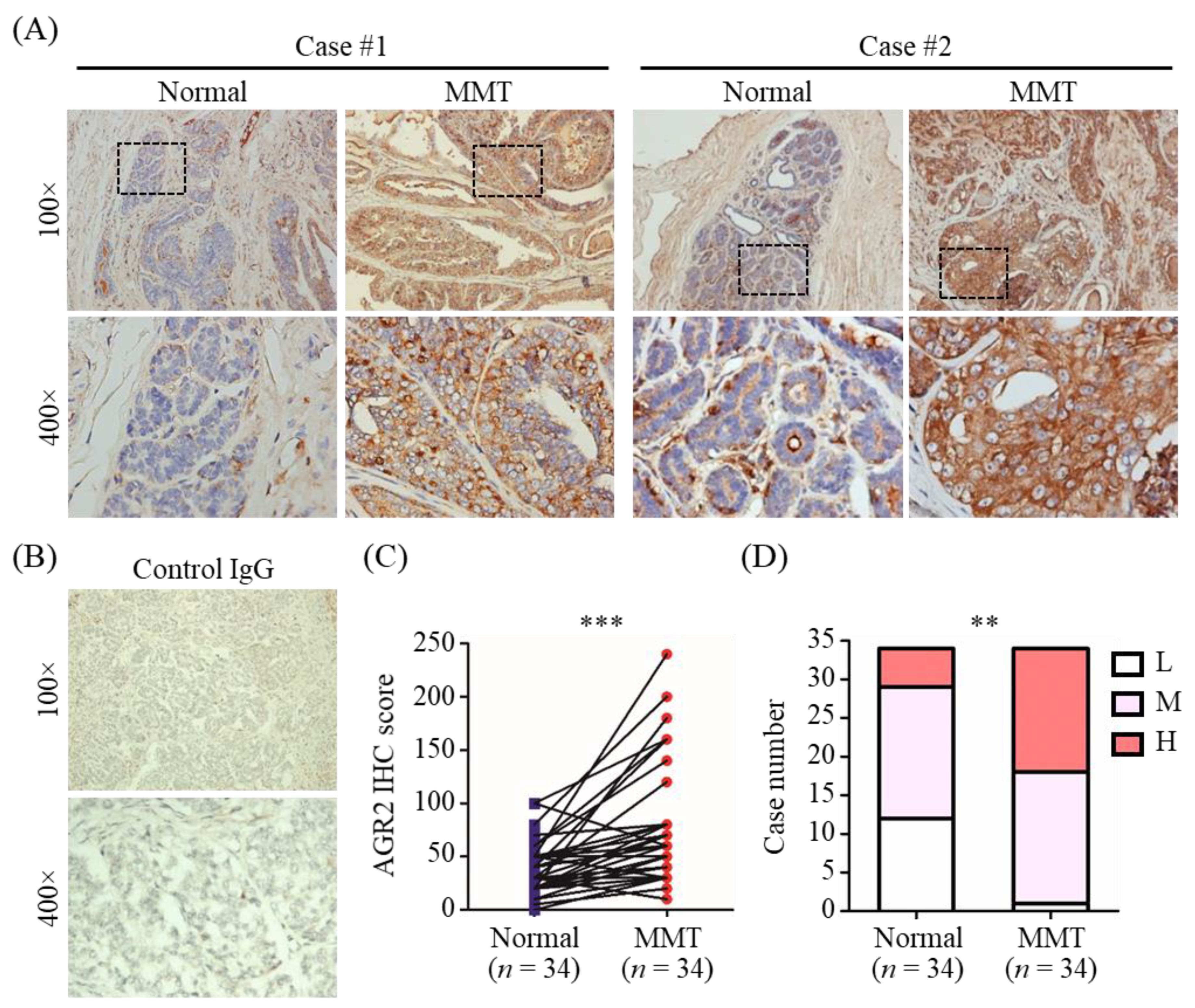
3.1. Elevated Protein Level of AGR2 in Canine Mammary Tumor Tissues
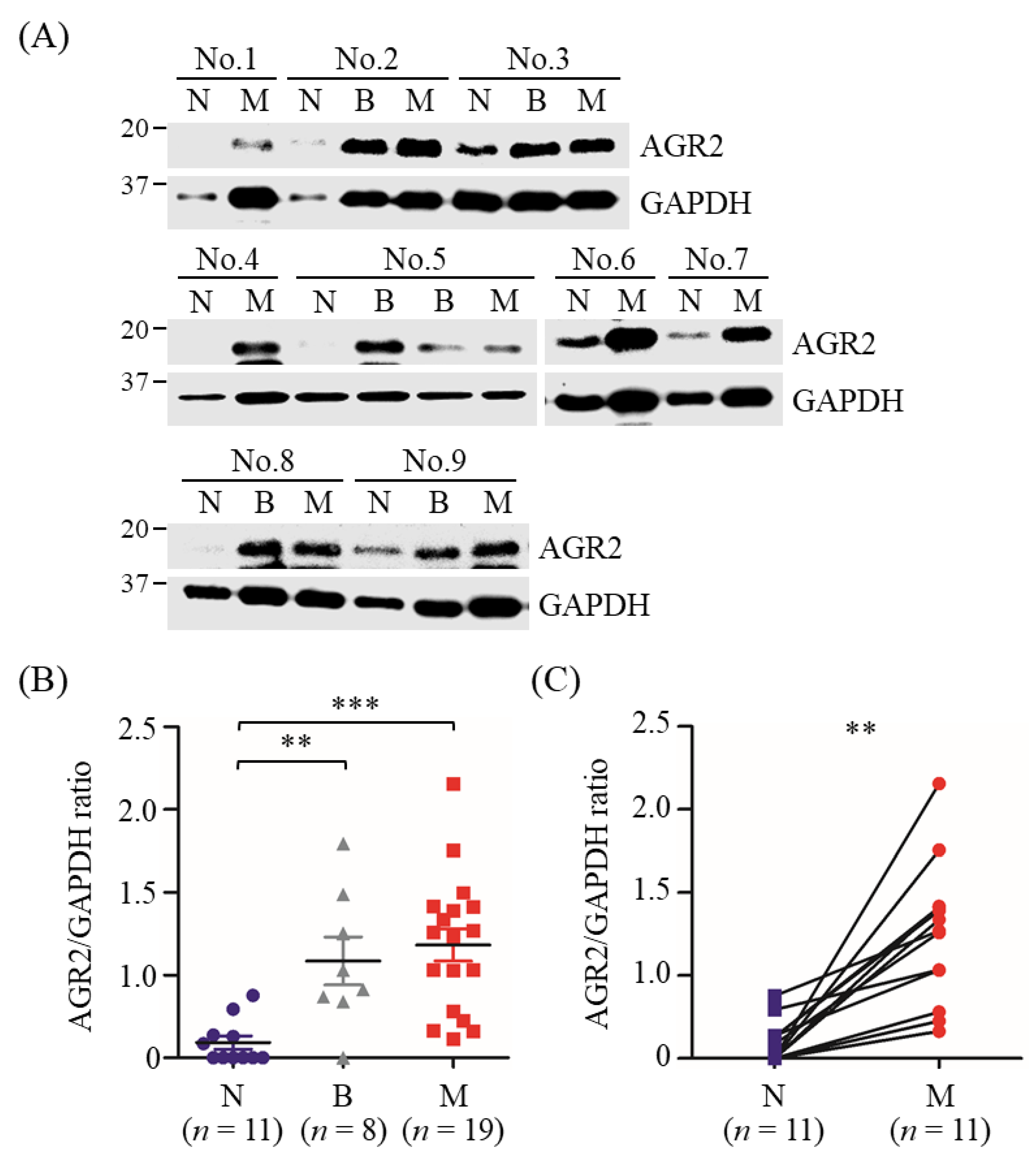
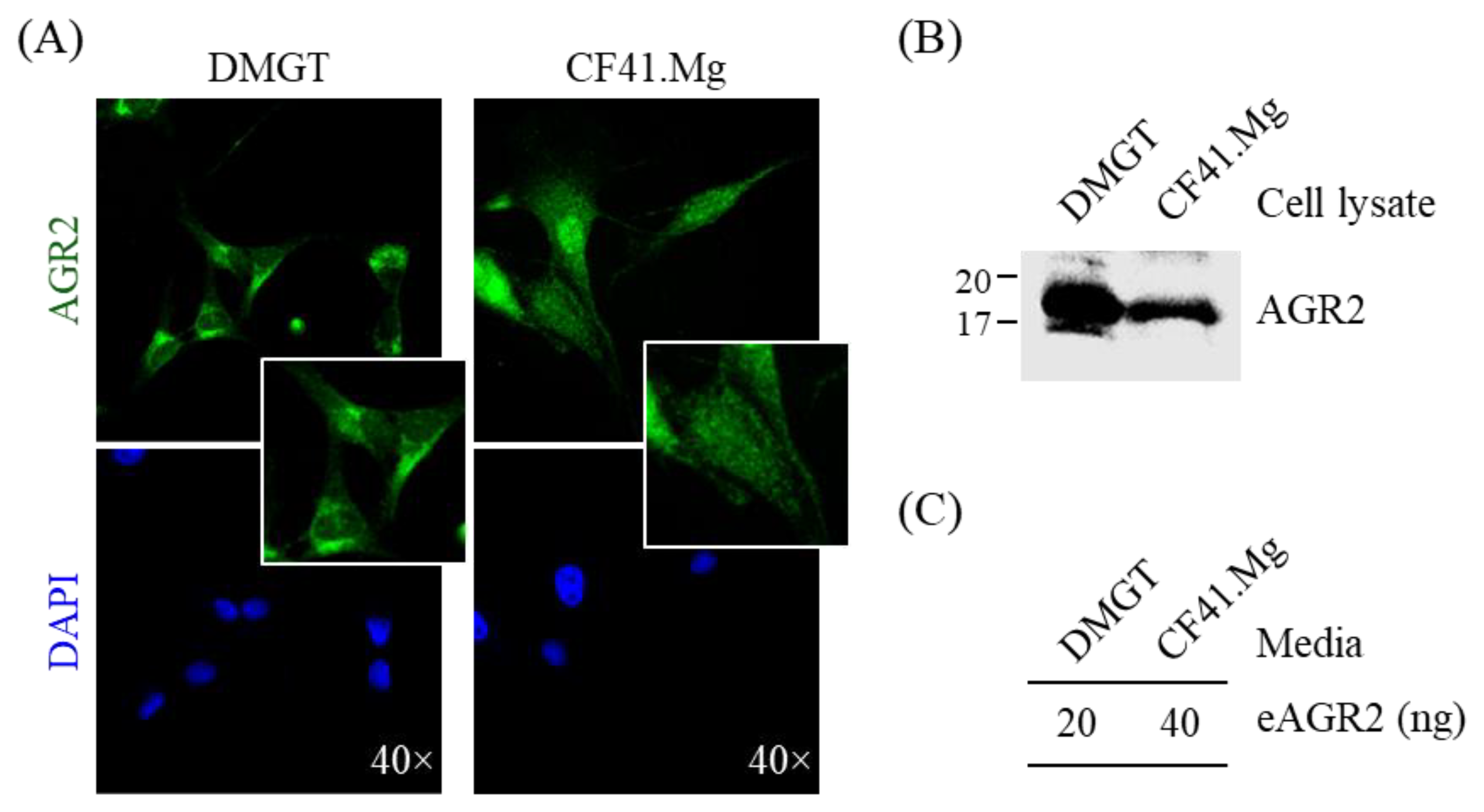
3.2. Detection of AGR2 in Cell Lysates and Serum-Free Conditioned Media of Canine MMT Cells
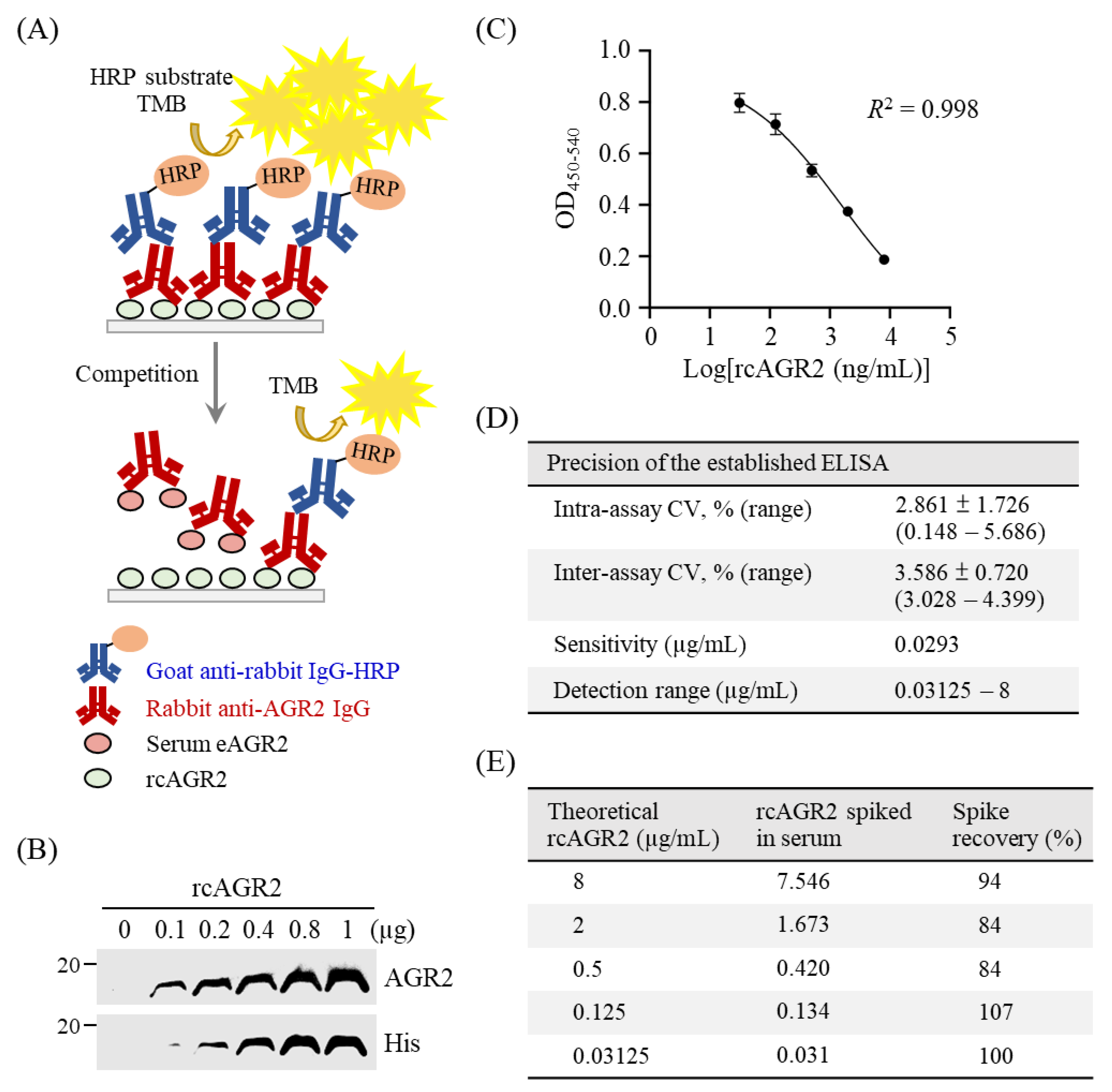
3.3. Establishment of a Competitive ELISA for eAGR2 Detection
3.4. Association of Serum eAGR2 Concentration with Stage and Metastasis of MMT
3.5. Significant Correlation of Serum eAGR2 Concentration with Unfavorable Overall Survival of MMT Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goldschmidt, M.; Pena, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.; De Andres, P.J.; Clemente, M.; Cuesta, P.; Perez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Wu, C.C.; Chang, S.C.; Zeng, G.Y.; Chu, H.W.; Huang, Y.; Liu, H.P. Proteome Analyses Reveal Positive Association of COL2A1, MPO, TYMS, and IGFBP5 with Canine Mammary Gland Malignancy. Proteom. Clin. Appl. 2019, 13, e1800151. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.C.; Patel, S.; Tyson, K.; Adam, P.J.; Schenker, M.; Loader, J.A.; Daviet, L.; Legrain, P.; Parekh, R.; Harris, A.L.; et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br. J. Cancer 2003, 88, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delom, F.; Nazaraliyev, A.; Fessart, D. The role of protein disulphide isomerase AGR2 in the tumour niche. Biol. Cell 2018, 110, 271–282. [Google Scholar] [CrossRef]
- Park, S.-W.; Zhen, G.; Verhaeghe, C.; Nakagami, Y.; Nguyenvu, L.T.; Barczak, A.J.; Killeen, N.; Erle, D.J. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA. 2009, 106, 6950–6955. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.; Wodziak, D.; Lowe, A.W. Epidermal growth factor receptor (EGFR) signaling requires a specific endoplasmic reticulum thioredoxin for the post-translational control of receptor presentation to the cell surface. J. Biol. Chem. 2015, 290, 8016–8027. [Google Scholar] [CrossRef] [Green Version]
- Higa, A.; Mulot, A.; Delom, F.; Bouchecareilh, M.; Nguyen, D.T.; Boismenu, D.; Wise, M.J.; Chevet, E. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J. Biol. Chem. 2011, 286, 44855–44868. [Google Scholar] [CrossRef] [Green Version]
- Alsereihi, R.; Schulten, H.J.; Bakhashab, S.; Saini, K.; Al-Hejin, A.M.; Hussein, D. Leveraging the Role of the Metastatic Associated Protein Anterior Gradient Homologue 2 in Unfolded Protein Degradation: A Novel Therapeutic Biomarker for Cancer. Cancers 2019, 11, 890. [Google Scholar] [CrossRef] [Green Version]
- Han, C.C.; Wan, F.S. New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis. J. Breast Cancer 2018, 21, 354–362. [Google Scholar] [CrossRef]
- Maurel, M.; Obacz, J.; Avril, T.; Ding, Y.P.; Papadodima, O.; Treton, X.; Daniel, F.; Pilalis, E.; Hörberg, J.; Hou, W.; et al. Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Zhao, F.; Edwards, R.; Dizon, D.; Afrasiabi, K.; Mastroianni, J.R.; Geyfman, M.; Ouellette, A.J.; Andersen, B.; Lipkin, S.M. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/-mice. Dev. Biol. 2010, 338, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumartin, L.; Alrawashdeh, W.; Trabulo, S.M.; Radon, T.P.; Steiger, K.; Feakins, R.M.; di Magliano, M.P.; Heeschen, C.; Esposito, I.; Lemoine, N.R.; et al. ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene 2017, 36, 3094–3103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiemann, K.; Garri, C.; Lee, S.B.; Malihi, P.D.; Park, M.; Alvarez, R.M.; Yap, L.P.; Mallick, P.; Katz, J.E.; Gross, M.E.; et al. Loss of ER retention motif of AGR2 can impact mTORC signaling and promote cancer metastasis. Oncogene 2019, 38, 3003–3018. [Google Scholar] [CrossRef] [PubMed]
- Hrstka, R.; Nenutil, R.; Fourtouna, A.; Maslon, M.M.; Naughton, C.; Langdon, S.; Murray, E.; Larionov, A.; Petrakova, K.; Muller, P.; et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene 2010, 29, 4838–4847. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Q.; Chen, H.; Hu, L.; Negi, H.; Zheng, Y.; Ahmed, Y.; Wu, Z.; Li, D. Binding of anterior gradient 2 and estrogen receptor-alpha: Dual critical roles in enhancing fulvestrant resistance and IGF-1-induced tumorigenesis of breast cancer. Cancer Lett. 2016, 377, 32–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, F.; Zhang, F.; Cui, Y.; Wang, Q.; Liu, H.; Wu, Y. miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. J. Exp. Clin. Cancer Res. 2019, 38, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Xu, Q.; Yuan, Q.; Jia, M.; Niu, H.; Liu, X.; Zhang, J.; Young, C.Y.; Yuan, H. Proteasome inhibition boosts autophagic degradation of ubiquitinated-AGR2 and enhances the antitumor efficiency of bevacizumab. Oncogene 2019, 38, 3458–3474. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, Z.; Chen, H.; Zhu, Q.; Gao, G.; Hu, L.; Negi, H.; Kamle, S.; Li, D. Induction of anterior gradient 2 (AGR2) plays a key role in insulin-like growth factor-1 (IGF-1)-induced breast cancer cell proliferation and migration. Med. Oncol. 2015, 32, 577. [Google Scholar] [CrossRef] [Green Version]
- Fessart, D.; Domblides, C.; Avril, T.; Eriksson, L.A.; Begueret, H.; Pineau, R.; Malrieux, C.; Dugot-Senant, N.; Lucchesi, C.; Chevet, E.; et al. Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. Elife 2016, 5. [Google Scholar] [CrossRef]
- Guo, J.; Gong, G.; Zhang, B. Identification and prognostic value of anterior gradient protein 2 expression in breast cancer based on tissue microarray. Tumour Biol. 2017, 39, 1010428317713392. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.B.; Tao, K.X.; Hu, J.; Liu, Z.B.; Ding, X.L.; Chu, Y.N.; Cui, J.Y.; Shuai, X.M.; Gao, J.B.; Cai, K.L.; et al. The prognostic value of AGR2 expression in solid tumours: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 15500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrstka, R.; Bouchalova, P.; Michalova, E.; Matoulkova, E.; Muller, P.; Coates, P.J.; Vojtesek, B. AGR2 oncoprotein inhibits p38 MAPK and p53 activation through a DUSP10-mediated regulatory pathway. Mol. Oncol. 2016, 10, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Fessart, D.; de Barbeyrac, C.; Boutin, I.; Grenier, T.; Richard, E.; Begueret, H.; Bernard, D.; Chevet, E.; Robert, J.; Delom, F. Extracellular AGR2 triggers lung tumour cell proliferation through repression of p21(CIP1). Biochim. Biophys. Acta. Mol. Cell. Res. 2020, 1868, 118920. [Google Scholar] [CrossRef]
- Tian, S.; Hu, J.; Tao, K.; Wang, J.; Chu, Y.; Li, J.; Liu, Z.; Ding, X.; Xu, L.; Li, Q.; et al. Secreted AGR2 promotes invasion of colorectal cancer cells via Wnt11-mediated non-canonical Wnt signaling. Exp. Cell Res. 2018, 364, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, Q.; Yu, X.; Merugu, S.B.; Mangukiya, H.B.; Smith, N.; Li, Z.; Zhang, B.; Negi, H.; Rong, R.; et al. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene 2017, 36, 5098–5109. [Google Scholar] [CrossRef]
- Jia, M.; Guo, Y.; Zhu, D.; Zhang, N.; Li, L.; Jiang, J.; Dong, Y.; Xu, Q.; Zhang, X.; Wang, M.; et al. Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-kappaB pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Wayner, E.A.; Quek, S.I.; Ahmad, R.; Ho, M.E.; Loprieno, M.A.; Zhou, Y.; Ellis, W.J.; True, L.D.; Liu, A.Y. Development of an ELISA to detect the secreted prostate cancer biomarker AGR2 in voided urine. Prostate 2012, 72, 1023–1034. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, Z.; Jiang, Y.; Lu, J.; Xie, H.; Tang, F. AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin. Chim. Acta 2018, 484, 323–327. [Google Scholar] [CrossRef]
- Moidu, N.A.; Nisa Syakila, A.R.; Syafruddin, S.E.; Low, T.Y.; Mohtar, M.A. Secretion of pro-oncogenic AGR2 protein in cancer. Heliyon 2020, 6, e05000. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000, 56, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2016, 14, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Chang, C.C.; Chang, T.J.; Wong, M.L. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998–2002). J. Am. Vet. Med. Assoc. 2005, 227, 1625–1629. [Google Scholar] [CrossRef]
- de Araujo, M.R.; Campos, L.C.; Ferreira, E.; Cassali, G.D. Quantitation of the Regional Lymph Node Metastatic Burden and Prognosis in Malignant Mammary Tumors of Dogs. J. Vet. Intern. Med. 2015, 29, 1360–1367. [Google Scholar] [CrossRef]
- Kani, K.; Malihi, P.D.; Jiang, Y.; Wang, H.; Wang, Y.; Ruderman, D.L.; Agus, D.B.; Mallick, P.; Gross, M.E. Anterior gradient 2 (AGR2): Blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype. Prostate 2013, 73, 306–315. [Google Scholar] [CrossRef] [PubMed]


| Benign Mammary Tumor (BMT) | Malignant Mammary Tumor (MMT) | |||||
|---|---|---|---|---|---|---|
| Group | Case Number | Median (Range) Age, Year | Median (Range) B.W., Kg | Case Number | Median (Range) Age, Year | Median (Range) B.W., Kg |
| Total | 21 | 8.4 (3–16) | 5.4 (1.28–38) | 81 | 10 (2–16) | 7.1 (1.7–44.6) |
| Breed | ||||||
| Pedigree | 19 | 8 (3–15) | 5.4 (1.28–38) | 66 | 10 (2–16) | 5.59 (1.7–3.8) |
| Mixed | 2 | 14.5 (13–16) | 18.4 (13.4–23.4) | 15 | 13 (8–16) | 15.6 (2.4–44.6) |
| Neuter | ||||||
| Yes | 6 | 10 (9–13) | 7.2 (2.72–19.4) | 34 | 9 (7–14) | 7.1 (2.1–44.6) |
| No | 15 | 8 (3–16) | 5.4 (1.28–38) | 47 | 10 (4–16) | 5.9 (1.7–30.4) |
| MMT subtype | ||||||
| Simple carcinoma | - | - | 45 | 10.5 (2–16) | 7.89 (1.95–44.6) | |
| Complex carcinoma | - | - | - | 26 | 10 (7–16) | 7.1 (3.3–29.8) |
| Multiple MMTs 1 | - | - | 10 | 9 (7–16) | 5 (2.4–30.4) | |
| Clinical stage | ||||||
| I | - | - | 33 | 9 (2–16) | 4.8 (1.7–36.8) | |
| II | - | - | 15 | 11 (8–16) | 6.5 (1.95–44.6) | |
| III | - | - | 16 | 12 (8–16) | 13 (2.7–29.8) | |
| IV | - | - | 8 | 12.5 (8–15) | 11.9 (4.2–38) | |
| V | - | - | 9 | 10 (3.5–14) | 12.9 (3.8–26.4) | |
| Characteristics | Case Number | eAGR2 (µg/mL) 1 | p-Value |
|---|---|---|---|
| Age (years) 2 | |||
| BMT | |||
| <10 | 12 | 4.34 ± 1.934 | 0.831 |
| ≥10 | 9 | 4.54 ± 1.879 | - |
| MMT | |||
| <10 | 33 | 5.58 ± 2.788 | 0.419 |
| ≥10 | 48 | 6.23 ± 2.957 | - |
| Total | |||
| <10 | 45 | 4.91 ± 2.625 | 0.199 |
| ≥10 | 57 | 5.32 ± 2.869 | - |
| Body weight (kg) 2 | |||
| <6.7 | 51 | 4.91 ± 2.265 | 0.103 |
| ≥6.7 | 51 | 5.43 ± 3.162 | - |
| Neuter status 2 | |||
| No | 62 | 5.11 ± 3.021 | 0.739 |
| Yes | 40 | 5.24 ± 2.370 | - |
| MMT subtype 3 | |||
| Simple carcinoma | 45 | 6.32 ± 2.878 | 0.168 |
| Complex carcinoma | 26 | 5.86 ± 3.072 | - |
| Multiple MMTs | 10 | 4.66 ± 2.224 | - |
| Metastasis 2 | |||
| No | 85 | 5.20 ± 2.324 | 0.002 ** |
| Yes | 17 | 7.93 ± 3.682 | - |
| Stage 3 | |||
| I/II | 48 | 4.76 ± 2.459 | 0.0007 *** |
| III/IV | 24 | 5.29 ± 2.734 | - |
| V | 9 | 8.81 ± 2.762 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.H.-C.; Chang, S.-C.; Huang, Y.; Liu, H.-P. Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors. Animals 2021, 11, 2923. https://doi.org/10.3390/ani11102923
Yuan SH-C, Chang S-C, Huang Y, Liu H-P. Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors. Animals. 2021; 11(10):2923. https://doi.org/10.3390/ani11102923
Chicago/Turabian StyleYuan, Stephen Hsien-Chi, Shih-Chieh Chang, Yenlin Huang, and Hao-Ping Liu. 2021. "Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors" Animals 11, no. 10: 2923. https://doi.org/10.3390/ani11102923






