Effects of a Combination of Lysolecithin, Synthetic Emulsifier, and Monoglycerides on Growth Performance, Intestinal Morphology, and Selected Carcass Traits in Broilers Fed Low-Energy Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broilers, Diets, and Housing
2.2. Growth Performance Evaluation
2.3. Carcass Traits and Meat Characteristics
2.4. Intestinal Morphology
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Traits and Meat Characteristics
3.3. Intestinal Morphology
4. Discussion
4.1. Growth Performance
4.2. Carcass Traits and Meat Characteristics
4.3. Intestinal Morphology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, C.A.; Duong, T.; Latham, R.E.; Shirley, R.B.; Lee, J.T. Increasing amino acid density improves growth performance and processing yield in Cobb 700 × MV broilers. J. Appl. Poult. Res. 2020, 29, 465–478. [Google Scholar] [CrossRef]
- Johnson, C.A.; Duong, T.; Latham, R.E.; Shirley, R.B.; Lee, J.T. Effects of amino acid and energy density on growth performance and processing yield of mixed-sex Cobb 700 × MV broiler chickens. J. Appl. Poult. Res. 2020, 29, 269–283. [Google Scholar] [CrossRef]
- Jansen, M. Modes of Action of Lysophospholipids as Feed Additives on Fat Digestion in Broilers. Ph.D. Thesis, Catholic Univ. of Leuven, Leuven, Belgium, 2 December 2015. Available online: https://limo.libis.be/primo-explore/fulldisplay?docid=LIRIAS1673616&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1 (accessed on 2 September 2021).
- Wealleans, A.L.; Jansen, M.; Di Benedetto, M. Addition of lysolecithin to broiler diets improves growth performance across fat levels and sources. Br. Poult. Sci. 2020, 61, 51–56. [Google Scholar] [CrossRef]
- Joshi, A.; Paratkar, S.G.; Thorat, B.N. Modification of Lecithin by Physical, Chemical and Enzymatic Methods. Eur. J. Lipid Sci. Technol. 2006, 108, 363–373. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Tomás, M.C. Update on Vegetable Lecithin and Phospholipid Technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Buyse, J.; Scholey, D.; Van Campenhout, L.; Burton, E.; Pritchard, S.; Di Benedetto, M.; Nuyens, F.; Jansen, M. Lysolecithin but not lecithin improves nutrient digestibility and growth rates in young broilers. Br. Poult. Sci. 2020, 61, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Zampiga, M.; Meluzzi, A.; Sirri, F. Effect of dietary supplementation of lysophospholipids on productive performance, nutrient. digestibility and carcass quality traits of broiler chickens. Ital. J. Anim. Sci. 2016, 15, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, G.A.; Poutahidis, T.; Chalvatzi, S.; Di Benedetto, M.; Hardas, A.; Tsiouris, V.; Georgopoulou, I.; Arsenos, G.; Fortomaris, P.D. Effects of lysolecithin supplementation in low-energy diets on growth performance, nutrient digestibility, viscosity and intestinal morphology of broilers. Br. Poult. Sci. 2018, 59, 232–239. [Google Scholar] [CrossRef]
- Haetinger, V.S.; Dalmoro, Y.K.; Godoy, G.L.; Lang, M.B.; De Souza, M.B.; Aristimunha, P.; Stefanello, C. Optimizing cost, growth performance and nutrient absorption with a bio-emulsifier based on lysophospholipids for broiler chickens. Poult. Sci. 2021, 100, 101025. [Google Scholar] [CrossRef]
- Jansen, M.; Nuyens, F.; Buyse, J.; Leleu, S.; Van Campenhout, L. Interaction between fat type and lysolecithin supplementation in broiler feeds. Poult. Sci. 2015, 94, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Boontiam, W.; Jung, B.; Kim, Y.Y. Effects of lysophospholipid supplementation to lower nutrient diets on growth performance, intestinal morphology, and blood metabolites in broiler chickens. Poult. Sci. 2017, 96, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Lecithin. In Kirk-Othmer Encyclopedia of Chemical Technology, 14th ed.; Grant, H., Ed.; John Wiley & Sons, Inc: New York, NY, USA, 2000; Volume 15, pp. 192–209. [Google Scholar]
- Mandalari, G.; Adel-Patient, K.; Barkholt, V.; Baro, C.; Bennett, L.; Bublin, M.; Gaier, S.; Graser, G.; Ladics, G.S.; Mierzejewska, D.; et al. In vitro digestibility of beta-casein and beta-lactoglobulin under simulated human gastric and duodenal conditions: A multi-laboratory evaluation. Regul. Toxicol. Pharmacol. 2009, 55, 372–381. [Google Scholar] [CrossRef]
- Brautigan, D.L.; Li, R.; Kubicka, E.; Turner, S.D.; Garcia, J.S.; Weintraut, M.L.; Wong, E.A. Lysolecithin as feed additive enhances collagen expression and villus length in the jejunum of broiler chickens. Poult. Sci. 2017, 96, 2889–2898. [Google Scholar] [CrossRef]
- Chen, C.; Jung, B.; Kim, W.K. Effects of lysophospholipid on growth performance, carcass yield, intestinal development, and bone quality in broilers. Poult. Sci. 2019, 98, 3902–3913. [Google Scholar] [CrossRef]
- Zaefarian, F.; Romero, L.F.; Ravindran, V. Influence of high dose of phytase and an emulsifier on performance, apparent metabolisable energy and nitrogen retention in broilers fed on diets containing soy oil or tallow. Br. Poult. Sci. 2015, 56, 590–597. [Google Scholar] [CrossRef]
- Polycarpo, G.V.; Burbarelli, M.F.C.; Carao, A.C.P.; Merseguel, C.E.B.; Dadalt, J.C.; Maganha, S.R.L.; Sousa, R.L.M.; Cruz-Polycarpo, V.C.; Albuquerque, R.D. Effects of lipid sources, lysophospholipids and organic acids in maize-based broiler diets on nutrient balance, liver concentration of fat-soluble vitamins, jejunal microbiota and performance. Br. Poul. Sci. 2016, 57, 788–798. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poult. Sci. 2017, 96, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Boontiam, W.; Hyun, Y.K.; Jung, B.; Kim, Y.Y. Effects of lysophospholipid supplementation to reduced energy, crude protein, and amino acid diets on growth performance, nutrient digestibility, and blood profiles in broiler chickens. Poult. Sci. 2019, 98, 6693–6701. [Google Scholar] [CrossRef] [PubMed]
- Classen, H.L. Response of Broiler Chickens to Dietary Energy and its Relationship to Amino Acid Nutrition. In Proceedings of the 24th Australian Poultry Science Symposium, Sydney, Australia, 17 February 2013; pp. 107–114. Available online: http://bibliotecavirtual.corpmontana.com/bitstream/123456789/3939/1/M003401.pdf (accessed on 2 September 2021).
- Arbor Acres Broiler Nutrition Specifications 2019. Available online: https://eu.aviagen.com/assets/Tech_Center/AA_Broiler/AABroilerNutritionSpecs2019-EN.pdf (accessed on 14 October 2021).
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Arbor Acres Broiler Performance Objectives 2019. Available online: https://es.aviagen.com/assets/Tech_Center/AA_Broiler/AASF-AAFF-BroilerPO2019-EN.pdf (accessed on 14 October 2021).
- Kidd, M.T.; McDaniel, C.D.; Branton, S.L.; Miller, E.R.; Boren, B.B.; Fancher, B.I. Increasing amino acid density improves live performance and carcass yields of commercial broilers. J. Appl. Poult. Res. 2004, 13, 593–604. [Google Scholar] [CrossRef]
- Dozier, W.A.; Gordon, R.W.; Anderson, J.; Kidd, M.T.; Corzo, A.; Branton, S.L. Growth, meat yield, and economic responses of broilers provided three- and four-phase schedules formulated to moderate and high nutrient density during a fifty-six-day production period. J. Appl. Poult. Res. 2006, 15, 312–325. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ravindran, V. Effect of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: Growth performance and digestibility of energy, minerals, and amino acids. Br. Poult. Sci. 2008, 49, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Wang, W.B.; Liu, L.; Wang, C.; Feng, W.; Luo, Q.P.; Han, R.; Wang, X. Effects of fat type and emulsifier in feed on growth performance, slaughter traits, and lipid metabolism of Cherry Valley ducks. Poult. Sci. 2019, 98, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Siyal, F.A.; Babazadeh, D.; Wang, C.; Arain, M.A.; Saeed, M.; Ayasan, T.; Zhang, L.; Wang, T. Emulsifiers in the poultry industry. Worlds Poult. Sci. J. 2017, 73, 611–620. [Google Scholar] [CrossRef]
- Adams, K.L.; Jensen, A.H. Comparative utilization of in-seed fats and the respective extracted fats by the young pig. J. Anim. Sci. 1984, 59, 1557–1565. [Google Scholar] [CrossRef]
- Kil, D.Y.; Sauber, T.E.; Jones, D.B.; Stein, H.H. Effect of the form of dietary fat and the concentration of dietary neutral detergent fiber on ileal and total tract endogenous losses and apparent and true digestibility of fat by growing pigs. J. Anim. Sci. 2010, 88, 2959–2967. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.G.; Kil, D.Y.; Stein, H.H. In growing pigs, the true ileal and total tract digestibility of acid hydrolyzed ether extract in extracted corn oil is greater than in intact sources of corn oil or soybean oil. J. Anim. Sci. 2013, 91, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Priyadarshi, A.; Gautam, B.; Saikhlai, K. Effect of dietary supplementation of lysophospholipids and phospholipids blend on performance and carcass quality traits of broilers fed energy deficient diet. J. Anim. Health Behav. Sci. 2019, 3, 115. [Google Scholar]
- Thornhill, A. Evaluation of a Lysophospholipid Using Two Oils on Performance, Carcass Composition and Organ Characteristics of Broilers. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. Available online: https://scholar.sun.ac.za/handle/10019.1/109147?show=full (accessed on 3 September 2021).
- Maynard, C.W.; Latham, R.E.; Brister, R.; Owens, C.M.; Rochell, S.J. Effects of dietary energy and amino acid density during finisher and withdrawal phases on live performance and carcass characteristics of Cobb MV × 700 broilers. J. Appl. Poult. Res. 2019, 28, 729–742. [Google Scholar] [CrossRef]
- Melegy, T.; Khaled, N.F.; El-Bana, R.; Abdellatif, H. Dietary fortification of a natural biosurfactant, lysolecithin in broiler. Afr. J. Agric. Res. 2010, 5, 2886–2892. [Google Scholar]
- Mohammadigheisar, M.; Kim, H.S.; Kim, I.H. Effect of inclusion of lysolecithin or multi-enzyme in low energy diet of broiler chickens. J. Appl. Anim. Res. 2018, 46, 1198–1201. [Google Scholar] [CrossRef] [Green Version]
- Nahashon, S.N.; Adefope, N.; Amenyenu, A.; Wright, D. Effects of dietary metabolizable energy and crude protein concentrations on growth performance and carcass characteristics of French Guinea broilers. Poult. Sci. 2005, 84, 337–344. [Google Scholar] [CrossRef]
- Marcu, A.; Vacaru-Opriş, I.; Marcu, A.; Nicula, M.; Dronca, D.; Kelciov, B. Effect of different levels of dietary protein and energy on the growth and slaughter performance at „Hybro PN+” broiler chickens. Sci. Pap. Anim. Sci. Biotechnol. 2012, 45, 424–431. [Google Scholar]
- Zhang, B.; Haitao, L.; Zhao, D.; Guo, Y.; Barri, A. Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids, and apparent metabolizable energy content. Anim. Feed. Sci. Technol. 2011, 163, 177–184. [Google Scholar] [CrossRef]
- Kassim, H.; Suwanpradit, S. The effects of dietary protein levels on the carcass composition of starter and grower broilers. Asian-Australas. J. Anim. Sci. 1996, 9, 261–266. [Google Scholar] [CrossRef]
- Rabie, M.H.; Szilagyi, M. Effects of L-carnitine supplementation of diets differing in energy levels on performance, abdominal fat content, and yield and composition of edible meat of broilers. Br. J. Nutr. 1998, 80, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.P.; Xie, M.; Wang, W.W.; Hou, S.S.; Huang, W. Effects of dietary energy on growth performance and carcass quality of white growing pekin ducks from two to six weeks of age. Poult. Sci. 2008, 87, 1162–1164. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, J.N.; Hou, S.S.; Huang, W. The apparent metabolizable energy requirement of White Pekin ducklings from hatch to 3 weeks of age. Anim. Feed. Sci. Technol. 2010, 157, 95–98. [Google Scholar] [CrossRef]
- Raju, M.V.L.N.; Rama Rao, S.V.; Chakrabarti, P.P.; Ra, B.V.S.K.; Panda, A.K.; Prabhavathi Devi, B.L.A.; Sujatha, V.; Reddy, J.R.C.; Shyam Sunder, G.; Prasad, R.B.N. Rice bran lysolecithin as a source of energy in broiler chicken diet. Br. Poult. Sci. 2011, 52, 769–774. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Nourmohammadi, R.; Nazarizadeh, H.; Latshaw, J.D. Effects of lysolecithin and xylanase supplementation on the growth performance, nutrient digestibility and lipogenic gene expression in broilers fed low-energy wheat-based diets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1564–1573. [Google Scholar] [CrossRef]
- Sanz, M.; Flores, A.; Lopez-Bote, C.J. The metabolic use of calories from dietary fat in broilers is affected by fatty acid saturation. Br. Poult. Sci. 2000, 41, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Flores, A.; Perez De Ayala, P.; Lopez-Bote, C.J. Higher lipid accumulation in broilers fed on saturated fats than in those fed unsaturated fats. Br. Poult. Sci. 1999, 40, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, R.; Tres, A.; Sala, R.; Guardiola, F.; Barroeta, A.C. Evolution of lipid classes and fatty acid digestibility along the gastrointestinal tract of broiler chickens fed different fat sources at different ages. Poult. Sci. 2019, 98, 1341–1353. [Google Scholar] [CrossRef]
- Khonyoung, D.; Yamauchi, K.; Suzuki, K. Influence of dietary fat sources and lysolecithin on growth performance, visceral organ size, and histological intestinal alteration in broiler chickens. Livest. Sci. 2015, 176, 111–120. [Google Scholar] [CrossRef]
- Lairon, D. Digestion and absorption of lipids. In Designing Functional Foods, 1st ed.; McClements, D.J., Decker, E.A., Eds.; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 68–93. [Google Scholar]
- Park, J.H.; Nguyen, D.H.; Kim, I.H. Effects of exogenous lysolecithin emulsifier supplementation on the growth performance, nutrient digestibility, and blood lipid profiles of broiler chickens. J. Poult. Sci. 2018, 55, 190–194. [Google Scholar] [CrossRef] [PubMed]
| 0–10 Days | 11–21 Days | 22–30 Days | |
|---|---|---|---|
| Ingredients (g/kg) | |||
| Corn | 545.7 | 567.0 | 619.6 |
| Soybean meal, 47% | 359.7 | 330.0 | 291.1 |
| Extruded full-fat soybeans 2 | 53.3 | 77.0 | 70.0 |
| Limestone | 12.2 | 11.2 | 9.4 |
| Corn gluten meal, 60% | 10.0 | - | - |
| Monocalcium phosphate | 7.1 | 4.6 | 3.1 |
| Sodium chloride | 2.5 | 2.5 | 2.5 |
| L-Lysine HCl | 3.0 | 1.9 | - |
| DL-Methionine | 2.6 | 2.2 | 1.8 |
| L-Threonine | 1.7 | 1.4 | 0.3 |
| Vitamin and mineral premix 3 | 2.0 | 2.0 | 2.0 |
| NSP enzyme 4 | 0.1 | 0.1 | 0.1 |
| Phytase 5 | 0.1 | 0.1 | 0.1 |
| Calculated nutrient composition (%, as fed basis) | |||
| Dry matter | 88.06 | 87.97 | 87.84 |
| ME, kcal/kg | 2900 | 2950 | 3000 |
| Crude protein | 24.00 | 22.92 | 21.00 |
| Crude fat | 3.61 | 4.07 | 4.04 |
| Crude fibre | 2.77 | 2.86 | 2.56 |
| Lysine | 1.43 | 1.29 | 1.13 |
| Methionine | 0.58 | 0.52 | 0.50 |
| Methionine + cysteine | 0.88 | 0.82 | 0.84 |
| Threonine | 0.94 | 0.88 | 0.84 |
| Arginine | 1.42 | 1.37 | 1.38 |
| Tryptophan | 0.25 | 0.25 | 0.25 |
| Ca | 0.96 | 0.88 | 0.80 |
| Available phosphorous | 0.48 | 0.43 | 0.40 |
| Na | 0.16 | 0.16 | 0.16 |
| Cl | 0.23 | 0.21 | 0.18 |
| Control | LEX 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| Body weight at hatch | 40.17 | 40.33 | 0.260 | 0.6753 |
| 0–7 days | ||||
| BW 3, day 7 | 193 | 195 | 1.883 | 0.3694 |
| BWG 3, g | 153 | 155 | 1.716 | 0.3567 |
| FI 3, g | 150 | 150 | 1.411 | 0.7960 |
| FCR 3 | 0.982 | 0.969 | 0.0083 | 0.3159 |
| 7–14 days | ||||
| BW, day 14 | 542 | 556 | 4.854 | 0.0752 |
| BWG, g | 349 | 361 | 3.604 | 0.0540 |
| FI, g | 399 | 402 | 4.879 | 0.6921 |
| FCR | 1.143 | 1.114 | 0.0072 | 0.0228 |
| 14–21 days | ||||
| BW, day 21 | 962 | 1008 | 6.171 | 0.0007 |
| BWG, g | 420 | 452 | 4.901 | 0.0016 |
| FI, g | 602 | 617 | 3.856 | 0.0246 |
| FCR | 1.440 | 1.369 | 0.011 | 0.0019 |
| 21–28 days | ||||
| BW, day 28 | 1530 | 1602 | 14.798 | 0.0087 |
| BWG, g | 568 | 594 | 10.863 | 0.1338 |
| FI, g | 907 | 904 | 9.343 | 0.7847 |
| FCR | 1.600 | 1.532 | 0.0269 | 0.1117 |
| Hatch–Catch (30 days) | ||||
| BW at catch | 1581 | 1668 | 19.295 | 0.0133 |
| FI, g | 2250 | 2271 | 15.446 | 0.3725 |
| FCR | 1.464 | 1.399 | 0.0112 | 0.0027 |
| Control | LEX 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| Slaughter weight, g | 1696 | 1720 | 17.616 | 0.4240 |
| Carcass weight, g | 1179 | 1226 | 11.825 | 0.0060 |
| Carcass yield, % | 69.54 | 71.48 | 0.672 | 0.0431 |
| Wing, % | 7.12 | 7.33 | 0.058 | 0.0123 |
| Fillet, % | 15.93 | 16.30 | 0.154 | 0.0971 |
| Tender, % | 3.20 | 3.29 | 0.045 | 0.1619 |
| Breast meat, % | 19.13 | 19.59 | 0.177 | 0.0724 |
| Drum, % | 10.35 | 10.27 | 0.087 | 0.5144 |
| Thighs, % | 17.21 | 17.16 | 0.126 | 0.7685 |
| Skin, % | 1.89 | 1.81 | 0.031 | 0.0737 |
| Abdominal fat, % | 1.30 | 1.14 | 0.046 | 0.0121 |
| Gizzard, % | 0.94 | 0.98 | 0.025 | 0.2582 |
| Heart, % | 0.54 | 0.56 | 0.015 | 0.3163 |
| Liver, % | 2.40 | 2.23 | 0.040 | 0.0040 |
| Control | LEX 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| Fat content, g/100 g | 5.50 | 4.96 | 0.241 | 0.1390 |
| Moisture, g/100 g | 73.43 | 74.72 | 0.290 | 0.0078 |
| Control | LEX 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
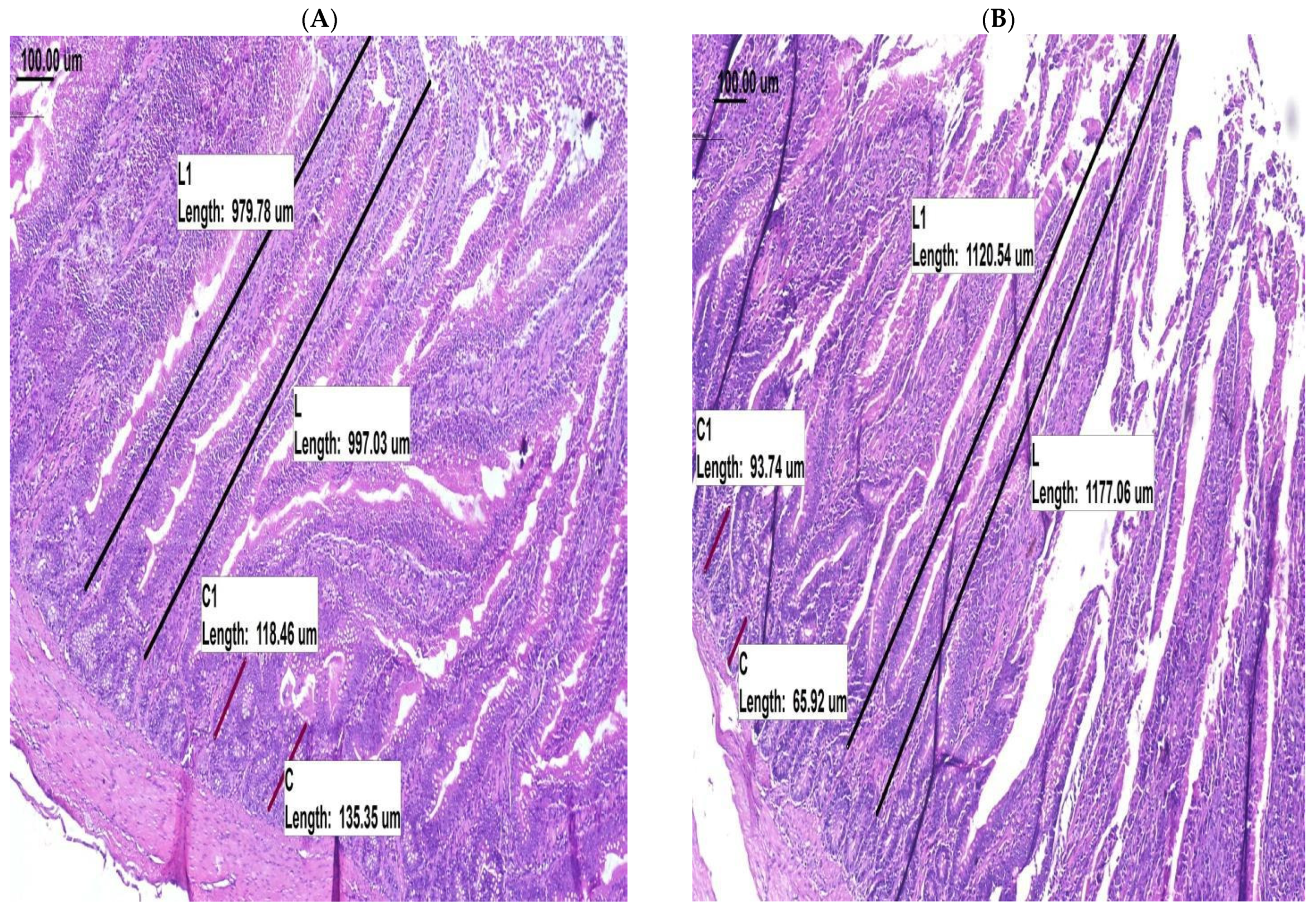
| Villus height, μm | 1015.88 | 1248.73 | 40.049 | 0.0021 |
| Crypt depth, μm | 145.33 | 102.67 | 7.534 | 0.0025 |
| Villus:crypt ratio | 7.06 | 12.45 | 0.701 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazalah, A.; Abd-Elsamee, M.; Ibrahim, M.; Abdelgayed, S.S.; Abdelkader, M.; Gonzalez-Sanchez, D.; Wealleans, A. Effects of a Combination of Lysolecithin, Synthetic Emulsifier, and Monoglycerides on Growth Performance, Intestinal Morphology, and Selected Carcass Traits in Broilers Fed Low-Energy Diets. Animals 2021, 11, 3037. https://doi.org/10.3390/ani11113037
Ghazalah A, Abd-Elsamee M, Ibrahim M, Abdelgayed SS, Abdelkader M, Gonzalez-Sanchez D, Wealleans A. Effects of a Combination of Lysolecithin, Synthetic Emulsifier, and Monoglycerides on Growth Performance, Intestinal Morphology, and Selected Carcass Traits in Broilers Fed Low-Energy Diets. Animals. 2021; 11(11):3037. https://doi.org/10.3390/ani11113037
Chicago/Turabian StyleGhazalah, Abdallah, Mamdouh Abd-Elsamee, Moataz Ibrahim, Sherein S. Abdelgayed, Mohamed Abdelkader, David Gonzalez-Sanchez, and Alexandra Wealleans. 2021. "Effects of a Combination of Lysolecithin, Synthetic Emulsifier, and Monoglycerides on Growth Performance, Intestinal Morphology, and Selected Carcass Traits in Broilers Fed Low-Energy Diets" Animals 11, no. 11: 3037. https://doi.org/10.3390/ani11113037
APA StyleGhazalah, A., Abd-Elsamee, M., Ibrahim, M., Abdelgayed, S. S., Abdelkader, M., Gonzalez-Sanchez, D., & Wealleans, A. (2021). Effects of a Combination of Lysolecithin, Synthetic Emulsifier, and Monoglycerides on Growth Performance, Intestinal Morphology, and Selected Carcass Traits in Broilers Fed Low-Energy Diets. Animals, 11(11), 3037. https://doi.org/10.3390/ani11113037







