Investigating Genetic and Phenotypic Variability of Queen Bees: Morphological and Reproductive Traits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sampling and Transport
2.2. Freeze Immobilization
2.3. Morphological Measures
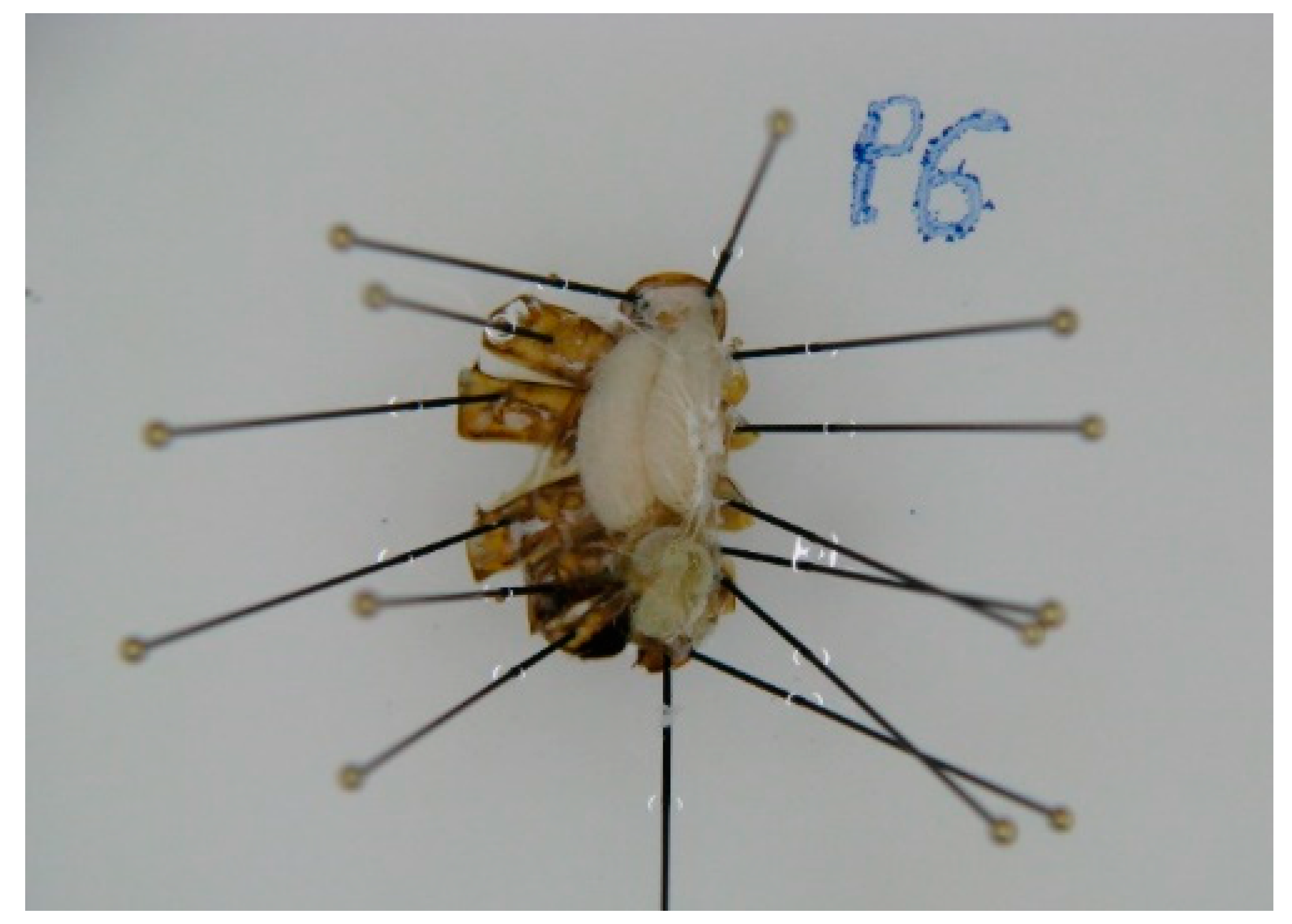
2.4. Abdomen Dissection
2.5. Spermatheca Extraction and Analysis
2.6. Ovarioles Count
2.7. Statistical Analysis
3. Results and Discussion
3.1. Defects and Descriptive Statistics
3.2. Heritabilities, Genetic and Phenotypic Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, UK, 1971. [Google Scholar]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, UK, 1987. [Google Scholar]
- Hoover, S.E.; Keeling, C.I.; Winston, M.L.; Slessor, K.N. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 2003, 90, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, T. Brood pheromone regulates foraging activity of honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, T. Cued in: Honey bee pheromones as information flow and colony decision-making. Apidologie 2004, 35, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Gary, N.E. Honey productivity of honey bee colonies in relation to body weight, attractiveness and fecundity of the queen. J. Apic. Res. 1983, 22, 209–213. [Google Scholar] [CrossRef]
- Tarpy, D.R. Genetic diversity within honey bee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Tarpy, D.R.; vanEngelsdorp, D.; Pettis, J.S. Genetic diversity affects colony survivorship in commercial honey bee colonies. Naturwissenschaften 2013, 100, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Rangel, J.; Keller, J.J.; Tarpy, D.R. The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insectes Sociaux 2013, 60, 65–73. [Google Scholar] [CrossRef]
- Kaftanoglu, O.; Duzenli, A.; Kumova, U. A study on determination the effects of queen rearing season on queen quality under Cukurova region conditions. Turk. J. Vet. Anim. Sci. 1988, 16, 567–577. [Google Scholar]
- Laidlaw, H.H., Jr. Contemporary Queen Rearing; Dadant & Sons Inc.: Hamilton, ON, Canada, 1992. [Google Scholar]
- Bieńkowska, M.; Panasiuk, B.; Węgrzynowicz, P.; Gerula, D. The effect of different thermal conditions on drone semen quality and number of spermatozoa entering the spermatheca of queen bee. J. Apic. Sci. 2011, 55, 161–168. [Google Scholar]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Vilde, J. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Buchler, R.; Berg, S.; Ritter, W.; Muhlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef] [Green Version]
- Brodschneider, R.; Moosbeckhofer, R.; Crailsheim, K. Surveys as a tool to record winter losses of honey bee colonies: A two-year case study in Austria and South Tyrol. J. Apic. Res. 2010, 49, 23–30. [Google Scholar] [CrossRef]
- Spleen, A.M.; Lengerich, E.J.; Rennich, K.; Caron, D.; Rose, R.; Pettis, J.S.; Henson, M.; Wilkes, J.T.; Wilson, M.; Stitzinger, J.; et al. A national survey of managed honey bee 2011–2012 winter colony losses in the United States: Results from the Bee Informed Partnership. J. Apic. Res. 2013, 52, 44–53. [Google Scholar] [CrossRef] [Green Version]
- vanEngelsdorp, D.; Tarpy, D.R.; Lengerich, E.J.; Pettis, J.S. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 2013, 108, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- vanEngelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A Survey of honey bee colony losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef] [PubMed]
- Hoopingarner, R.; Farrar, C. Genetic control of size in queen honey bees. J. Econ. Entomol. 1959, 52, 547–548. [Google Scholar] [CrossRef]
- Dodologlu, A.; Gene, F. Comparison of some features of queens reared from different honey bee (Apis mellifera L.) genotypes. J. Appl. Anim. Res. 2003, 24, 105–109. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Goodman, R.D.; Allaway, M.A. On the relative importance of queens and workers to honey production. Apidologie 1990, 21, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kahya, Y.; Gençer, H.V.; Woyke, J. Weight at emergence of honey bee (Apis mellifera caucasica) queens and its effect on live weights at the pre and post mating periods. J. Apic. Res. 2008, 47, 118–125. [Google Scholar] [CrossRef]
- Delaney, D.A.; Keller, J.J.; Caren, J.R.; Tarpy, D.R. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 2011, 42, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hatjina, F.; Bienkowska, M.; Charistos, L.; Chlebo, R.; Costa, C.; Drazic, M.; Filipi, J.; Gregorc, A.; Ivanova, E.N.; Kezić, N.; et al. A review of methods used in some European countries for assessing the quality of honey bee queens through their physical characters and the performance of their colonies. J. Apic. Res. 2014, 53, 337–363. [Google Scholar] [CrossRef] [Green Version]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Assessing the mating ‘health’ of commercial honey bee queens. J. Econ. Entomol. 2012, 105, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Woyke, J. Correlations between the age at which honey bee brood was grafted, characteristics of the resultant queens, and results of insemination. J. Apic. Res. 1971, 10, 45–55. [Google Scholar] [CrossRef]
- Collins, A.M.; Pettis, J.S. Correlation of queen size and spermathecal contents and effects of miticide exposure during development. Apidologie 2013, 44, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Szabo, T.I.; Mills, P.F.; Heikel, D.T. Effects of honey bee queen weight and air temperature on the initiation of oviposition. J. Apic. Res. 1987, 26, 73–78. [Google Scholar]
- Szabo, T.I. Relationship between weight of honey-bee queens (Apis mellifera L.) at emergence and at the cessation of egg laying. Amer Bee J. 1973, 13, 127–135. [Google Scholar]
- Yadava, R.P.S.; Smith, M.V. Aggressive behavior of Apis mellifera L. workers towards introduced queens. III. Relationship between the attractiveness of the queen and worker aggression. Can. J. Zool. 1971, 4, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Guerra, J.C.V.; Kalvelage, H.; Espindola, E. Maternal influence on the acceptance of virgin queens introduced into Africanized honey bee (Apis mellifera) colonies. Genet. Mol. Res. 2004, 3, 441–445. [Google Scholar] [PubMed]
- Szabo, T.I.; Townsend, G.F. Behavioural studies on queen introduction in the honey bee 1. effect of the age of workers (from a colony with a laying queen) on their behaviour towards an introduced virgin queen. J. Apic. Res. 1974, 13, 19–25. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insectes Sociaux 2011, 58, 569–574. [Google Scholar] [CrossRef]
- Amiri, E.; Strand, M.K.; Rueppel, O.; Tarpy, D.R. Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects 2017, 8, 48. [Google Scholar] [CrossRef]
- Jackson, T.J.; Tarpy, R.D.; Fahrbach, E.S. Histological estimates of ovariole number in honey bee queens, Apis mellifera, reveal lack of correlation with other queen quality measures. J. Insect Sci. 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; van Englesdorp, D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Richard, F.J.; Tarpy, D.R.; Grozinger, C.M. Effects of insemination quantity on honey bee queen physiology. PLoS ONE 2007, 2, e980. [Google Scholar] [CrossRef] [PubMed]
- Kocher, S.D.; Richard, F.J.; Tarpy, D.R.; Grozinger, C.M. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera). BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Niño, E.L.; Tarpy, D.R.; Grozinger, C.M. Differential effects of insemination volume and substance on reproductive changes in honey bee queens (Apis mellifera L.). Insect Mol. Biol. 2013, 22, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Škerl, M.I.S. Characteristics of honey bee (Apis Mellifera Carnica Pollman, 1879) queens reared in Slovenian commercial breeding stations. J. Apic. Sci. 2015, 59, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Gilley, D.C.; Tarpy, D.R.; Land, B.B. Effect of queen quality on interactions between workers and dueling queens in honey bee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2003, 55, 190–196. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Hatch, S.; Fletcher, D.J.C. The influence of queen age and quality during queen replacement in honey bee colonies. Anim. Behav. 2000, 59, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedej, S.; Hartfelder, K.; Aumeier, P.; Rosenkranz, P.; Engels, W. Caste determination is a sequential process: Effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica). J. Apic. Res. 1998, 37, 183–190. [Google Scholar] [CrossRef]
- Hatch, S.; Tarpy, R.D.; Fletcher, C.D.J. Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality. Insect Sociaux 1999, 46, 372–377. [Google Scholar] [CrossRef]
- Porporato, M.; Grillone, G.; Patetta, A.; Manino, A.; Laurino, D. Survey of the health status of some honey bee queens in Italy. J. Apic. Sci. 2015, 59, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years 361 of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.-L.; Jaffe, R.; et al. Miscellaneous standard methods for Apis mellifera research. J. Apic. Res. 2013, 52, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Brascamp, E.W.; Bijma, P. Methods to estimate breeding values in honey bees. Genet. Sel. Evol. 2014, 46, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brascamp, E.W.; Willam, A.; Boigenzahn, C.; Bijma, P.; Veerkamp, R.F. Heritabilities and genetic correlations for honey yield, gentleness, calmness and swarming behaviour in Austrian honey bees. Apidologie 2016, 47, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Butler, D. Asreml: Asreml Fits the Linear Mixed Model. R Package Version 3.0. 2009. Available online: www.vsni.co.uk (accessed on 25 January 2017).
- Wolak, M.E. Nadiv: An R package to create relatedness matrices for estimating non-additive genetic variances in animal models. Methods Ecol. Evol. 2012, 3, 792–796. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Mrode, A.R. Linear Models for the Prediction of Animal Breeding Values, 3rd ed.; CABI: Wallingford, UK, 2014; pp. 34–56. [Google Scholar]
- Es’kov, E.K.; Es’kova, M.D. Factors influencing wing size and body weight variation in the Western honey bee. Russ. J. Ecol. 2013, 44, 433–438. [Google Scholar] [CrossRef]
- Corbella, E.; Gonçalves, L.S. Relationship between weight at emergence, number of ovarioles, and spermathecal volume of africanized honey bees queens Apis mellifera L. Br. J. Genet. 1982, 5, 835–840. [Google Scholar]

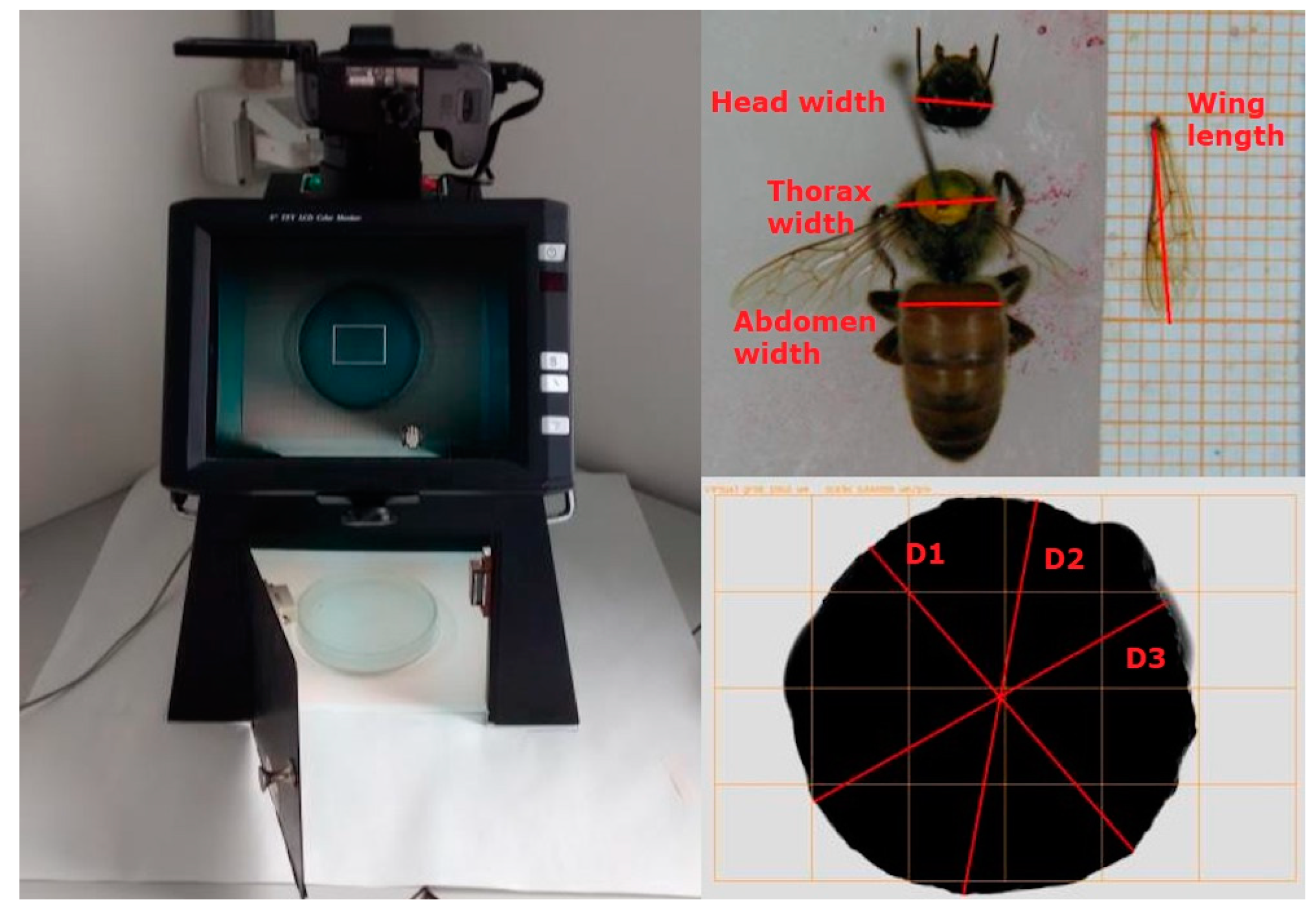

| Trait | Abbreviation | Unit | N | Mean | SD | CV (%) |
|---|---|---|---|---|---|---|
| Body weight | bw | mg | 147 | 195.90 | 19.84 | 10.1 |
| Head weight | hw | mg | 147 | 13.22 | 1.83 | 13.8 |
| Thorax weight | tw | mg | 147 | 77.93 | 8.17 | 10.5 |
| Abdomen weight | aw | mg | 147 | 104.76 | 15.28 | 14.6 |
| Head width | hwi | mm | 147 | 3.67 | 0.18 | 4.9 |
| Thorax width | twi | mm | 147 | 4.67 | 0.26 | 5.6 |
| Abdomen width | awi | mm | 147 | 4.80 | 0.21 | 4.4 |
| Wing length | wl | mm | 147 | 10.07 | 0.62 | 6.2 |
| Spermatheca diameter | sd | mm | 147 | 1.32 | 0.18 | 13.6 |
| Spermatheca volume | sv | µl | 147 | 1.27 | 0.53 | 41.7 |
| Number of Ovarioles | o | n | 147 | 141 | 25 | 17.9 |
| Number of Sperms | sp | n million | 143 | 3.6 | 2.9 | 82.8 |
| Trait | Body Weight | Head Weight | Thorax Weight | Abdomen Weight | Head Width | Thorax Width | Abdomen Width | Wing Length | Diameter Spermatheca | Volume Spermatheca | Ovarioles Number | Sperm Count |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight | 0.54 (0.34) | 0.80 (0.31) | 0.92 (0.30) | 0.84 (0.17) | 0.47 (0.50) | 0.34 (0.50) | −0.22 (1.10) | 0.17 (0.62) | 0.23 (0.76) | 0.40 (0.40) | −0.13 (0.50) | −0.13 (0.52) |
| Head weight | 0.39 (0.10) | 0.51 (0.35) | 0.99 (0.36) | 0.61 (0.45) | 0.97 (0.46) | 0.34 (0.53) | −0.56 (1.05) | 0.92 (0.71) | −0.44 (0.68) | −0.44 (0.50) | 0.28 (0.50) | 0.23 (0.57) |
| Thorax weight | 0.67 (0.06) | 0.19 (0.11) | 0.50 (0.39) | 0.98 (0.69) | −0.01 (0.79) | 0.83 (0.31) | 0.54 (0.75) | 0.74 (0.38) | 0.78 (0.39) | 0.61 (0.41) | −0.18 (0.53) | 0.15 (0.58) |
| Abdomen weight | 0.88 (0.03) | 0.31 (0.10) | 0.29 (0.10) | 0.46 (0.34) | 0.47 (0.54) | −0.14 (0.68) | −0.60 (1.19) | −0.45 (0.71) | −0.24 (1.05) | 0.06 (0.54) | −0.15 (0.53) | −0.3 (0.52) |
| Head width | 0.36 (0.09) | 0.31 (0.10) | 0.28 (0.10) | 0.27 (0.10) | 0.26 (0.27) | 0.21 (0.67) | 0.15 (1.08) | 0.96 (0.48) | 0.89 (0.48) | 0.7 (0.52) | 0.55 (0.58) | 0.63 (0.56) |
| Thorax width | 0.34 (0.10) | 0.30 (0.10) | 0.28 (0.11) | 0.26 (0.10) | 0.32 (0.09) | 0.42 (0.32) | 0.74 (0.36) | 0.98 (0.31) | 0.59 (0.57) | 0.44 (0.43) | 0.02 (0.53) | −0.17 (0.56) |
| Abdomen width | 0.39 (0.09) | 0.17 (0.10) | 0.26 (0.10) | 0.33 (0.10) | 0.25 (0.09) | 0.80 (0.04) | 0.13 (0.26) | 0.33 (0.90) | −0.22 (2.07) | 0.44 (0.65) | −0.59 (0.89) | 0.26 (0.96) |
| Wing length | 0.34 (0.10) | 0.16 (0.10) | 0.36 (0.10) | 0.20 (0.11) | 0.26 (0.10) | 0.39 (0.09) | 0.30 (0.09) | 0.30 (0.29) | 0.43 (0.81) | 0.40 (0.50) | 0.79 (0.59) | 0.57 (0.52) |
| Diameter spermatheca | 0.21 (0.10) | 0.04 (0.12) | 0.18 (0.12) | 0.20 (0.10) | 0.08 (0.12) | 0.15 (0.11) | 0.13 (0.10) | 0.10 (0.10) | 0.17 (0.34) | 0.99 (0.02) | −0.42 (0.64) | −0.96 (0.72) |
| Volume spermatheca | 0.22 (0.13) | 0.03 (0.13) | 0.11 (0.12) | 0.18 (0.13) | 0.02 (0.12) | 0.16 (0.13) | 0.16 (0.12) | 0.09 (0.12) | 0.97 (0.01) | 0.88 (0.39) | −0.31 (0.42) | −0.70 (0.56) |
| Ovarioles number | 0.01 (0.12) | 0.15 (0.12) | −0.003 (0.12) | −0.02 (0.12) | 0.08 (0.11) | 0.04 (0.12) | 0.03 (0.12) | 0.02 (0.12) | −0.01 (0.12) | −0.05 (0.13) | 0.70 (0.35) | 0.08 (0.48) |
| Sperm count | 0.03 (0.12) | 0.03 (0.12) | 0.09 (0.12) | −0.01 (0.12) | 0.10 (0.11) | 0.04 (0.12) | 0.08 (0.11) | 0.17 (0.11) | −0.07 (0.12) | −0.07 (0.13) | 0.05 (0.12) | 0.57 (0.35) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchini, E.; De Iorio, M.G.; Turri, F.; Pizzi, F.; Laurino, D.; Porporato, M.; Rizzi, R.; Pagnacco, G. Investigating Genetic and Phenotypic Variability of Queen Bees: Morphological and Reproductive Traits. Animals 2021, 11, 3054. https://doi.org/10.3390/ani11113054
Facchini E, De Iorio MG, Turri F, Pizzi F, Laurino D, Porporato M, Rizzi R, Pagnacco G. Investigating Genetic and Phenotypic Variability of Queen Bees: Morphological and Reproductive Traits. Animals. 2021; 11(11):3054. https://doi.org/10.3390/ani11113054
Chicago/Turabian StyleFacchini, Elena, Maria Grazia De Iorio, Federica Turri, Flavia Pizzi, Daniela Laurino, Marco Porporato, Rita Rizzi, and Giulio Pagnacco. 2021. "Investigating Genetic and Phenotypic Variability of Queen Bees: Morphological and Reproductive Traits" Animals 11, no. 11: 3054. https://doi.org/10.3390/ani11113054
APA StyleFacchini, E., De Iorio, M. G., Turri, F., Pizzi, F., Laurino, D., Porporato, M., Rizzi, R., & Pagnacco, G. (2021). Investigating Genetic and Phenotypic Variability of Queen Bees: Morphological and Reproductive Traits. Animals, 11(11), 3054. https://doi.org/10.3390/ani11113054








