Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Persons Performing the Heart Ultrasound Examination
2.2. Ultrasound Device
2.3. Animals
2.3.1. Case No. 1
2.3.2. Case No. 2
2.3.3. Case No. 3
2.3.4. Case No. 4
2.3.5. Case No. 5
2.3.6. Case No. 6
2.3.7. Case No. 7
3. Results
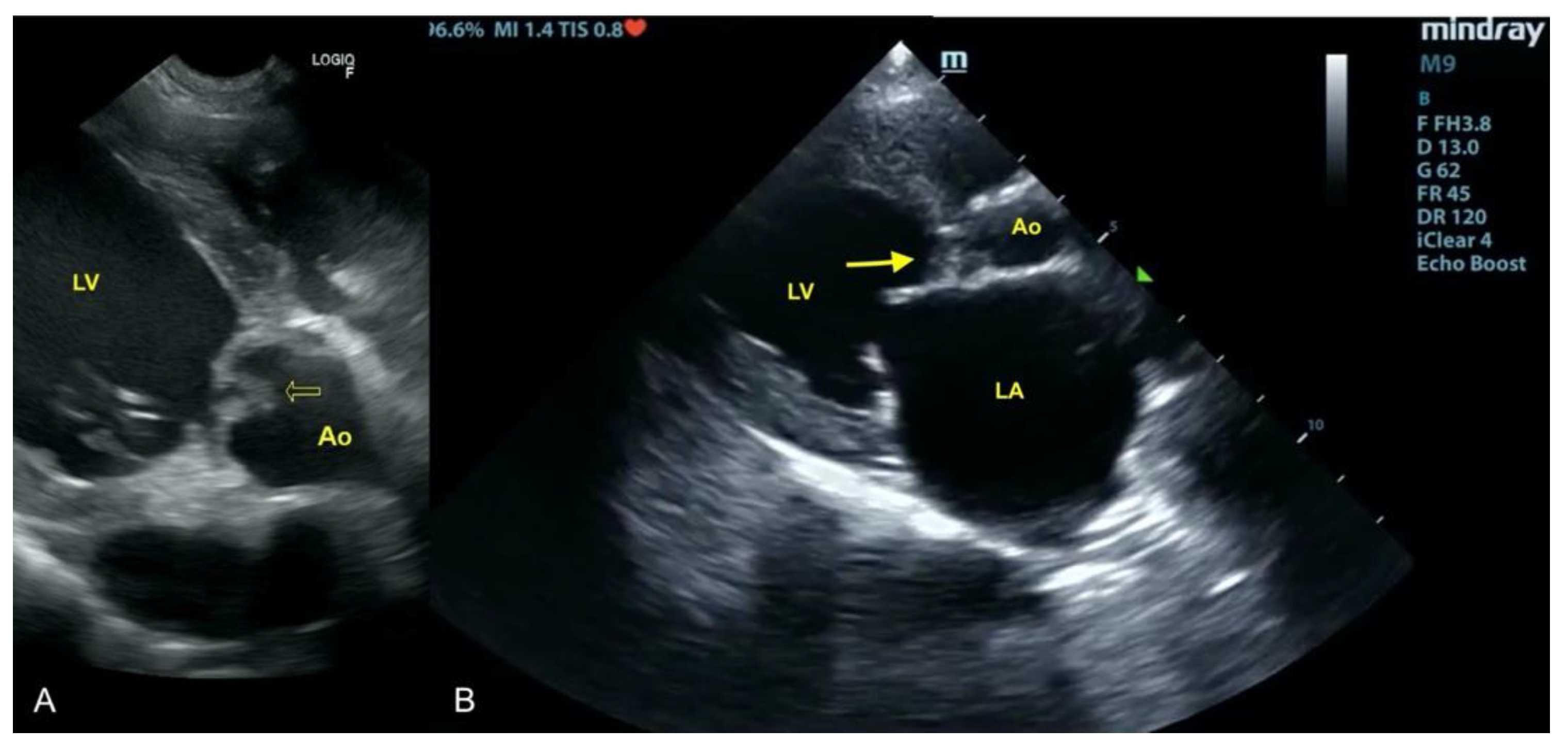
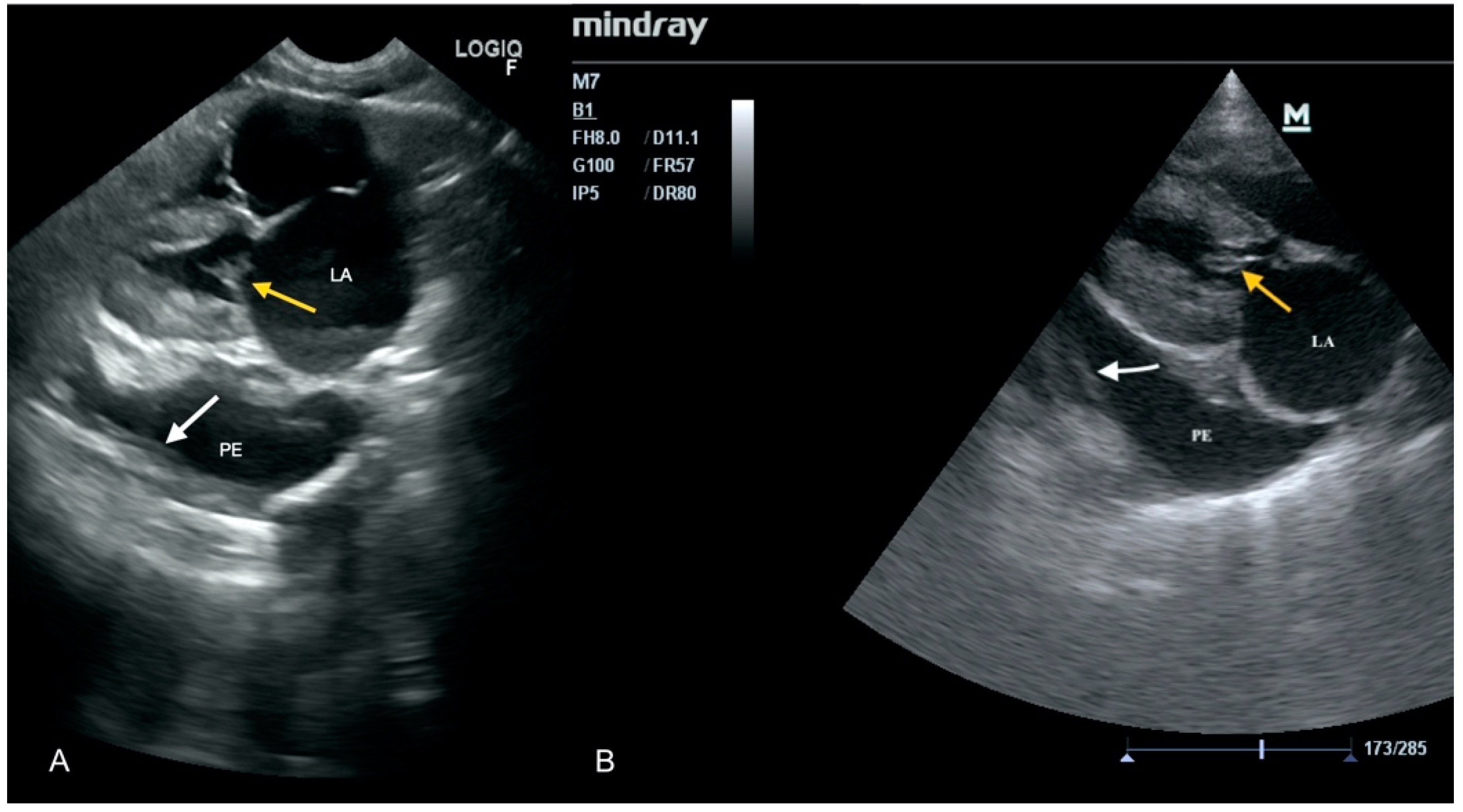
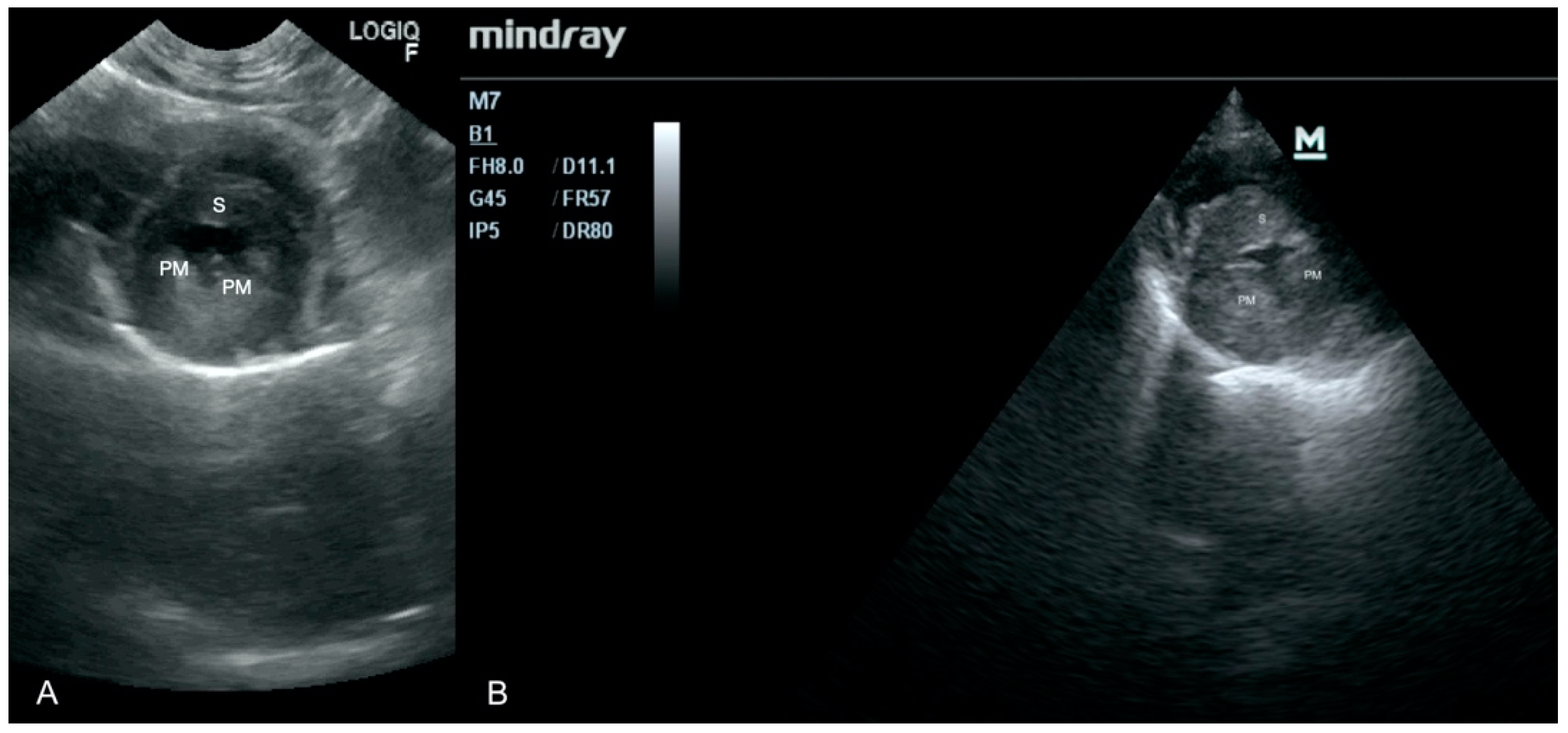
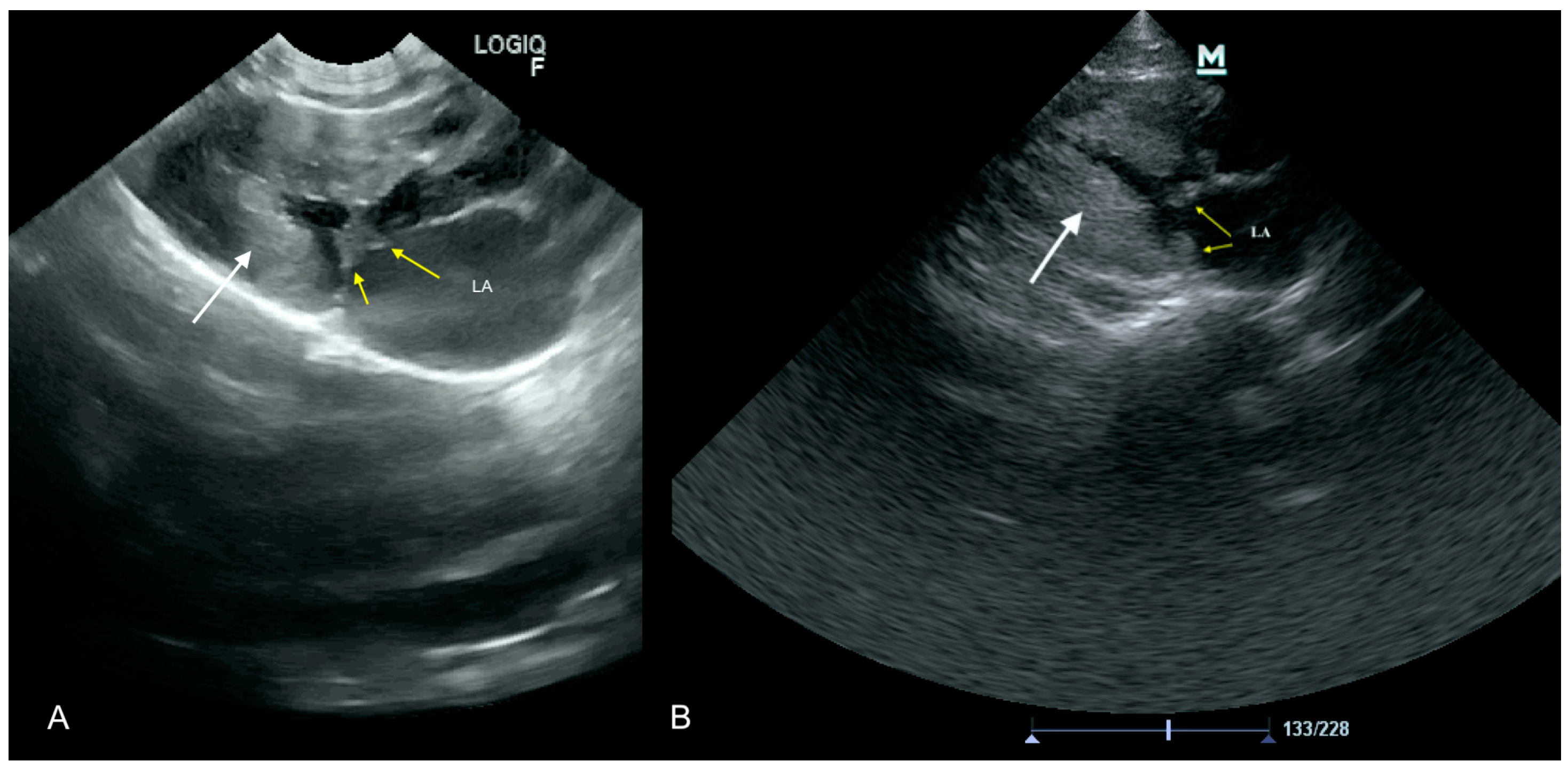
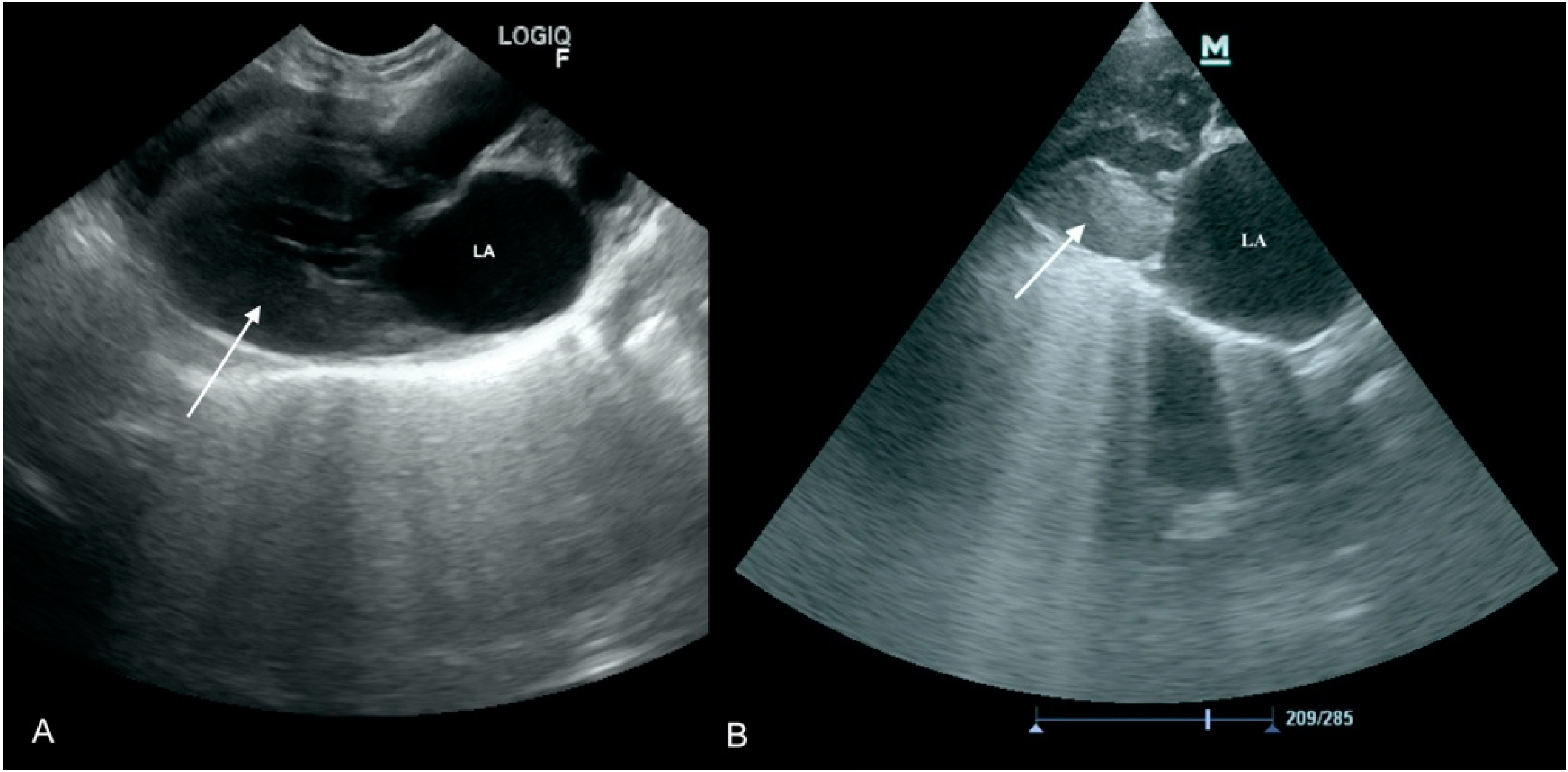
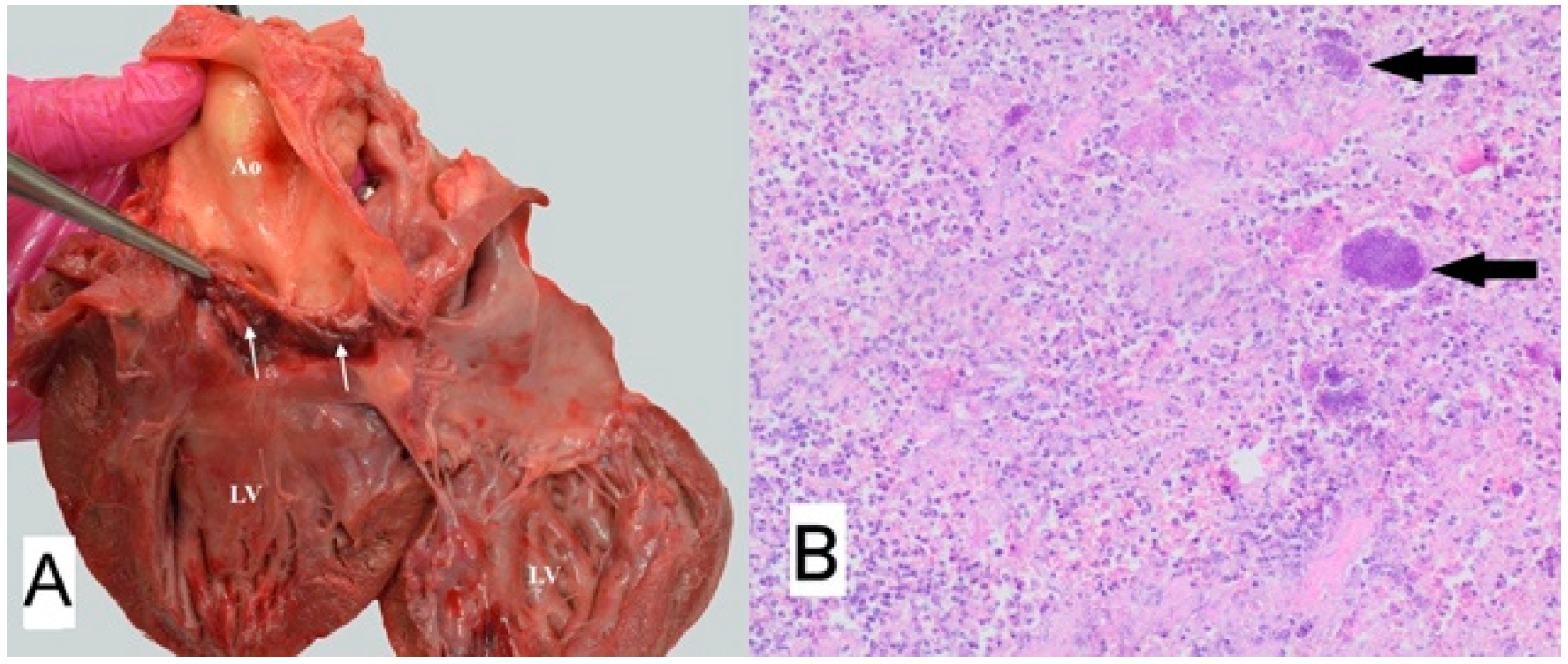
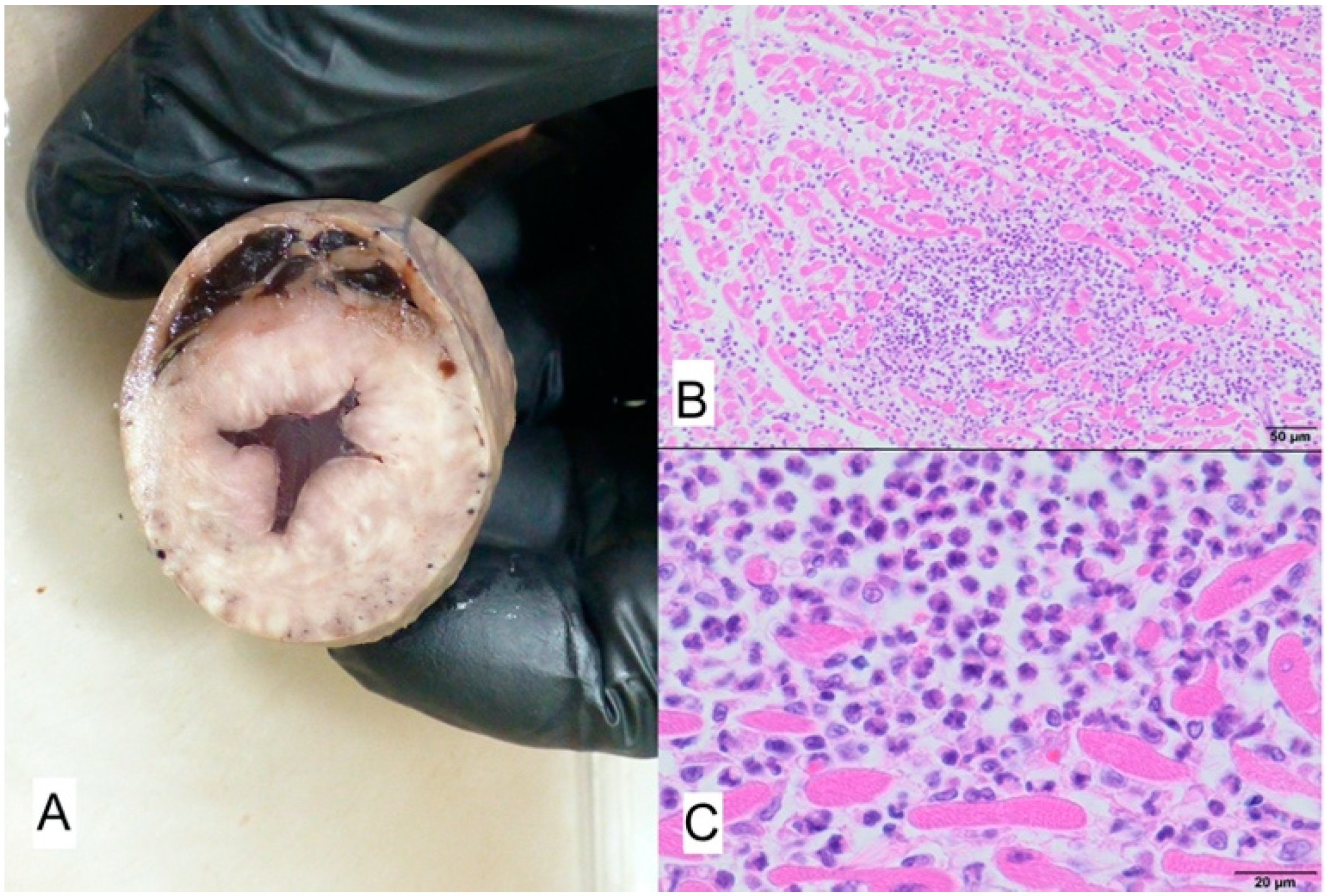
3.1. Case No. 1
3.2. Case No. 2
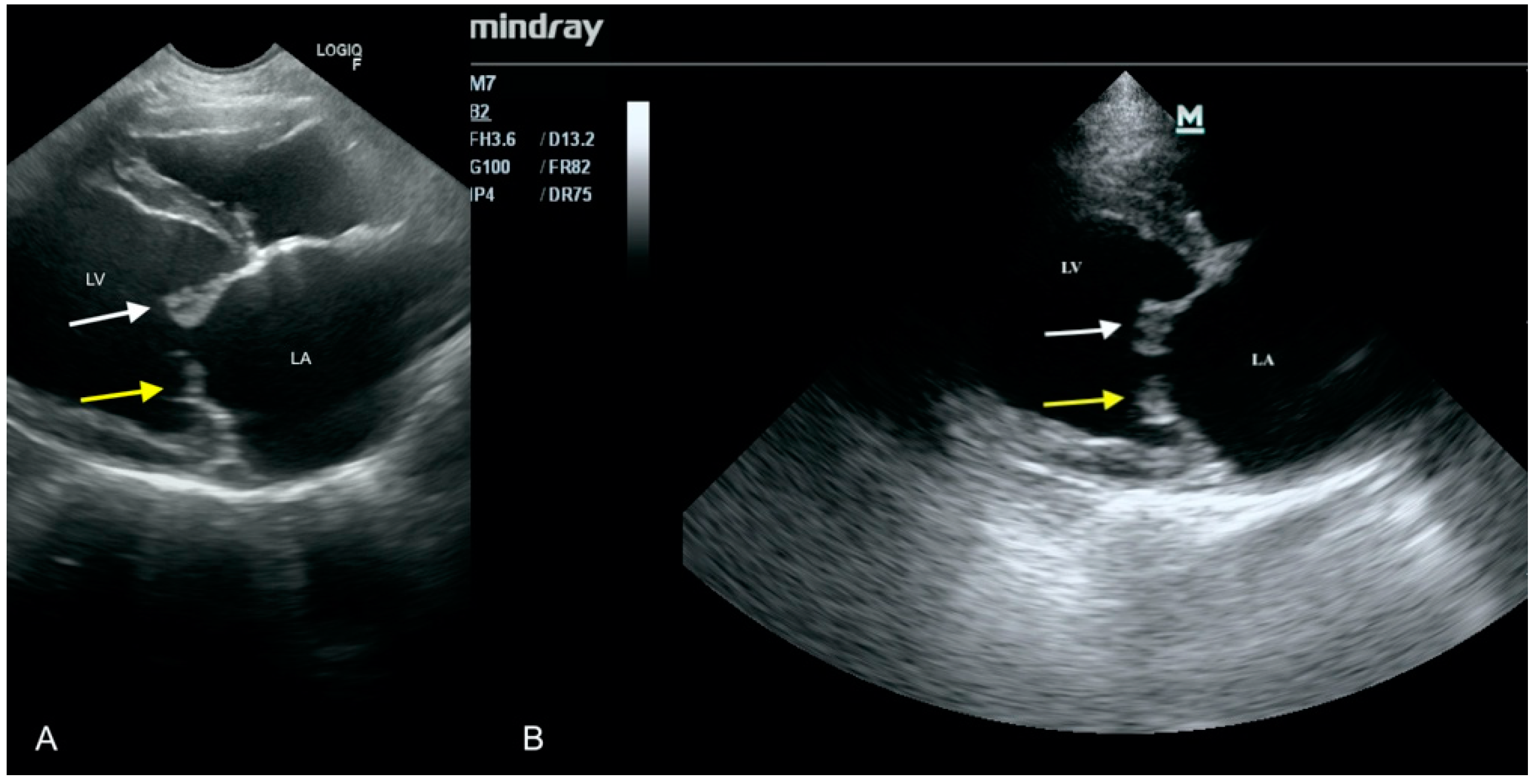
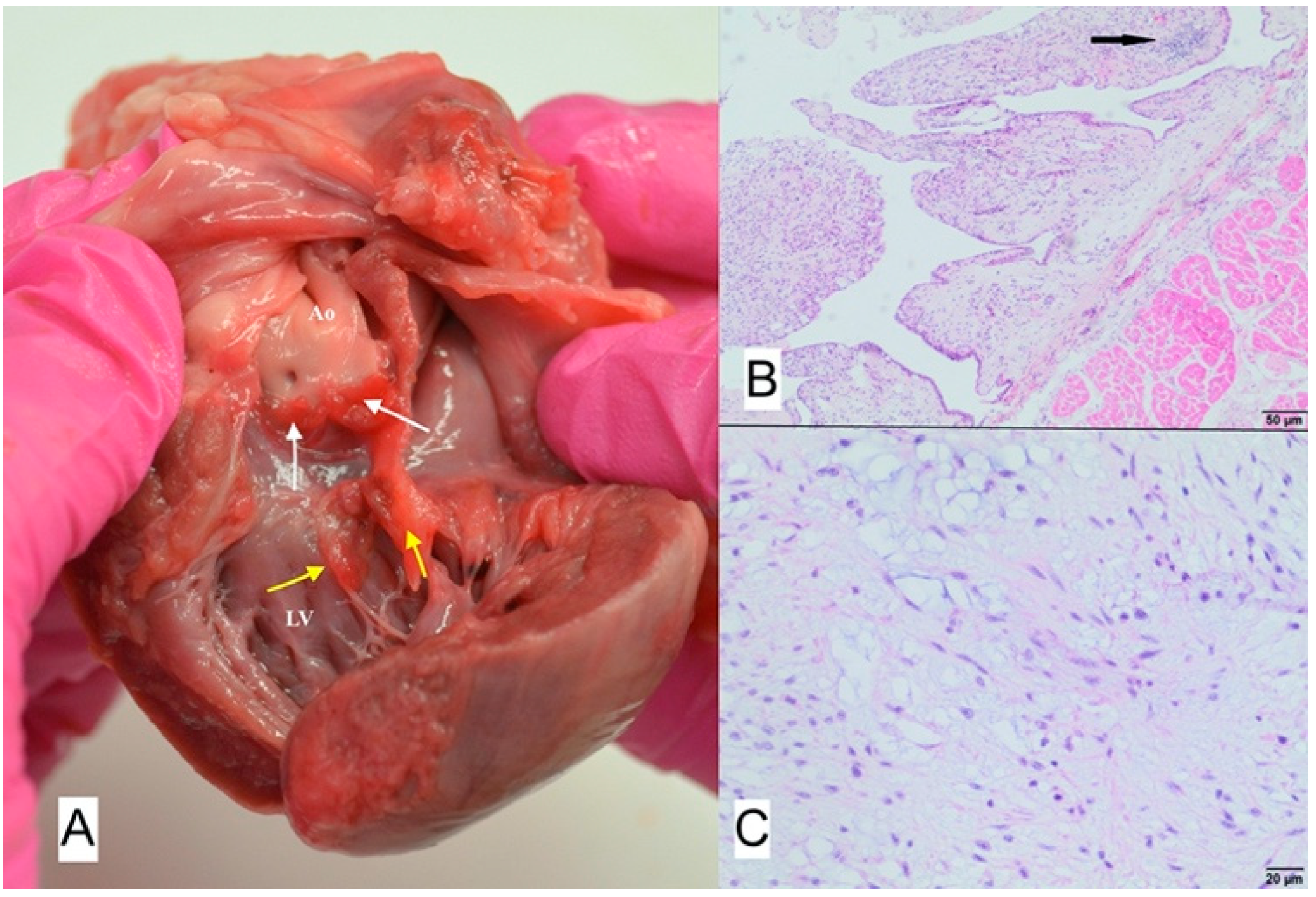
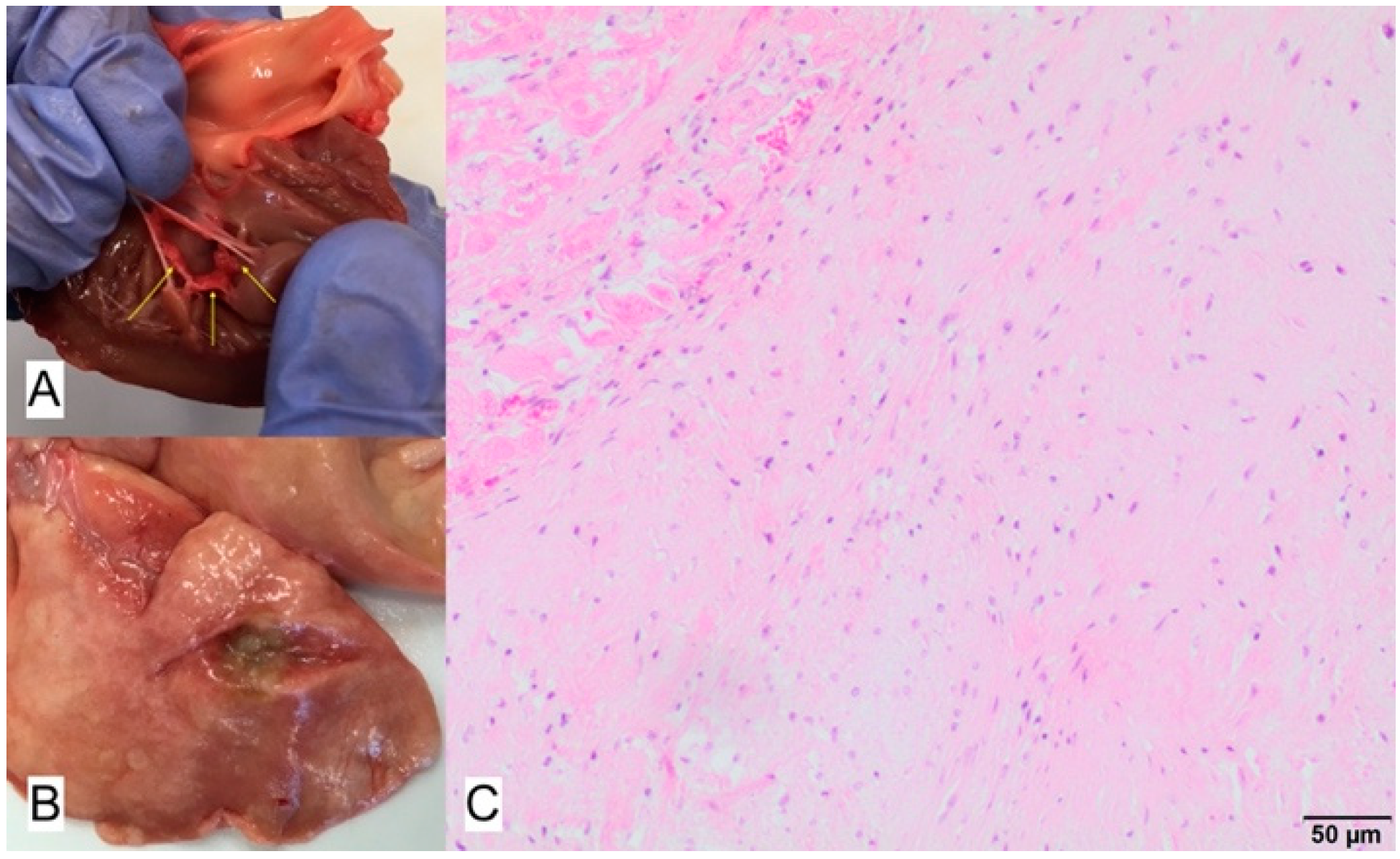
3.3. Case No. 3
3.4. Case No. 4
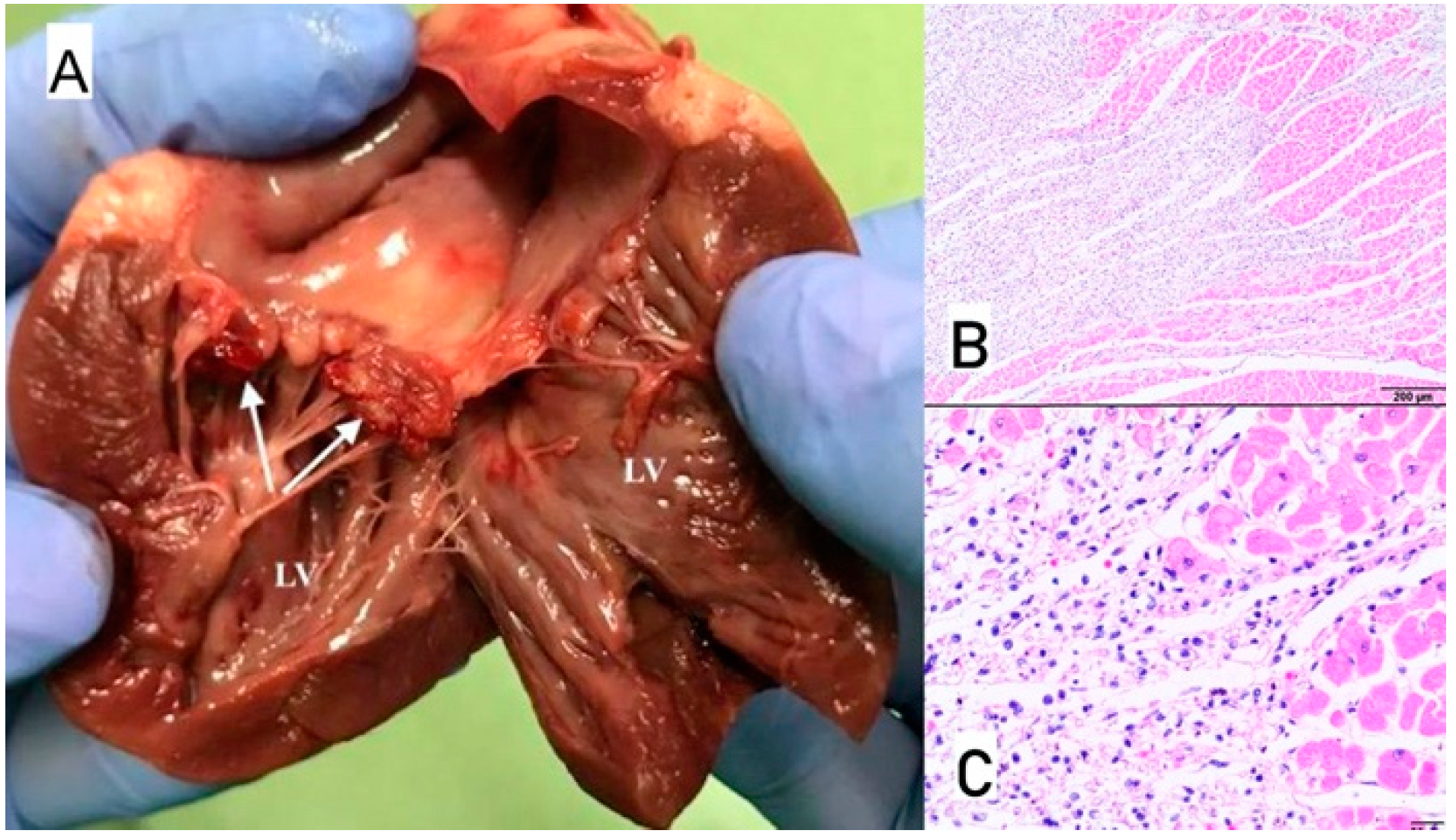
3.5. Case No. 5
3.6. Case No. 6
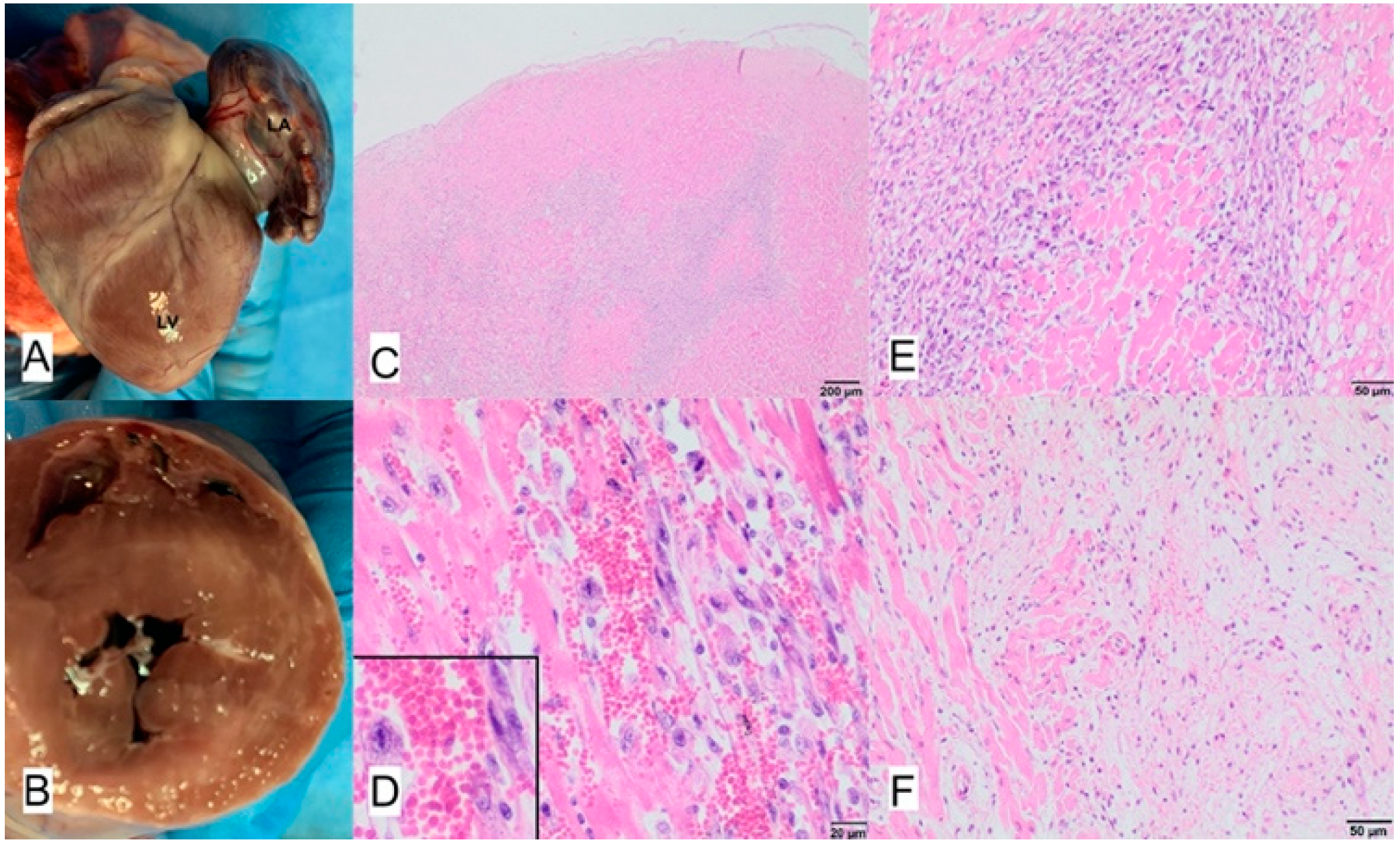
3.7. Case No. 7
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zachary, J.F. Pathologic Basis of Veterinary Disease, 6th ed.; Elsevier: St. Louis, MO, USA, 2017; ISBN 978-0-323-35775-3. [Google Scholar]
- Balakrishnan, N.; Alexander, K.; Keene, B.; Kolluru, S.; Fauls, M.L.; Rawdon, I.; Breitschwerdt, E.B. Successful Treatment of Mitral Valve Endocarditis in a Dog Associated with ‘Actinomyces Canis -like’ Infection. J. Vet. Cardiol. 2016, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.D.; Kienle, R.D. Small Animal Cardiovascular Medicine; Mosby: St. Louis, MO, USA, 1998; ISBN 978-0-8151-5140-1. [Google Scholar]
- Aoki, T.; Sunahara, H.; Sugimoto, K.; Ito, T.; Kanai, E.; Fujii, Y. Infective Endocarditis of the Aortic Valve in a Border Collie Dog with Patent Ductus Arteriosus. J. Vet. Med. Sci. 2015, 77, 331–336. [Google Scholar] [CrossRef]
- Felker, G.M.; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Baughman, K.L.; Hare, J.M. Echocardiographic Findings in Fulminant and Acute Myocarditis. J. Am. Coll. Cardiol. 2000, 36, 227–232. [Google Scholar] [CrossRef]
- Coffin, S.T.; Benton, S.M.; Lenihan, D.J.; Naftilan, A.J.; Mendes, L.A.; Lenihan, D.J. Eosinophilic Myocarditis—An Unusual Cause of Left Ventricular Hypertrophy. Am. J. Med. Sci. 2015, 349, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Sagrera, M.R.; Sala, M.F.; Barcena, J.P.; Garcia, J.B.; Canellas, C.F.; Juve, J.I. Acute Myocarditis and Left Ventricular “Hypertrophy”. Echocardiography 2000, 17, 567–570. [Google Scholar] [CrossRef]
- Lai, W.W.; Mertens, L.; Cohen, M.; Geva, T. (Eds.) Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult, 2nd ed.; John Wiley & Sons Ltd.: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2015; ISBN 978-1-118-74221-1. [Google Scholar]
- Janus, I.; Noszczyk-Nowak, A.; Nowak, M.; Cepiel, A.; Ciaputa, R.; Pasławska, U.; Dzięgiel, P.; Jabłońska, K. Myocarditis in Dogs: Etiology, Clinical and Histopathological Features (11 Cases: 2007–2013). Ir. Vet. J. 2014, 67, 28. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Kittleson, M.D.; Chomel, B.B.; MacDonald, K.A.; Pesavento, P.A. Clinicopathologic Findings and Outcome in Dogs with Infective Endocarditis: 71 Cases (1992–2005). J. Am. Vet. Med. Assoc. 2006, 228, 1735–1747. [Google Scholar] [CrossRef]
- Szatmári, V. Incidence of Postoperative Implant-Related Bacterial Endocarditis in Dogs That Underwent Trans-Catheter Embolization of a Patent Ductus Arteriosus without Intra- and Post-Procedural Prophylactic Antibiotics. Vet. Microbiol. 2017, 207, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.-D.; Altet, L.; Maggi, R.G.; Altimira, J.; Roura, X. First Description of Bartonella Koehlerae Infection in a Spanish Dog with Infective Endocarditis. Parasites Vectors 2017, 10, 247. [Google Scholar] [CrossRef]
- Roura, X.; Santamarina, G.; Tabar, M.-D.; Francino, O.; Altet, L. Polymerase Chain Reaction Detection of Bartonella Spp. in Dogs from Spain with Blood Culture-Negative Infectious Endocarditis. J. Vet. Cardiol. 2018, 20, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Vicent, L.; Saldivar, H.G.; Bouza, E.; Muñoz, P.; Cuerpo, G.; de Alarcón, A.; Vidal, B.; Cobo, M.; Goenaga, M.Á.; Carrasco-Chinchilla, F.; et al. Prognostic Implications of a Negative Echocardiography in Patients with Infective Endocarditis. Eur. J. Intern. Med. 2018, 52, 40–48. [Google Scholar] [CrossRef]
- Brandão, T.J.D.; Januario-da-Silva, C.A.; Correia, M.G.; Zappa, M.; Abrantes, J.A.; Dantas, A.M.R.; Golebiovski, W.; Barbosa, G.I.F.; Weksler, C.; Lamas, C.C. Histopathology of Valves in Infective Endocarditis, Diagnostic Criteria and Treatment Considerations. Infection 2017, 45, 199–207. [Google Scholar] [CrossRef]
- MacDonald, K.A.; Chomel, B.B.; Kittleson, M.D.; Kasten, R.W.; Thomas, W.P.; Pesavento, P. A Prospective Study of Canine Infective Endocarditis in Northern California (1999–2001): Emergence of Bartonella as a Prevalent Etiologic Agent. J. Vet. Intern. Med. 2004, 18, 56–64. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Imaging the Itis: Endocarditis, Myocarditis, and Pericarditis. Curr. Opin. Cardiol. 2019, 34, 57–64. [Google Scholar] [CrossRef]
- Von Reyn, C.F. Infective Endocarditis: An Analysis Based on Strict Case Definitions. Ann. Intern. Med. 1981, 94, 505. [Google Scholar] [CrossRef] [PubMed]
- Seif, D.; Meeks, A.; Mailhot, T.; Perera, P. Emergency Department Diagnosis of Infective Endocarditis Using Bedside Emergency Ultrasound. Cr. Ultrasound J. 2013, 5, 1. [Google Scholar] [CrossRef]
- Pathak, N.J.; Ng, L.; Saul, T.; Lewiss, R.E. Focused Cardiac Ultrasound Diagnosis of Right-Sided Endocarditis. Am. J. Emerg. Med. 2013, 31, 998.e3–998.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, B.; Zhou, J.; Kirkpatrick, J.; Xie, M.; Johri, A.M. Bedside Focused Cardiac Ultrasound in COVID-19 from the Wuhan Epicenter: The Role of Cardiac Point-of-Care Ultrasound, Limited Transthoracic Echocardiography, and Critical Care Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Blaivas, M. Unexpected Finding of Myocardial Depression in 2 Healthy Young Patients with COVID-19 Pneumonia: Possible Support for COVID-19-related Myocarditis. J. Am. Coll. Emerg. Physicians Open 2020, 1, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Murchan, S.; Kaufmann, M.E.; Deplano, A.; de Ryck, R.; Struelens, M.; Zinn, C.E.; Fussing, V.; Salmenlinna, S.; Vuopio-Varkila, J.; El Solh, N.; et al. Harmonization of Pulsed-Field Gel Electrophoresis Protocols for Epidemiological Typing of Strains of Methicillin-Resistant Staphylococcus Aureus: A Single Approach Developed by Consensus in 10 European Laboratories and Its Application for Tracing the Spread of Related Strains. J. Clin. Microbiol. 2003, 41, 1574–1585. [Google Scholar] [CrossRef]
- Fischer, B.G.; Baduashvili, A. Cardiac Point-of-Care Ultrasound for the Diagnosis of Infective Endocarditis in a Patient with Non-Specific Rheumatologic Symptoms and Glomerulonephritis. Am. J. Case Rep. 2019, 20, 542–547. [Google Scholar] [CrossRef]
- Muñoz, P.; Bouza, E.; Marín, M.; Alcalá, L.; Rodríguez Créixems, M.; Valerio, M.; Pinto, A.; on behalf of the Group for the Management of Infective Endocarditis of the Gregorio Marañón Hospital. Heart Valves Should Not Be Routinely Cultured. J. Clin. Microbiol. 2008, 46, 2897–2901. [Google Scholar] [CrossRef]
- Greub, G.; Lepidi, H.; Rovery, C.; Casalta, J.-P.; Habib, G.; Collard, F.; Fournier, P.-E.; Raoult, D. Diagnosis of Infectious Endocarditis in Patients Undergoing Valve Surgery. Am. J. Med. 2005, 118, 230–238. [Google Scholar] [CrossRef]
- Morris, A.J.; Drinković, D.; Pottumarthy, S.; MacCulloch, D.; Kerr, A.R.; West, T. Bacteriological Outcome after Valve Surgery for Active Infective Endocarditis: Implications for Duration of Treatment after Surgery. Clin. Infect. Dis. 2005, 41, 187–194. [Google Scholar] [CrossRef]
- MacDonald, K. Infective Endocarditis in Dogs: Diagnosis and Therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Spina, G.S.; Sampaio, R.O.; Branco, C.E.; Miranda, G.B.; Rosa, V.E.E.; Tarasoutchi, F. Incidental Histological Diagnosis of Acute Rheumatic Myocarditis: Case Report and Review of the Literature. Front. Pediatr. 2014, 2, 126. [Google Scholar] [CrossRef][Green Version]
- Collins, J.A.; Zhang, Y.; Burke, A.P. Pathologic Findings in Native Infective Endocarditis. Pathol. Res. Pract. 2014, 210, 997–1004. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaluś-Jordanow, O.; Stabińska-Smolarz, M.; Czopowicz, M.; Moroz, A.; Mickiewicz, M.; Łobaczewski, A.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Rzewuska, M.; Sapierzyński, R.; et al. Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats. Animals 2021, 11, 3162. https://doi.org/10.3390/ani11113162
Szaluś-Jordanow O, Stabińska-Smolarz M, Czopowicz M, Moroz A, Mickiewicz M, Łobaczewski A, Chrobak-Chmiel D, Kizerwetter-Świda M, Rzewuska M, Sapierzyński R, et al. Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats. Animals. 2021; 11(11):3162. https://doi.org/10.3390/ani11113162
Chicago/Turabian StyleSzaluś-Jordanow, Olga, Marta Stabińska-Smolarz, Michał Czopowicz, Agata Moroz, Marcin Mickiewicz, Andrzej Łobaczewski, Dorota Chrobak-Chmiel, Magdalena Kizerwetter-Świda, Magdalena Rzewuska, Rafał Sapierzyński, and et al. 2021. "Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats" Animals 11, no. 11: 3162. https://doi.org/10.3390/ani11113162
APA StyleSzaluś-Jordanow, O., Stabińska-Smolarz, M., Czopowicz, M., Moroz, A., Mickiewicz, M., Łobaczewski, A., Chrobak-Chmiel, D., Kizerwetter-Świda, M., Rzewuska, M., Sapierzyński, R., Grzegorczyk, M., Świerk, A., & Frymus, T. (2021). Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats. Animals, 11(11), 3162. https://doi.org/10.3390/ani11113162






