Phytogenic Ingredients from Hops and Organic Acids Improve Selected Indices of Welfare, Health Status Markers, and Bacteria Composition in the Caeca of Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
2.2. Litter Analysis
2.3. Gait-Scoring Observations
2.4. Foot Pad Dermatitis Observation
2.5. Sampling Procedures
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
3.1. Performance Response
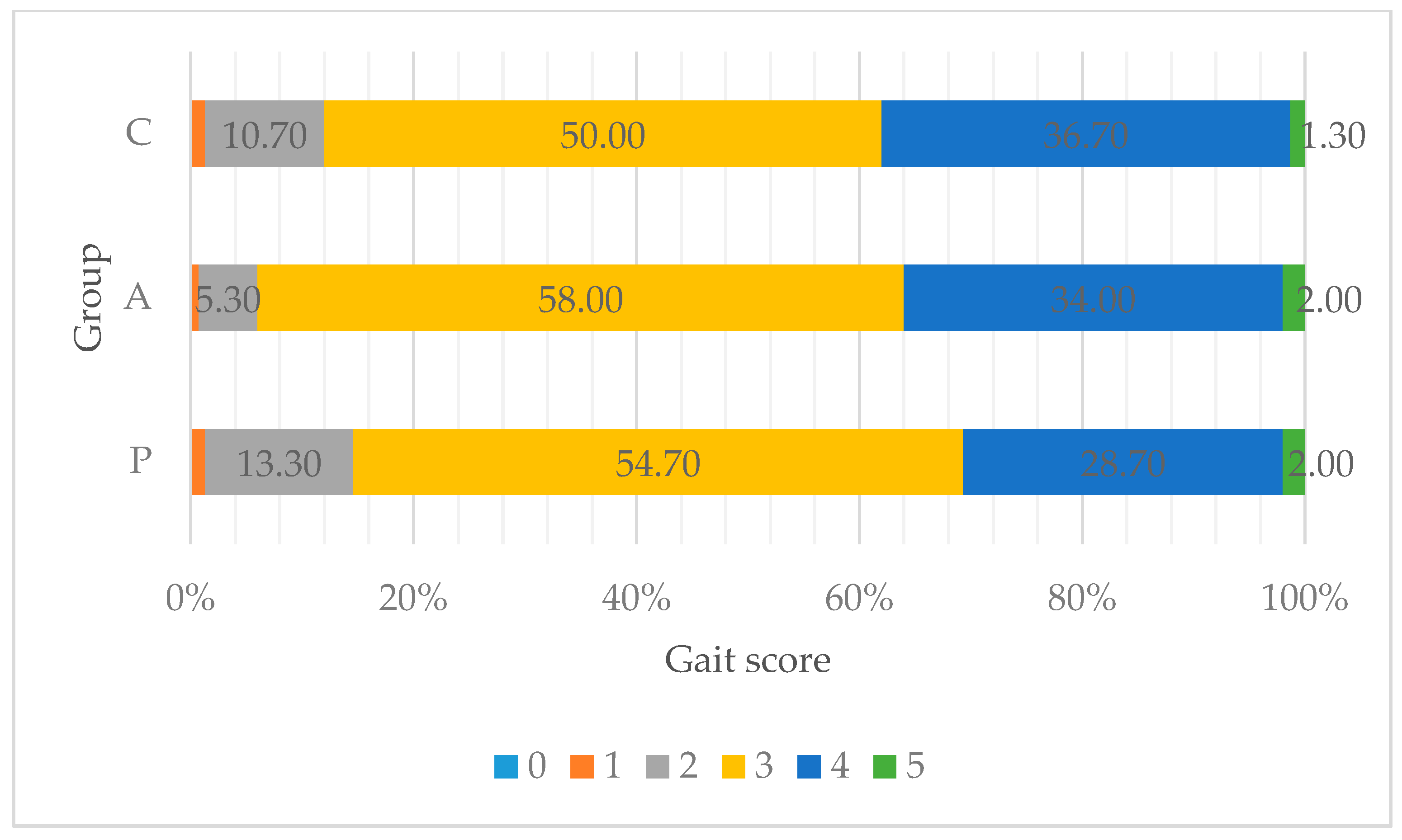
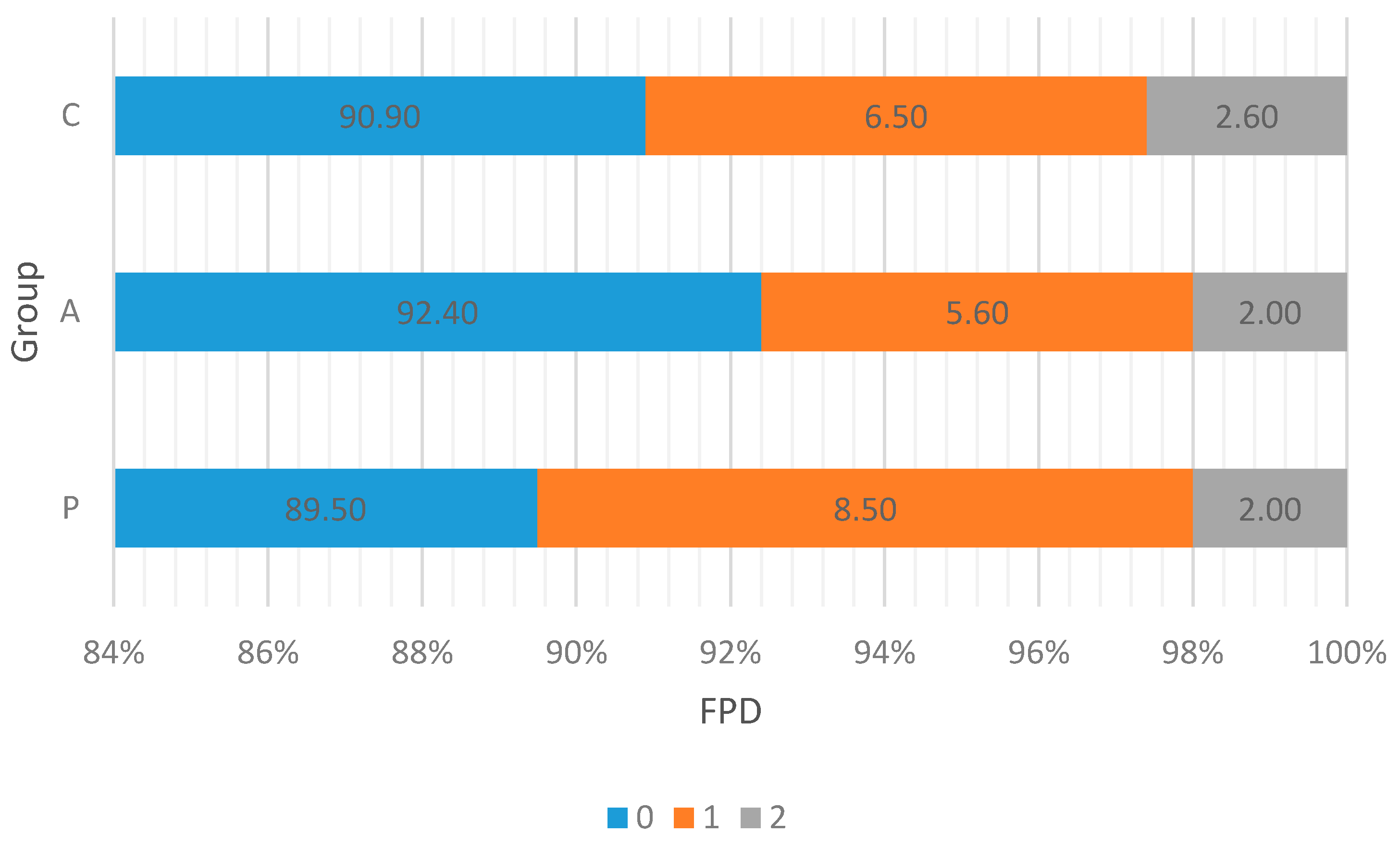
3.2. Walking Ability and Foot Pad Condition
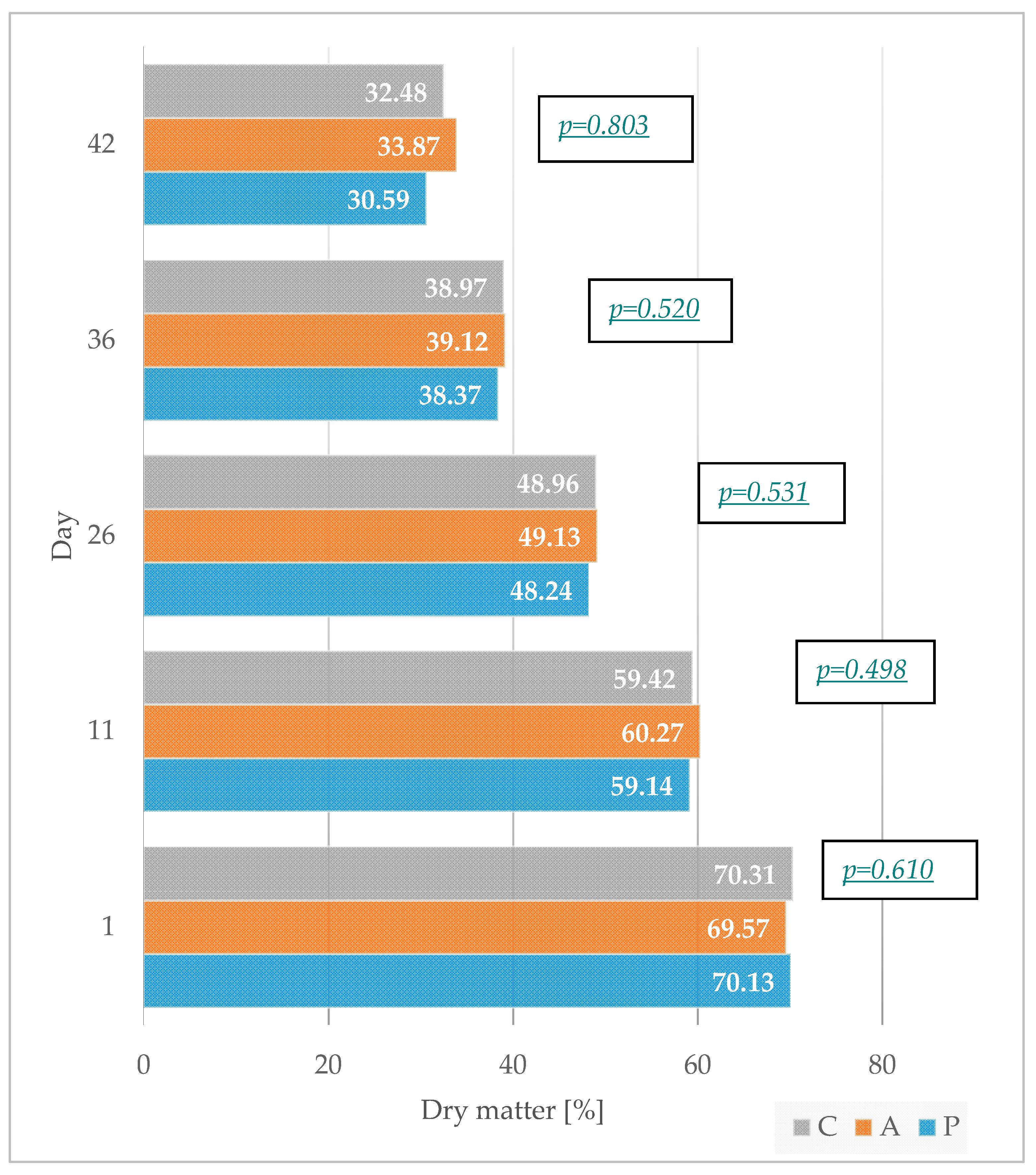
3.3. Determination of Litter Dry Matter
3.4. Bacterial Composition in the Caecal Digesta
3.5. Blood Indices of Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Ognik, K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef]
- Perić, L.; Milošević, N.; Žikić, D.; Bjedov, S.; Cvetković, D.; Markov, S.; Mohnl, M.; Steiner, T. Effects of probiotic and phytogenic products on performance, gut morphology and cecal microflora of broiler chickens. Arch. Anim. Breed. 2010, 53, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Weber, G.M.; Michalczuk, M.; Huyghebaert, G.; Juin, H.; Kwakernaak, C.; Gracia, M.I. Effects of a blend of essential oil compounds and benzoic acid on performance of broiler chickens as revealed by a meta-analysis of 4 growth trials in various locations. Poult. Sci. 2012, 91, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Patra, A.K. Influence of Plant Bioactive Compounds on Intestinal Epithelial Barrier in Poultry. Mini-Rev. Med. Chem. 2019, 20, 566–577. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczka, P.; Szkopek, D.; Kinsner, M.; Fotschki, B.; Juśkiewicz, J.; Banach, J. Cannabis-derived cannabidiol and nanoselenium improve gut barrier function and affect bacterial enzyme activity in chickens subjected to C. perfringens challenge. Vet. Res. 2020, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. The Review on Antimicrobial Resistance: Tackling drug-resistant infections globally. Arch. Pharm. Pract. 2016, 7, 110. [Google Scholar]
- Sjofjan, O.; Adli, D.N.; Harahap, R.P.; Jayanegara, A.; Utama, D.T.; Seruni, A.P. The effects of lactic acid bacteria and yeasts as probiotics on the growth performance, relative organ weight, blood parameters, and immune responses of broiler: A metaanalysis. F1000Research 2021, 10, 183. [Google Scholar] [CrossRef]
- Kupryś-Caruk, M.; Michalczuk, M.; Chablowska, B.; Stefanska, I.; Kotyrba, D.; Parzeniecka-Jaworska, M. Efficacy and safety assessment of microbiological feed additive for chicken broilers in tolerance studies. J. Vet. Res. 2018, 62, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Krauze, M.; Abramowicz, K.; Ognik, K. The Effect of Addition of Probiotic Bacteria (Bacillus Subtilis or Enterococcus faecium) or Phytobiotic Containing Cinnamon Oil to Drinking Water on the Health and Performance of Broiler Chickens. Ann. Anim. Sci. 2020, 20, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Suriya, R.; Zulkifli, I.; Alimon, A.R. The effect of dietary inclusion of herbs as growth promoter in broiler chickens. J. Anim. Vet. Adv. 2012, 11, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Ijaz, U.Z.; Kelly, C.; Linton, M.; Sloan, W.T.; Green, B.D.; Lavery, U.; Dorrell, N.; Wren, B.W.; Richmond, A.; et al. Impact of industrial production system parameters on chicken microbiomes: Mechanisms to improve performance and reduce Campylobacter. Microbiome 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- BenSassi, N.; Vas, J.; Vasdal, G.; Averós, X.; Estévez, I.; Newberry, R.C. On-farm broiler chicken welfare assessment using transect sampling reflects environmental inputs and production outcomes. PLoS ONE 2019, 14, e0214070. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, E.M.; Fairchild, B.D. Footpad dermatitis in poultry. Poult. Sci. 2010, 89, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. The nutrition of poultry as a factor affecting litter quality and foot pad dermatitis—An updated review. J. Anim. Physiol. Anim. Nutr. 2017, 101, e14–e20. [Google Scholar] [CrossRef] [PubMed]
- De Jong, I.C.; van Harn, J.; Gunnink, H.; Hindle, V.A.; Lourens, A. Footpad dermatitis in Dutch broiler flocks: Prevalence and factors of influence. Poult. Sci. 2012, 91, 1569–1574. [Google Scholar] [CrossRef]
- Ząbek, K.; Szkopek, D.; Michalczuk, M.; Konieczka, P. Dietary phytogenic combination with hops and a mixture of a free butyrate acidifier and gluconic acid maintaining the health status of the gut and performance in chickens. Animals 2020, 10, 1335. [Google Scholar] [CrossRef]
- Aviagen, R. 308 Broiler Nutrition Specifications. Aviagen 2007, 6, 1–8. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting Assoc; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 15, pp. 136–138. [Google Scholar]
- Kestin, S.C.; Knowles, T.G.; Tinch, A.E.; Gregory, N.G. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992, 131, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A. Animal welfare indicators and their use in society. Welf. Prod. Anim. Assess. Manag. RISKS 2009, 5, 371–389. [Google Scholar]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amit-Romach, E.; Sklan, D.; Uni, Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 2004, 83, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsen, H.Y.; Lin, C.K.; Chi, W.R. Development and use of 16S rRNA gene targeted PCR primers for the identification of Escherichia coli cells in water. J. Appl. Microbiol. 1998, 85, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kastner, S.; Perreten, V.; Bleuler, H.; Hugenschmidt, G.; Lacroix, C.; Meile, L. Antibiotic susceptibility patterns and resistance genes of starter cultures and probiotic bacteria used in food. Syst. Appl. Microbiol. 2006, 29, 145–155. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2005, 169, 350–369. [Google Scholar] [CrossRef]
- El-Wahab, A.A.; Aziza, A.; El-Adl, M. Impact of dietary excess methionine and lysine with or without addition of L-carnitine on performance, blood lipid profile and litter quality in broilers. Asian J. Anim. Vet. Adv. 2015, 10, 191–202. [Google Scholar] [CrossRef]
- Sood, U.; Gupta, V.; Kumar, R.; Lal, S.; Fawcett, D.; Rattan, S.; Poinern, G.E.J.; Lal, R. Chicken Gut Microbiome and Human Health: Past Scenarios, Current Perspectives, and Futuristic Applications. Indian J. Microbiol. 2020, 60, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tellez, G.; Escobar, J. Identification of biomarkers for footpad dermatitis development and wound healing. Front. Cell. Infect. Microbiol. 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, M.M. REVIEW: Footpad dermatitis (FPD) in chickens. Am. Korean J. Food Health Converg. 2020, 6, 11–16. [Google Scholar]
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2007, 59, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Probiotics in Animal Nutrition: Production, Impact and Regulation; FAO Animal Production and Health: Rome, Italy, 2016. [Google Scholar]
- Vizzier Thaxton, Y.; Christensen, K.D.; Mench, J.A.; Rumley, E.R.; Daugherty, C.; Feinberg, B.; Parker, M.; Siegel, P.; Scanes, C.G. Symposium: Animal welfare challenges for today and tomorrow. Poult. Sci. 2016, 95, 2198–2207. [Google Scholar] [CrossRef]
- Williams, T.; Athrey, G. Cloacal SWABS are unreliable sources for estimating lower gastro-intestinal tract microbiota membership and structure in broiler chickens. Microorganisms 2020, 8, 718. [Google Scholar] [CrossRef]
- Qaid, M.; Albatshan, H.; Shafey, T.; Hussein, E.; Abudabos, A.M. Effect of stocking density on the performance and immunity of 1-to 14-d- old broiler chicks. Rev. Bras. Cienc. Avic. 2016, 18, 683–692. [Google Scholar] [CrossRef]
- Michalczuk, M.; Jóźwik, A.; Damaziak, K.; Zdanowska-Sasiadek, Z.; Marzec, A.; Gozdowski, D.; Strzałkowska, N. Age-related changes in the growth performance, meat quality, and oxidative processes in breast muscles of three chicken genotypes. Turkish J. Vet. Anim. Sci. 2016, 40, 389–398. [Google Scholar] [CrossRef]
- Konieczka, P.; Barszcz, M.; Choct, M.; Smulikowska, S. The interactive effect of dietary n-6: N-3 fatty acid ratio and vitamin E level on tissue lipid peroxidation, DNA damage in intestinal epithelial cells, and gut morphology in chickens of different ages. Poult. Sci. Sci. 2018, 97, 149–158. [Google Scholar] [CrossRef]
- Niknafs, S.; Roura, E. Nutrient sensing, taste and feed intake in avian species. Nutr. Res. Rev. 2018, 31, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guo, Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim. Feed Sci. Technol. 2007, 132, 240–249. [Google Scholar] [CrossRef]
- Midilli, M.; Alp, M.; Kocabaǧli, N.; Muǧlali, Ö.H.; Turan, N.; Yilmaz, H.; Çakir, S. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers. S. Afr. J. Anim. Sci. 2008, 38, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Froebel, L.K.; Jalukar, S.; Lavergne, T.A.; Lee, J.T.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019, 98, 6668–6676. [Google Scholar] [CrossRef] [PubMed]

| Item | Starter | Grower I | Grower II | Finisher |
|---|---|---|---|---|
| Ingredient (%): | ||||
| Wheat | 35 | 35.03 | 36.6 | 30.12 |
| Maize | 24.2 | 30.00 | 30.42 | 38 |
| Soybean meal | 0.0 | 28.6 | 25 | 22 |
| Rapeseed meal | 3.0 | 0.0 | 0.0 | 2.0 |
| Sunflower oil | 1.3 | 1.9 | 3.0 | 3.2 |
| Sunflower meal | 0.0 | 2.0 | 3.0 | 3.0 |
| Limestone | 1.36 | 0.91 | 0.7 | 0.57 |
| Monocalciumphosphate | 0.7 | 0.38 | 0.12 | 0.06 |
| Methionine | 0.34 | 0.28 | 0.26 | 0.2 |
| Sulfate of lysine | 0.4 | 0.42 | 0.42 | 0.4 |
| NaCl | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium sulfite | 0.2 | 0.2 | 0.2 | 0.2 |
| Threonine | 0.06 | 0.08 | 0.08 | 0.05 |
| Vitamin−mineral premix 1 | 0.50 | 0.50 | 0.50 | 0.50 |
| Nutritional composition: | ||||
| EM * (kcal/kg) | 2901.2 | 3014.5 | 3108.5 | 3160.8 |
| Protein (%) | 22.36 | 20.51 | 19.45 | 18.43 |
| Fat (%) | 3.25 | 3.94 | 5.02 | 5.50 |
| Score | Description |
|---|---|
| 0 | No lesions. |
| 1 | Superficial lesions, color lesions with diameter not exceeding 0.5 cm. |
| 2 | Deep lesions with a scab and ulceration, color lesions with diameter of 0.5 cm or greater. |
| Bacteria | Primers | Sequence (5′-3′) | Reference |
|---|---|---|---|
| Total bacteria | Forward | CGTGCCAGCCGCGGTAATACG | Amit-Romach et al. [25] |
| Reverse | GGGTTGCGCTCGTTGCGGGAC TTAACCCAACAT | ||
| Clostridium | Forward | AAAGGAAGATTAATACCGCATAA | |
| Reverse | ATCTTGCGACCGTACTCCCC | Amit-Romach et al. [25] | |
| Lactobacillus | Forward | CATCCAGTGCAAACCTAAGAG | |
| Reverse | GATCCGCTTGCCTTCGCA | Wang et al. [26] | |
| Escherichia coli | Forward | GGGAGTAAAGTTAATACCTTTGCTC | |
| Reverse | TTCCCGAAGGCACATTCT | Tsen et al. [27] | |
| Bifidobacterium | Forward | CGGGTGCTICCCACTTTCATG | |
| Reverse | GATTCTGGCTCAGGATGAACG | Kastner et al. [28] |
| Indices | Group 1 | Medium | SE | p-Value |
|---|---|---|---|---|
| ALT (U/L) | C | 6.3 | 0.75 | 0.101 |
| A | 6.9 | 0.82 | ||
| P | 4.8 | 0.42 | ||
| AST (U/L) | C | 422.1 | 49.3 | 0.318 |
| A | 508.5 | 69.79 | ||
| P | 399.9 | 30.8 | ||
| Ca (mmol/L) | C | 2.51 | 0.29 | 0.730 |
| A | 2.68 | 0.05 | ||
| P | 2.53 | 0.06 | ||
| Cl (mmol/L) | C | 106.7 | 2.21 | 0.179 |
| A | 110.3 | 0.98 | ||
| P | 110.5 | 1.26 | ||
| GGT (U/L) | C | 18.30 b | 1.25 | 0.049 |
| A | 22.97 a | 1.13 | ||
| P | 22.35 a | 1.7 | ||
| GLU (mmol/L) | C | 14.6 | 0.49 | 0.377 |
| A | 14.44 | 0.28 | ||
| P | 15.21 | 0.41 | ||
| K (mmol/L) | C | 8.02 b | 0.96 | 0.011 |
| A | 5.36 a | 0.14 | ||
| P | 6.00 a | 0.39 | ||
| LAC (mmol/L) | C | 8.06 b | 1.07 | 0.012 |
| A | 5.24 a | 0.51 | ||
| P | 5.22 a | 0.32 | ||
| Mg (mmol/L) | C | 0.98 | 0.19 | 0.895 |
| A | 0.91 | 0.02 | ||
| P | 0.92 | 0.04 | ||
| Na (mmol/L) | C | 148.0 | 2.34 | 0.265 |
| A | 149.3 | 0.96 | ||
| P | 152.1 | 1.66 | ||
| P (mmol/L) | C | 2.53 b | 0.23 | 0.040 |
| A | 2.13 a | 0.04 | ||
| P | 2.03 a | 0.04 | ||
| TP (g/L) | C | 30.45 | 1.44 | 0.884 |
| A | 30.15 | 1.16 | ||
| P | 29.45 | 1.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalczuk, M.; Holl, E.; Möddel, A.; Jóźwik, A.; Slósarz, J.; Bień, D.; Ząbek, K.; Konieczka, P. Phytogenic Ingredients from Hops and Organic Acids Improve Selected Indices of Welfare, Health Status Markers, and Bacteria Composition in the Caeca of Broiler Chickens. Animals 2021, 11, 3249. https://doi.org/10.3390/ani11113249
Michalczuk M, Holl E, Möddel A, Jóźwik A, Slósarz J, Bień D, Ząbek K, Konieczka P. Phytogenic Ingredients from Hops and Organic Acids Improve Selected Indices of Welfare, Health Status Markers, and Bacteria Composition in the Caeca of Broiler Chickens. Animals. 2021; 11(11):3249. https://doi.org/10.3390/ani11113249
Chicago/Turabian StyleMichalczuk, Monika, Elisabeth Holl, Anne Möddel, Artur Jóźwik, Jan Slósarz, Damian Bień, Katarzyna Ząbek, and Paweł Konieczka. 2021. "Phytogenic Ingredients from Hops and Organic Acids Improve Selected Indices of Welfare, Health Status Markers, and Bacteria Composition in the Caeca of Broiler Chickens" Animals 11, no. 11: 3249. https://doi.org/10.3390/ani11113249
APA StyleMichalczuk, M., Holl, E., Möddel, A., Jóźwik, A., Slósarz, J., Bień, D., Ząbek, K., & Konieczka, P. (2021). Phytogenic Ingredients from Hops and Organic Acids Improve Selected Indices of Welfare, Health Status Markers, and Bacteria Composition in the Caeca of Broiler Chickens. Animals, 11(11), 3249. https://doi.org/10.3390/ani11113249






