Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Ethical Clearance
2.2. Materials
2.3. Encapsulation of the Tannin Extract
2.4. Animals and Treatments
2.5. Bodyweight and Testicular Measurements
2.6. Ambient Temperature
2.7. Semen Collection
2.8. Semen Evaluations
2.8.1. Subjective Evaluation
2.8.2. Objective Evaluation
2.9. Blood Collection and Analysis
2.9.1. Oxidative Status
2.9.2. Hormonal Status
2.10. Statistical Analyses
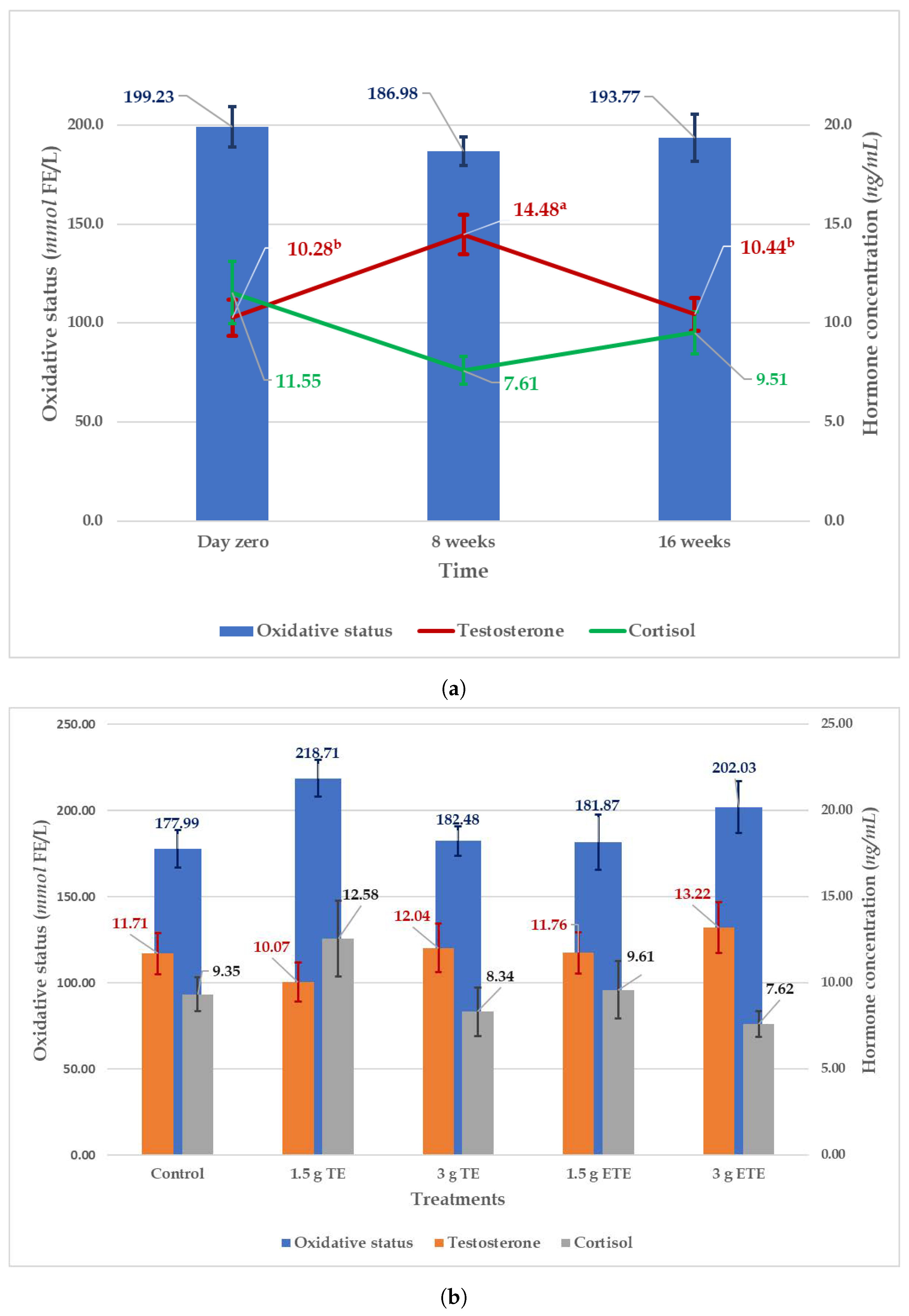
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Experimental Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | 1.5 g TE | 3 g TE | 1.5 g ETE | 3 g ETE | ||
| Initial Bodyweight (kg) | 52.7 ± 1.85 | 52.3 ± 2.42 | 52.4 ± 1.59 | 53.5 ± 1.37 | 53.0 ± 2.05 | 0.991 |
| Initial scrotal circumference (cm) | 31.6 ± 1.52 | 31.9 ± 1.39 | 30.5 ± 0.94 | 30.7 ± 1.35 | 30.4 ± 0.52 | 0.860 |
| Initial testicular volume (cm) | 179.5 ± 22.61 | 186.3 ± 21.80 | 182.8 ± 17.93 | 184.5 ± 15.42 | 192.8 ± 13.64 | 0.991 |
| Initial testicular width (mm) | 59.7 ± 3.14 | 61.7 ± 2.47 | 61.4 ± 2.25 | 62.4 ± 2.40 | 61.7 ± 1.49 | 0.951 |
| Initial testicular length (mm) | 98.8 ± 4.40 | 96.9 ± 4.57 | 96.8 ± 4.17 | 97.5 ± 2.52 | 99.0 ± 4.26 | 0.992 |
| Initial semen volume (mL) | 0.7 ± 0.11 | 0.7 ± 0.18 | 1.0 ± 0.14 | 0.9 ± 0.23 | 0.8 ± 0.19 | 0.728 |
| Intial semen pH | 5.8 ± 0.17 | 5.9 ± 0.37 | 5.7 ± 0.33 | 6.5 ± 0.62 | 6.5 ± 0.22 | 0.355 |
| Initial sperm concentration (10/mL) | 3.8 ± 0.90 | 3.9 ± 0.75 | 3.9 ± 0.69 | 3.9 ± 0.91 | 3.5 ± 0.80 | 0.997 |
| Initial Semen colour (1–4) | 3.2 ± 0.31 | 3.2 ± 0.48 | 3.7 ± 0.21 | 2.8 ± 0.40 | 3.5 ± 0.22 | 0.471 |
| Initial Sperm mass motility (1–5) | 3.3 ± 0.21 | 3.3 ± 0.56 | 3.5 ± 0.43 | 3.3 ± 0.61 | 3.8 ± 0.17 | 0.907 |
| Initial progressive motility (%) | 56.2 ± 4.86 | 54.5 ± 10.80 | 52.2 ± 9.31 | 51.1 ± 12.71 | 56.4 ± 6.71 | 0.991 |
References
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; HCLD, A.A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [PubMed]
- Baker, M.A.; Aitken, R.J. The importance of redox regulated pathways in sperm cell biology. Mol. Cell. Endocrinol. 2004, 216, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Tilmisany, A.M.; Al-Sawaf, H. Mechanisms of male infertility: Role of antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukusa, K.; Lehloenya, K. Selenium supplementation improves testicular characteristics and semen quality of Saanen bucks. Small Rumin. Res. 2017, 151, 52–58. [Google Scholar] [CrossRef]
- Huang, M.T.; Lee, C.Y.; Ho, C.T. Phenolic Compounds in Food and Their Effects on Health; ACS Symposium Series (USA); American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Brenes, A.; Viveros, A.; Goñí, I.; Centeno, C.; Sayago-Ayerdy, S.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. J. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Deng, Y.T.; Liang, G.; Shi, Y.; Li, H.L.; Zhang, J.; Mao, X.M.; Fu, Q.R.; Peng, W.X.; Chen, Q.X.; Shen, D.Y. Condensed tannins from Ficus altissima leaves: Structural, antioxidant, and antityrosinase properties. Process Biochem. 2016, 51, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, K.; Mingbin, L.; Zhao, J.; Xiong, B. Effects of chestnut tannins on the meat quality, welfare, and antioxidant status of heat-stressed lambs. Meat Sci. 2016, 116, 236–242. [Google Scholar] [CrossRef]
- Dey, A.; Dutta, N.; Pattanaik, A.K.; Sharma, K. Antioxidant status, metabolic profile and immune response of lambs supplemented with tannin rich Ficus infectoria leaf meal. Jpn. J. Vet. Res. 2015, 63, 15–24. [Google Scholar]
- Dschaak, C.; Williams, C.; Holt, M.; Eun, J.S.; Young, A.; Min, B. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Preparation of acacia tannin loaded lipid microparticles by solid-in-oil-in-water and melt dispersion methods, their characterization and evaluation of their effect on ruminal gas production In Vitro. PLoS ONE 2018, 13, e0206241. [Google Scholar] [CrossRef] [Green Version]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de Ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- Deaville, E.; Givens, D.; Mueller-Harvey, I. Chestnut and mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilisation and losses. Anim. Feed Sci. Technol. 2010, 157, 129–138. [Google Scholar] [CrossRef]
- Roux, J.; Kemp, G.; Wingfield, M. Diseases of black wattle in South Africa—A review. S. Afr. For. J. 1995, 174, 35–40. [Google Scholar] [CrossRef]
- El Kadili, S.; Raes, M.; Bister, J.L.; Archa, B.; Chentouf, M.; Kirschvink, N. Effect of season on sexual behavior, testicular measurements and seminal characteristics in “Beni arouss” North Moroccan bucks. Anim. Reprod. Sci. 2019, 201, 41–54. [Google Scholar] [CrossRef]
- Ghorbankhani, F.; Souri, M.; Moeini, M.; Mirmahmoudi, R. Effect of nutritional state on semen characteristics, testicular size and serum testosterone concentration in Sanjabi ram lambs during the natural breeding season. Anim. Reprod. Sci. 2015, 153, 22–28. [Google Scholar] [CrossRef]
- Zamiri, M.; Khodaei, H. Seasonal thyroidal activity and reproductive characteristics of Iranian fat-tailed rams. Anim. Reprod. Sci. 2005, 88, 245–255. [Google Scholar] [CrossRef]
- Cevik, M.; Yilmazer, C.; Kocyigit, A. Comparison of sexual performance and testicular characteristics of melatonin treated Kivircik and Charollais rams during the non-breeding season. Arq. Bras. Med. Vet. Zootec. 2017, 69, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Van Niekerk, W.; Hassen, A.; Snyman, L.; Rethman, N.F.; Coertze, R. Influence of mineral composition and rumen degradability of Atriplex nummularia (Hatfield Select F1) plants on selection preference of sheep. Afr. J. Range Forage Sci. 2009, 26, 91–96. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Characterization of starch and gum arabic-maltodextrin microparticles encapsulating acacia tannin extract and evaluation of their potential use in ruminant nutrition. Asian-Australas. J. Anim. Sci. 2019, 32, 977. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. Routine sheep and goat procedures. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 737–758. [Google Scholar] [CrossRef]
- Martinez, J.; Dominguez, B.; Barrientos, M.; Canseco, R.; Ortega, E.; Lamothe, C. Biometry and testicular growth influenced by nutrition on prepubertal Pelibuey lambs. J. Anim. Feed Sci. 2012, 2, 314–321. [Google Scholar]
- Steger, K.; Wrobel, K.H. Immunohistochemical demonstration of cytoskeletal proteins in the ovine testis during postnatal development. Anat. Embryol. 1994, 189, 521–530. [Google Scholar] [CrossRef]
- Barth, A.D.; Oko, R. Abnormal Morphology of Bovine Spermatozoa; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Verde, V.; Fogliano, V.; Ritieni, A.; Maiani, G.; Morisco, F.; Caporaso, N. Use of N, N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free Radic. Res. 2002, 36, 869–873. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Rizvi, S.I. N,N-Dimethyl-p-phenylenediamine dihydrochloride-based method for the measurement of plasma oxidative capacity during human aging. Anal. Biochem. 2013, 436, 165–167. [Google Scholar] [CrossRef]
- Palme, R. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Inst. J. Mammal Biol. 1997, 62, 192–197. [Google Scholar]
- Palme, R. Biotin-streptavidin enzyme immunoassay for the determination of oestrogens and androgens in boar faeces. In Advances of Steroid Analysis ’93; Akadémiai Kiadó: Görög, Budapest, 1994; pp. 111–117. [Google Scholar]
- Kafi, M.; Safdarian, M.; Hashemi, M. Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams. Small Rumin. Res. 2004, 53, 133–139. [Google Scholar] [CrossRef]
- Belkhiri, Y.; Bouzebda-Afri, F.; Bouzebda, Z.; Mouffok, C.; Djaout, A. Seasonal variations in reproductive parameters of Ouled Djellal rams in the East of Algeria. Indian J. Anim. Res. 2017, 53, 1407–1413. [Google Scholar]
- Zamiri, M.; Khalili, B.; Jafaroghli, M.; Farshad, A. Seasonal variation in seminal parameters, testicular size, and plasma testosterone concentration in Iranian Moghani rams. Small Rumin. Res. 2010, 94, 132–136. [Google Scholar] [CrossRef]
- Worlddata.info. Average Length of Daylight in Pretoria. Available online: https://www.worlddata.info/africa/south-africa/sunset.php (accessed on 28 June 2020).
- Batailler, M.; Chesneau, D.; Derouet, L.; Butruille, L.; Segura, S.; Cognié, J.; Dupont, J.; Pillon, D.; Migaud, M. Pineal-dependent increase of hypothalamic neurogenesis contributes to the timing of seasonal reproduction in sheep. Sci. Rep. 2018, 8, 6188. [Google Scholar] [CrossRef]
- Avdi, M.; Leboeuf, B.; Terqui, M. Advanced breeding and “buck effect” in indigenous Greek goats. Livest. Prod. Sci. 2004, 87, 251–257. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, Y.; Du, M.; Liu, W.; Ren, Y.; Zhang, C.; Zhang, J. The effect of dietary grape pomace supplementation on epididymal sperm quality and testicular antioxidant ability in ram lambs. Theriogenology 2017, 97, 50–56. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, M.; Saleemi, M.; Gul, S.; Ahmad, N.; Umar, S. Protective effects of vitamin E on sodium arsenite-induced toxicity, testicular measurements and histopathological studies of testes in Teddy goat bucks. Andrologia 2017, 49, e12699. [Google Scholar] [CrossRef]
- Elbaz, H.; Abdel Razek, E. Effect of vitamin E and selenium injections on the testes and accessory sex glands of barki rams during non-breed-ing season. Adv. Anim. Vet. Sci. 2019, 7, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Yue, D.; Yan, L.; Luo, H.; Xu, X.; Jin, X. Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 2010, 118, 217–222. [Google Scholar] [CrossRef]
- Ozer Kaya, S.; Gur, S.; Erisir, M.; Kandemir, F.M.; Benzer, F.; Kaya, E.; Turk, G.; Sonmez, M. Influence of vitamin E and vitamin E-selenium combination on arginase activity, nitric oxide level and some spermatological properties in ram semen. Reprod. Domest. Anim. 2020, 55, 162–169. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Kesavan, M.; Rai, B.; Balamurugan, P.; Arulkumar, S. Effect of area specific mineral mixture and antioxidants supplementation on semen evaluation of Barbari bucks. J. Entomol. Zool. Stud. 2020, 8, 720–723. [Google Scholar]
- Attia, K.A.H.; El-Raghi, A.; Fouda, S.F. Relieve the negative effects of heat stress on semen quality, reproductive efficiency and oxidative capacity of rabbit bucks using different natural antioxidants. Anim. Biosci. 2020, 34, 844–854. [Google Scholar]
- Cofré-Narbona, E.J.; Peralta-Troncoso, O.A.; Urquieta-Mangiola, B.E.; Raggi-Saini, L.A.; Benavides-Aguila, N.; Parraguez-Gamboa, V.H. Improvement of antioxidant status and semen quality by oral supplementation with vitamins c and e in rams. Rev. Cient. 2016, 26, 156–163. [Google Scholar]
- Piagentini, M.; Silva, D.; Dell’Aqua, C.; Moya-Araujo, C.; Codognoto, V.; Ramos, A.; Oba, E. Effect of selenium supplementation on semen characteristics of Brazil’s ram. Reprod. Domest. Anim. 2017, 52, 355–358. [Google Scholar] [CrossRef]
- Butt, M.A.; Shahid, M.Q.; Bhatti, J.A.; Khalique, A. Effect of dietary vitamin e and selenium supplementation on physiological responses and reproductive performance in holstein friesian bulls during humid hot summer. Pak. Vet. J. 2019, 39, 593–597. [Google Scholar] [CrossRef]
- Stefanov, R.; Chervenkov, M.; Anev, G.; Maksimović, N.; Andreeva, M.; Ivanova, T.; Milovanović, A. Effect of supplementation with inorganic and organic selenium on sperm quality and quantity in north-east Bulgarian merino rams. Biotechnol. Anim. Husb. 2018, 34, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Steiner, A.; Hansen, K.; Diamond, M.; Coutifaris, C.; Cedars, M.; Legro, R.; Usadi, R.; Baker, V.; Coward, R.; Santoro, N.; et al. Antioxidants in the treatment of male factor infertility: Results from the double blind, multi-center, randomized controlled Males, Antioxidants, and Infertility (MOXI) trial. Hum. Reprod. 2018, 33, i30. [Google Scholar]
- Barbagallo, F.; Vignera, S.L.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T. Cytoplasmic droplets: The good, the bad or just confusing? Hum. Reprod. 2005, 20, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuster, C.; Althouse, G. A technique for preserving retained distal cytoplasmic droplets in situ for immunofluorescence evaluation of ejaculated porcine spermatozoa. Prep. Biochem. Biotechnol. 2003, 33, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Matoušek, J.; Staněk, R. Immunosuppressive and leucolytic effect of phospholipid binding protein in bull seminal plasma. Anim. Reprod. Sci. 1993, 31, 1–6. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [Green Version]
- Sarlós, P.; Egerszegi, I.; Balogh, O.; Molnár, A.; Cseh, S.; Rátky, J. Seasonal changes of scrotal circumference, blood plasma testosterone concentration and semen characteristics in Racka rams. Small Rumin. Res. 2013, 111, 90–95. [Google Scholar] [CrossRef]
- Casao, A.; Cebrián, I.; Asumpção, M.E.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 2010, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Flores-Gil, V.; de la Blanca, M.M.; Velázquez, R.; Toledano-Díaz, A.; Santiago-Moreno, J.; López-Sebastián, A. Influence of testosterone administration at the end of the breeding season on sperm cryoresistance in rams (Ovis aries) and bucks (Capra hircus). Domest. Anim. Endocrinol. 2020, 72, 106425. [Google Scholar] [CrossRef]
- McSweeney, C.; Palmer, B.; McNeill, D.; Krause, D. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Gerlach, K.; Pries, M.; Südekum, K.H. Effect of condensed tannin supplementation on in vivo nutrient digestibilities and energy values of concentrates in sheep. Small Rumin. Res. 2018, 161, 57–62. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of lipid-encapsulated acacia tannin extract on feed intake, nutrient digestibility and methane emission in sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef] [Green Version]
- Sinz, S.; Liesegang, A.; Kreuzer, M.; Marquardt, S. Do supplements of Acacia mearnsii and grapeseed extracts alone or in combination alleviate metabolic nitrogen load and manure nitrogen emissions of lambs fed a high crude protein diet? Arch. Anim. Nutr. 2019, 73, 306–323. [Google Scholar] [CrossRef]
- Salhab, S.; Zarkawi, M.; Wardeh, M.; Al-Masri, M.; Kassem, R. Development of testicular dimensions and size, and their relationship to age, body weight and parental size in growing Awassi ram lambs. Small Rumin. Res. 2001, 40, 187–191. [Google Scholar] [CrossRef]
- Elmaz, Ö.; Cirit, Ü.; Demir, H. Relationship of testicular development with age, body weight, semen characteristics and testosterone in Kivircik ram lambs. S. Afr. J. Anim. Sci. 2007, 37, 269–274. [Google Scholar] [CrossRef]
- Hassan, M.; Pervage, S.; Ershaduzzaman, M.; Talukder, M. Influence of age on the spermiogramic parameters of native sheep. J. Bangladesh Agric. Univ. 2009, 7, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Al-kawmani, A.A.; Alfuraiji, M.M.; Abou-Tarboush, F.M.; Alodan, M.A.; Farah, M.A. Developmental changes in testicular interstitium in the Najdi Ram Lambs. Saudi J. Biol. Sci. 2014, 21, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Allaoui, A.; Safsaf, B.; Tlidjane, M.; Djaalab, I.; Mansour, H.D. Effect of increasing levels of wasted date palm in concentrate diet on reproductive performance of Ouled Djellal breeding rams during flushing period. Vet. World 2018, 11, 712. [Google Scholar] [CrossRef]
- Yamamoto, M.; Katsuno, S.; Yokoi, K.; Hibi, H.; Miyake, K. The effect of varicocelectomy on testicular volume in infertile patients with varicoceles. Nagoya J. Med. Sci. 1995, 58, 47–50. [Google Scholar]
- Nugraha, C.; Herwijanti, E.; Novianti, I.; Furqon, A.; Septian, W.; Busono, W.; Suyadi, S. Correlations between age of Bali bull and semen production at National Artificial Insemination Center, Singosari-Indonesia. J. Indones. Trop. Anim. Agric. 2019, 44, 258–260. [Google Scholar] [CrossRef]
- Webb, E.C.; Dombo, M.; Roets, M. Seasonal variation in semen quality of Gorno Altai cashmere goats and South African indigenous goats. S. Afr. J. Anim. Sci. 2004, 34, 240–243. [Google Scholar]
- Anawalt, B.D. Approach to male infertility and induction of spermatogenesis. J. Clin. Endocrinol. Metab. 2013, 98, 3532–3542. [Google Scholar] [CrossRef] [Green Version]
- Darbandi, S.; Darbandi, M.; Khorshid, H.R.K.; Sadeghi, M.R.; Heidari, M.; Cheshmi, G.; Akhondi, M.M. The effect of paternal age on semen quality and fertilization outcome in men with normal sperm DNA compaction, reactive oxygen species, and total antioxidant capacity levels. Turk. J. Urol. 2019, 45, 164. [Google Scholar] [CrossRef]
- Moghaddam, G.; Pourseif, M.; Rafat, S. Seasonal variation in semen quantity and quality traits of Iranian crossbred rams. Slovak J. Anim. Sci. 2012, 45, 67–75. [Google Scholar]
- Sajjad, M.; Ali, S.; Ullah, N.; Anwar, M.; Akhter, S.; Andrabi, S. Blood serum testosterone level and its relationship with scrotal circumference and semen characteristics in Nili-Ravi buffalo bulls. Pak. Vet. J. 2007, 27, 63. [Google Scholar]
- Samavat, J.; Cantini, G.; Lorubbio, M.; Degl’Innocenti, S.; Adaikalakoteswari, A.; Facchiano, E.; Lucchese, M.; Maggi, M.; Saravanan, P.; Ognibene, A.; et al. Seminal but not Serum Levels of Holotranscobalamin are Altered in Morbid Obesity and Correlate with Semen Quality: A Pilot Single Centre Study. Nutrients 2019, 11, 1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, E.S.E.; Hafez, B. Reproduction in Farm Animals; John Wiley & Sons; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Aller, J.; Aguilar, D.; Vera, T.; Almeida, G.; Alberio, R. Seasonal variation in sexual behavior, plasma testosterone and semen characteristics of Argentine Pampinta and Corriedale rams. Span. J. Agric. Res. 2012, 10, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Nagy, S.; Hallap, T.; Johannisson, A.; Rodriguez-Martinez, H. Changes in plasma membrane and acrosome integrity of frozen-thawed bovine spermatozoa during a 4 h incubation as measured by multicolor flow cytometry. Anim. Reprod. Sci. 2004, 80, 225–235. [Google Scholar] [CrossRef]
- Palacios, C.; Álvarez, S.; Martín-Gil, J.; Abecia, J. Plasma hormonal levels of rams are affected by sexual activity and confinement in a semen collection centre. ARC J. Anim. Vet. Sci. 2016, 2, 15–21. [Google Scholar]
- Sengupta, P.; Dutta, S. Thyroid disorders and semen quality. Biomed. Pharmacol. J. 2018, 11, 01–10. [Google Scholar] [CrossRef]
- Singh, R.; Singh, K. Male Infertility: Understanding, Causes and Treatment; Springer: Singapore, 2017. [Google Scholar]
- Wdowiak, A.; Bien, A.; Iwanowicz-Palus, G.; Makara-Studzińska, M.; Bojar, I. Impact of emotional disorders on semen quality in men treated for infertility. Neuro Endocrinol. Lett. 2017, 38, 50–58. [Google Scholar]
- Deichsel, K.; Pasing, S.; Erber, R.; Ille, N.; Palme, R.; Aurich, J.; Aurich, C. Increased cortisol release and transport stress do not influence semen quality and testosterone release in pony stallions. Theriogenology 2015, 84, 70–75. [Google Scholar] [CrossRef]
| Factors | Parameters | ||||
|---|---|---|---|---|---|
| Bodyweight (kg) | Scrotal Circumference (cm) | Testicular Volume (cm) | Testicular Width (mm) | Testicular Length (mm) | |
| Season | |||||
| Autumn | 52.9 ± 0.35 | 29.8 ± 0.21 | 150.0 ± 3.44 | 56.5 ± 0.49 | 92.8 ± 0.80 |
| Winter | 52.3 ± 0.41 | 28.2 ± 0.20 | 101.2 ± 3.08 | 47.5 ± 0.52 | 87.8 ± 0.88 |
| p-value | 0.320 | <0.001 | <0.001 | <0.001 | < 0.001 |
| Treatments | |||||
| Control | 52.1 ± 0.69 | 28.8 ± 0.36 | 124.2 ± 6.51 | 51.7 ± 1.07 | 90.0 ± 1.42 |
| 1.5 g TE | 52.6 ± 0.77 | 29.6 ± 0.38 | 134.3 ± 6.72 | 53.3 ± 1.03 | 91.7 ± 1.53 |
| 3.0 g TE | 51.8 ± 0.38 | 28.7 ± 0.36 | 121.6 ± 5.81 | 51.9 ± 1.00 | 88.3 ± 1.23 |
| 1.5 g ETE | 52.9 ± 0.42 | 28.9 ± 0.35 | 118.5 ± 5.81 | 51.1 ± 0.97 | 88.3 ± 1.31 |
| 3.0 g ETE | 53.6 ± 0.60 | 29.0 ± 0.25 | 129.4 ± 6.11 | 52.1 ± 0.94 | 93.1 ± 1.23 |
| p-value | 0.220 | 0.299 | 0.233 | 0.409 | 0.040 |
| Variable | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | 1.5 g TE | 3 g TE | 1.5 g ETE | 3 g ETE | ||
| Semen volume (mL) | 2.0 ± 0.14 | 2.4 ± 0.20 | 1.6 ± 0.13 | 1.9 ± 0.17 | 2.2 ± 0.28 | 0.042 |
| Semen pH | 7.3 ± 0.04 | 7.3 ± 0.04 | 7.3 ± 0.07 | 7.3 ± 0.04 | 7.3 ± 0.05 | 0.610 |
| Subjective evaluation | ||||||
| Sperm concentration (/mL) | 2.9 ± 0.26 | 3.6 ± 0.31 | 3.0 ± 0.29 | 2.3 ± 0.16 | 3.1 ± 0.20 | 0.012 |
| Semen colour (1–4) | 3.8 ± 0.08 | 3.6 ± 0.15 | 3.8 ± 0.13 | 3.7 ± 0.09 | 3.9 ± 0.06 | 0.320 |
| Sperm mass motility (1–5) | 4.3 ± 0.11 | 4.3 ± 0.12 | 4.2 ± 0.13 | 4.3 ± 0.14 | 4.2 ± 0.12 | 0.822 |
| Sperm PM (%) | 75.0 ± 1.02 | 75.0 ± 1.75 | 70.8 ± 1.96 | 71.1 ± 2.58 | 73.2 ± 1.90 | 0.343 |
| Objective evaluation (SCA®) | ||||||
| Sperm total motility (%) | 77.7 ± 1.79 | 71.7 ± 3.36 | 74.7 ± 2.82 | 76.3 ± 2.79 | 78.6 ± 2.95 | 0.415 |
| - Rapid motility (%) | 47.1 ± 2.88 | 45.1 ± 3.60 | 44.1 ± 3.73 | 46.8 ± 3.32 | 55.9 ± 3.18 | 0.099 |
| - Medium motility (%) | 7.1 ± 0.57 | 6.6 ± 0.54 | 7.6 ± 0.68 | 7.5 ± 0.59 | 5.4 ± 0.65 | 0.077 |
| - Slow motility (%) | 23.6 ± 1.47 | 20.1 ± 1.25 | 23.0 ± 1.28 | 22.1 ± 1.17 | 17.3 ± 1.31 | 0.005 |
| Sperm PM (%) | 50.1 ± 2.80 | 48.0 ± 3.52 | 48.0 ± 3.60 | 50.2 ± 3.30 | 58.3 ± 3.12 | 0.143 |
| - Rapid PM (%) | 29.9 ± 2.36 | 29.2 ± 2.47 | 27.8 ± 2.45 | 28.9 ± 2.02 | 35.7 ± 2.58 | 0.154 |
| - Medium PM (%) | 20.2 ± 1.49 | 18.8 ± 1.61 | 20.3 ± 2.13 | 21.3 ± 1.92 | 22.7 ± 2.25 | 0.658 |
| Sperm non-PM (%) | 27.7 ± 1.77 | 23.7 ± 1.49 | 26.7 ± 1.54 | 26.1 ± 1.34 | 20.2 ± 1.67 | 0.007 |
| Immotiltiy (%) | 22.3 ± 1.79 | 28.4 ± 3.36 | 25.4 ± 2.82 | 23.7 ± 2.79 | 21.4 ± 2.95 | 0.415 |
| Variable | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | 1.5 g TE | 3 g TE | 1.5 g ETE | 3 g ETE | ||
| Viability (%) | 72.3 ± 2.77 | 72.6 ± 2.48 | 75.5 ± 2.23 | 72.1 ± 2.81 | 70.8 ± 3.00 | 0.791 |
| Acrosome integrity (%) | 90.6 ± 0.84 | 87.9 ± 1.36 | 86.4 ± 1.49 | 87.5 ± 2.04 | 87.0 ± 1.96 | 0.366 |
| Total abnormalities (%) | 9.2 ± 1.37 | 7.3 ± 1.54 | 13.1 ± 2.74 | 6.7 ± 0.99 | 6.9 ± 1.36 | 0.048 |
| Head abnormalities (%) | 0.2 ± 0.09 | 0.3 ± 0.12 | 0.5 ± 0.31 | 0.3 ± 0.13 | 0.2 ± 0.10 | 0.777 |
| Midpiece abnormalities (%) | 5.1 ± 0.70 | 5.2 ± 1.12 | 7.3 ± 1.86 | 4.2 ± 0.63 | 3.4 ± 0.60 | 0.124 |
| Tail abnormalities (%) | 2.7 ± 0.81 | 1.4 ± 0.60 | 2.6 ± 0.76 | 1.3 ± 0.33 | 3.0 ± 1.02 | 0.351 |
| Cytoplasmic droplets (%) | 1.3 ± 0.34 | 0.4 ± 0.18 | 2.7 ± 0.83 | 0.9 ± 0.37 | 0.4 ± 0.17 | 0.002 |
| Treatments | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Tannin Type | Tannin Level (g/day) | Testicular Length (mm) | Semen Volume (mL) | Sperm Concentration (/mL) | Slow Motility Sperm (%) | Non-Progressive Motility Sperm (%) | Sperm with Cytoplasmic Droplet (%) |
| TE | 1.5 | 91.9 ± 1.17 | 2.5± 0.61 | 3.6 ± 0.03 | 20.1 ± 1.53 | 23.7 ± 1.68 | 0.4 ± 1.44 |
| 3 | 88.8 ± 0.95 | 1.7 ± 0.72 | 3.1 ± 0.03 | 23.0 ± 1.41 | 26.7 ± 1.58 | 2.7 ± 2.64 | |
| ETE | 1.5 | 88.6 ± 1.02 | 1.9 ± 0.74 | 2.4 ± 0.02 | 22.1 ± 1.32 | 26.1 ± 1.39 | 0.9 ± 1.97 |
| 3 | 93.3 ± 0.93 | 2.1 ± 0.95 | 3.2 ± 0.02 | 17.3 ± 1.72 | 20.2 ± 2.03 | 0.4 ± 1.47 | |
| ANOVA | p-value | p-value | p-value | p-value | p-value | p-value | |
| Tannin Type | 0.663 | 0.830 | 0.032 | 0.151 | 0.191 | 0.051 | |
| Tannin level | 0.535 | 0.113 | 0.585 | 0.459 | 0.347 | 0.068 | |
| Tannin type × Tannin level | 0.003 | 0.010 | 0.006 | 0.003 | 0.004 | 0.003 | |
| Variable | Bodyweight | Testicular Volume | Scrotal Circumference | Semen Volume | Sperm Concentration | pH | Mass Motility | Progressive Motility | Viability | Acrosome Integrity | Testosterone |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Testicular volume | 0.320 *** | 1 | |||||||||
| Scrotal circumference | 0.388 *** | 0.728 *** | 1 | ||||||||
| Semen volume | 0.266 * | 0.319 ** | 0.409 *** | 1 | |||||||
| Sperm concentration | 0.046 | 0.257 * | 0.231 * | 0.205 *** | 1 | ||||||
| pH | −0.188 | −0.175 | −0.112 | −0.139 | −0.056 | 1 | |||||
| Mass motility | 0.030 | −0.077 | 0.001 | −0.047 | 0.017 | −0.243 * | 1 | ||||
| Progressive motility | −0.052 | 0.152 | 0.081 | 0.104 | 0.212 * | −0.152 | 0.421 *** | 1 | |||
| Viability | 0.164 | −0.061 | −0.092 | −0.243 ** | −0.008 | −0.143 | 0.282 ** | 0.178 * | 1 | ||
| Acrosome integrity | −0.005 | −0.117 | −0.078 | −0.273 ** | −0.006 | −0.216 * | 0.327 *** | 0.196 * | 0.608 *** | 1 | |
| Testosterone | 0.205 | 0.033 | 0.019 | 0.202 | −0.164 | −0.312 | 0.068 | −0.140 | 0.162 | 0.165 | 1 |
| Cortisol | −0.028 | 0.196 | 0.295 * | 0.191 | −0.107 | 0.085 | −0.530 ** | −0.437 * | −0.537 ** | −0.594 ** | −0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.; Lehloenya, K.; Mphaphathi, M.; Hassen, A. Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams. Animals 2021, 11, 3266. https://doi.org/10.3390/ani11113266
Ahmed O, Lehloenya K, Mphaphathi M, Hassen A. Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams. Animals. 2021; 11(11):3266. https://doi.org/10.3390/ani11113266
Chicago/Turabian StyleAhmed, Osman, Khoboso Lehloenya, Masindi Mphaphathi, and Abubeker Hassen. 2021. "Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams" Animals 11, no. 11: 3266. https://doi.org/10.3390/ani11113266
APA StyleAhmed, O., Lehloenya, K., Mphaphathi, M., & Hassen, A. (2021). Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams. Animals, 11(11), 3266. https://doi.org/10.3390/ani11113266








