Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management, Housing, and Experimental Design
2.2. Diets and Corticosterone Challenge
2.3. Growth Rate Parameters and Animal Sampling
2.4. Histopathological Test of the Small Intestinal Villi Components, Muscle Myofibrils, and Liver
2.5. Meat Quality Test
2.5.1. Tissue Sample Collections
2.5.2. Muscle pH Measurement
2.5.3. Measurement of Drip Loss
2.5.4. Measurement of Cooking Loss
2.5.5. Measurement of Meat Tenderness
2.5.6. Measurement of Color
2.5.7. Determination of Crude Protein
2.5.8. Determination of Fat (Ether Extract)
2.5.9. Determination of Energy
2.5.10. Moisture Contents and Dry Matter
2.6. Determination of Telomere Length Using Real-Time Quantitative PCR (qPCR) Analysis
2.7. Gene Expression Analysis of Mitochondrial DNA Related Genes and Acute Phase Protein Factors Using Reverse Transcriptase (RT)-PCR
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Buffy Coat Telomere Length
3.2. Small Intestinal Duodenal, Jejunal, and Ileal Villi Height and Crypt Depth
3.3. Muscle Myofibrils and Myofibril Bundle Diameters
3.4. Liver Histopathology
3.5. Meat Quality Traits
3.6. Mitochondrial Genes
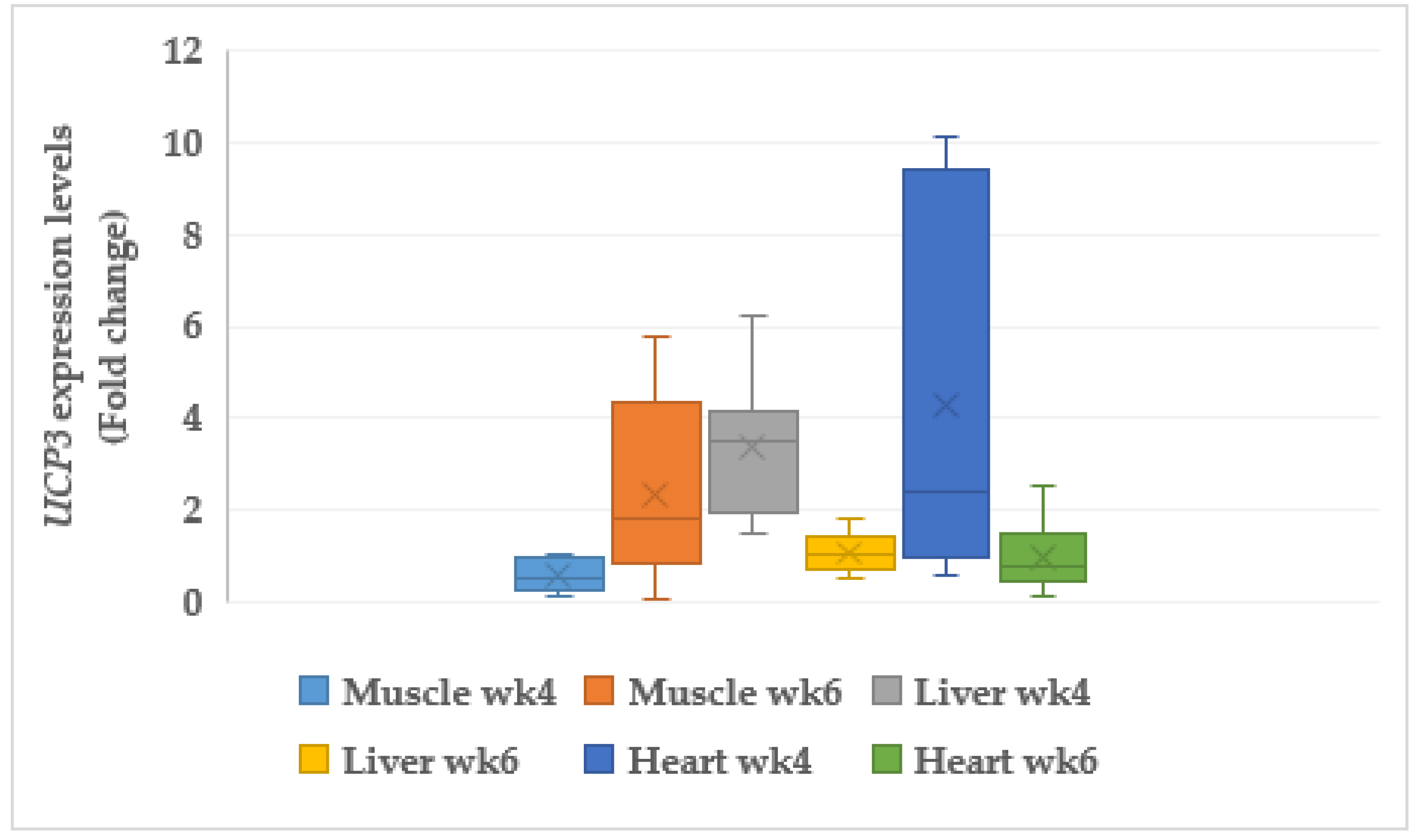
3.6.1. Uncoupling Protein 3 (UCP3)
3.6.2. Cytochrome C Oxidase (COX6A1)
3.7. Acute phase Protein
3.7.1. Serum Amyloid A like 1 (SAAL1)
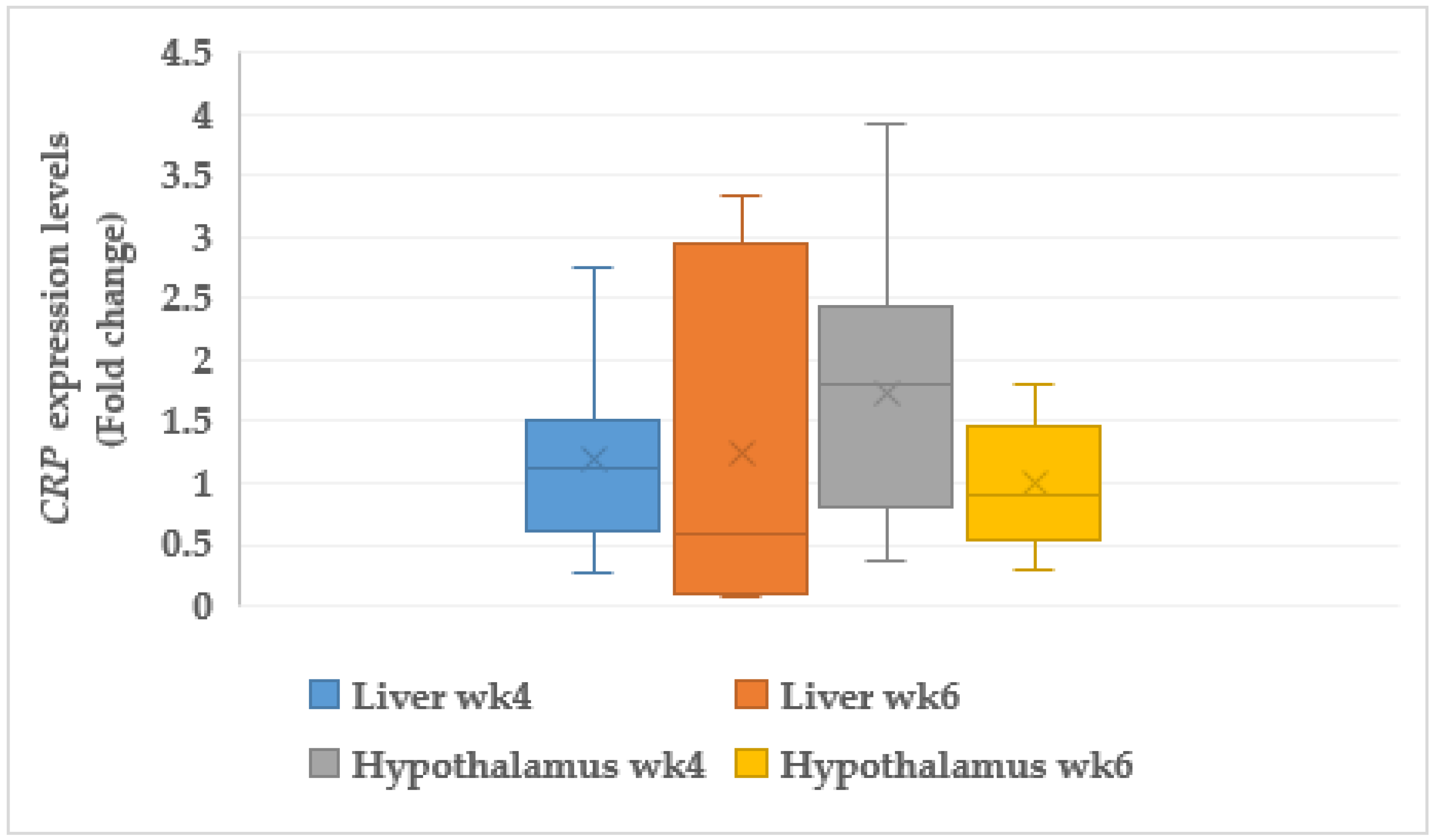
3.7.2. C-reactive Protein (CRP)
4. Discussion
4.1. Body Weight and Telomere Length
4.2. Intestinal Histopathology
4.3. Liver Histopathology
4.4. Muscle Histopathology
4.5. Meat Physical Components
4.6. Meat Biochemical Components
4.7. Relationship between Telomere Length and Meat Quality Traits
4.8. Mitochondria Genes
4.9. Acute Phase Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spratt, R.S.; McBride, B.W.; Bayley, H.S.; Leeson, S. Energy metabolism of broiler breeder hens. Contribution of issues to total heat production in fed and fasted hens. Poult. Sci. 1990, 69, 1348–1356. [Google Scholar] [CrossRef]
- Zulkifli, I.; Najafi, P.; Nurfarahin, A.J.; Soleimani, A.F.; Kumari, S.; Anna Aryan, A.; O’ Reilly, E.L.; Eckersalli, P.D. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult. Sci. 2014, 93, 3112–3118. [Google Scholar] [CrossRef]
- Hayash, K.; Kaneda, S.; Otsuka, A.; Tomita, Y. Effects of ambient temperature and thyroxine on protein turnover and oxygen consumption in chicken skeletal muscle. In Proceedings of the 19th World Poultry Congress, Amsterdam, The Netherlands, 19–24 September 1992; Volume 2, pp. 93–96. [Google Scholar]
- Gross, W.B.; Siegel, P.B.; DuBose, R.T. Some effects of feeding corticosterone to chicken. Poult. Sci. 1980, 59, 516–522. [Google Scholar] [CrossRef]
- Hu, X.F.; Guo, Y.M. Corticosterone administration alters small intestinal morphology and function of broiler chickens. Asian-Austr. J. Anim. Sci. 2008, 21, 1773–1778. [Google Scholar] [CrossRef]
- Gross, W.B.; Siegel, P.B. Some effects of feeding deoxycorticosterone to chickens. Poult. Sci. 1981, 60, 2232–2239. [Google Scholar] [CrossRef]
- Anama, A.; Eloy, R.; Benchet, P.; Clendinnen, G.; Grenier, J.F. Effects of oral and parenteral corticosteroids on intestinal villous morphology and brush border enzymes in the rat. Comp. Stud. 1979, 41, 83–88. [Google Scholar]
- Buts, J.P.; DeMeyer, R. Intestinal development in the suckling rat. Effect of weaning, diet composition, and glucocorticoids on thymidine kinase activity and DNA synthesis. Pediatr. Res. 1984, 18, 145–150. [Google Scholar] [CrossRef]
- Dong, H.; Lin, H.; Jiao, H.C.; Song, Z.G.; Zhao, J.P.; Jiang, K.J. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 189–195. [Google Scholar] [CrossRef]
- Klont, R.E.; Brocks, L.; Eikelenboom, G. Muscle fibre type and meat quality. Meat Sci. 1998, 48, S219–S229. [Google Scholar] [CrossRef]
- Kim, G.D.; Jeong, J.Y.; Moon, S.H.; Hwang, Y.H.; Park, G.B.; Joo, S.T. Effects of muscle fiber type on meat characteristics of chicken and duck breast muscle. 54th ICoMST 2008, 54, 10–15. [Google Scholar]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigm. J. Clin. Invest. 2013, 123, 996–1002. [Google Scholar] [CrossRef]
- Tomas, F.M. Effect of corticosterone on myofibrillar protein turnover in diabetic rats as assessed by Ntau-methylhistidine excretion. Biochem. J. 1982, 205, 593–601. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Kogut, M.H.; Arsenault, R.J. Editorial: Gut health: The new paradigm in food animal production. Front. Vet. Sci. 2016, 3, 71. [Google Scholar] [CrossRef]
- Nivedita, B.; Halcyon, G.S.; Kristin, L.; Russel, V.; Esha, B.; Lisa, A.B. Telomere and telomere dynamics: Relevance to cancer of the gastrointestinal tract. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 733–748. [Google Scholar]
- Pietro, C.; Viviane, V.; Calvo, P.; Jerome, S.; Anna-maria, K. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed Sci. Tech. 2018, 250, 9–31. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Chan, S.; Chang, J.; Fulton, T.B.; Krauskopf, A.; Mceachern, M.; Prescott, J.; Roy, J.; Smith, C.; Wang, H. Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 253–263. [Google Scholar] [CrossRef]
- Li, B.; Oestreich, S.; de Lange, T. Identification of human Rap1: Implications for telomere evolution. Cell 2000, 101, 471–483. [Google Scholar] [CrossRef]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Wong, J.Y.Y.; Vivo, I.D.; Lin, X.; Fang, S.C.; Christiani, D.C. The Relationship between Inflammatory Biomarkers and Telomere Length in an Occupational Prospective Cohort Study. PLoS ONE 2014, 9, e87348. [Google Scholar] [CrossRef]
- Herborn, K.A.; Heidinger, B.J.; Boner, W.; Noguera, J.C.; Adam, A.; Daunt, F.; Monaghan, P. Stress exposure in early post-natal life reduces telomere length: An experimental demonstration in a long-lived seabird. Proc. R. Soc. B: Biol. Sci. 2014, 281, 20133151. [Google Scholar] [CrossRef]
- Sebastiano, M.; Eens, M.; Angelier, F.; Pineau, K.; Chastel, O.; Costantini, D. Corticosterone, Inflammation, Immune Status and Telomere Length in frigatebird nestlings facing a severe herpesvirus infection. Conserv. Physiol. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. BMC 2011, 13, 3. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Diez-Roux, A.; Jenny, N.S.; Fitzpatrick, A.L.; Jacobs, D.R. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2008, 88, 1405–1412. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Kim, S.; Parks, C.G.; DeRoo, L.A.; Chen, H.; Taylor, J.A.; Cawthon, R.M.; Sandler, D.P. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol. Biomark. Prev. 2009, 18, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wray, C.; Tian, X.; Hasselgren, P.; Lu, J. Expression of uncoupling protein 3 is upregulated in skeletal muscle during sepsis. Am. J. Physiol. Endocrinol. Metab. 2003, 28, E512–E520. [Google Scholar] [CrossRef]
- Newmeyer, D.D.; Farscho, D.M.; Reed, J.C. Cell-free apoptosis in Xenopus egg extracts: Inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 1994, 79, 353–364. [Google Scholar] [CrossRef]
- Volanikis, J.E. Human C-reactive protein: Expression, structure and function. Mol. Immmunol. 2001, 38, 189197. [Google Scholar] [CrossRef]
- Hu, X.F.; Guo, Y.M.; Huang, B.Y.; Zhang, L.B.; Bun, S.; Liu, D. Effect corticosterone Administration on Small Intestinal Weight and Expression of Small Intestinal Nutrient Transporter mRNA of Broiler Chickens *. Asian-Austr. J. Anim. Sci. 2010, 23, 17. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H.; Abullah, N.R.; Ooi, M.F. Carcass, meat and bone quality of broiler chickens fed with postbiotic and prebiotic combination. Int. J. Probiotics Prebiotics 2015, 10, 23–30. [Google Scholar]
- Cavitt, L.C.; Youm, G.W.; Meullenet, J.F.; Owens, C.M.; Xiong, R. Prediction of poultry meat tenderness using razor blade shear, allo Kramer shear and sarcomere length. J. Food Sci. 2004, 69, 11–15. [Google Scholar] [CrossRef]
- Hunt, M.C. Meat color measurements. Proccedings of the Meat Conference, AMSA, Purdue University, Lafayette, Indiana, 6–8 October 1980; 1980; pp. 41–46. Available online: www.meatscience.org (accessed on 21 March 2021).
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression using real-time quantitative PCR and the 2-ΔΔCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis System Multiple Incorporation. In Users Guide Statistical Version; SAS Institute Inc: Cary, NC, USA, 2002. [Google Scholar]
- Falconer, D.S. Introduction to Quantitative Genetics, 3rd ed.; Longman Scientific and Technical: London, UK, 1989. [Google Scholar]
- Badmus, K.A.; Idrus, Z.; Goh, Y.M.; Qurni, S.A.; Mamat-Hamidi, K. Telomere Length and Regulatory Genes as Novel Stress Biomarkers and their Diversities in Broiler Chickens (Gallus gallus domesticus) Subjected to Corticosterone Feeding. Animals 2021, 11, 2759. [Google Scholar] [CrossRef] [PubMed]
- Elia, M. Organ and Tissue Contribution to Metabolic Rate. In Energy Metabolism. Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992; pp. 61–77. [Google Scholar]
- Holliday, M.A. Body Composition and Energy Needs During Growth. In Human Growth: A Comprehensive Treatise; Fulkner, F., Tanner, J.M., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 101–117. [Google Scholar]
- Vicuna, E.A.; Kuttappan, V.A.; Galarza-Seeber, R.; Latorre, J.D.; Faulkner, O.B.; Hargis, B.M.; Tellez, G.; Bielke, L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015, 94, 2075–2080. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Evans, M.E.; Fernandez-Estivariz, C.; Jones, D.P. Trophic and cytoprotective nutrition for intestinaladaptation, mucosal repair, and barrier function. Annu. Rev. Nutr. 2003, 23, 229–261. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kamisoyama, H.; Isshiki, Y. Effects of fasting and refeeding on structures of the intestinal villus and epithelial cell in White Leghorn hens. Br. Poult. Sci. 1996, 37, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Gal-Garber, O.; Mabjeesh, S.J.; Sklan, D.; Uni, Z. Partialsequence and expression of the gene for and activity of the sodium glucose transporter in the small intestine of fed, starved and refed chickens. J. Nutr. 2000, 130, 2174–2179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Howard, T.; Goodlad, R.A.; Walters, J.R.F.; Ford, D.; Hirst, B.H. Increased expression of specific intestinal amino acid and Peptide transporter mRNA in rats fed by TPN is reversed by GLP-2. J. Nutr. 2004, 134, 2957–2964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchell, M.A.; Carlisle, A.J. The effect of chronic exposure to elevated environmental temperature on intestinal morphology and nutrient absorption in the domestic fowl (Gallus domesticus). Comp. Biochem. Physiol. 1992, 101, 137–142. [Google Scholar] [CrossRef]
- Saeid, J.M.; Mohamed, A.B.; Al-Baddy, M.A. Effect of garlic powder (Allium sativum) and black seed (Nigella sativa) on broiler growth performance and intestinal morphology. Iran. J. Appl. Anim. Sci. 2013, 3, 185–188. [Google Scholar]
- Nasir, A.; Moudgal, R.P.; Singh, N.B. Involvement of corticosterone in food intake, food passage time and in vivo uptake of nutrients in the chicken (Gallus domesticus). Br. Poult. Sci. 1999, 40, 517–522. [Google Scholar] [CrossRef]
- Mathew, A.; Evan, B.J. Practice of Toxicologic Pathology. Hascheck and Rousseaux’s Handbook of Toxicologic pathology, 3rd ed.; Elsevier: New York, NY, USA, 2013; Volume 46, pp. 2089–2095. [Google Scholar]
- Balcom, J.H.; Keck, T.; Warshaw, A.L.; Antoniu, B.; Graeme-Cook, F.; Fernandez-del Castillo, C. Telomerase activity in periampullary tumors correlates with aggressive malignancy. Ann. Surg. 2001, 234, 344–350. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M. The roles of telomeres and telomerase in cell life span. Mutat. Res. 1996, 366, 45–63. [Google Scholar] [CrossRef]
- Thomas, B.G.; Kleiner, D.E. Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Metabolism 2016, 65, 1080–1086. [Google Scholar] [CrossRef]
- Donati, B.; Valenti, L. Telomeres, NAFLD and Chronic Liver Disease. Int. J. Mol. Sci. 2016, 3, 383. [Google Scholar] [CrossRef]
- Ramírez, R.; Carracedo, J.; Jiménez, R.; Canela, A.; Herrera, E.; Aljama, P.; Blasco, M.A. Massive telomere loss is an early event of DNA damage-induced apoptosis. J. Biol. Chem. 2003, 278, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Telomeres, telomerase and cancer. Sci. Am. 1996, 274, 92–97. [Google Scholar] [CrossRef]
- DePinho, R.A. The age of cancer. Nature 2000, 408, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Yu, M.C.; Mack, T.M. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int. J. Cancer 1997, 70, 512–517. [Google Scholar] [CrossRef]
- Broberg, K.; Bjork, J.; Paulsson, K.; Hoglund, M.; Albin, M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. J. Carcinog. 2005, 26, 1263–1271. [Google Scholar] [CrossRef]
- Shao, L.; Wood, C.G.; Zhang, D.; Tannir, N.M.; Matin, S.; Dinnet, C.P.; Wu, X. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J. Urol. 2007, 178, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Choi, Y.Y.; Lee, W.K.; Choi, J.E.; Cha, S.I.; Kim, Y.J.; Kim, C.H.; Kam, S.; Jung, T.H.; Park, J.Y. Telomere length and the risk of lung cancer. Cancer Sci. 2008, 99, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Risques, R.A.; Vaughan, T.L.; Li, X.; Odze, R.D.; Blount, P.L.; Ayub, K.; Rabinovitch, P.S. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Santiago, S.; Mercedes, M.; Hamish, N.M.; Vernon, R.Y. Effect of corticosterone and its route of administration on muscle protein breakdown, measured in vivo by urinary excretion of N-methylhistidine in rats; Response to different levels of dietary protein and energy. Metabolism 1981, 30, 798–804. [Google Scholar]
- Ishamri, I.; Seon-Tea, J. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Korean J. Food Sci. An. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- McKee, S.R.; Sams, A.R. The effect of seasonal heat stress on rigor development and the incidence of pale, exudative turkey meat. Poult. Sci. 1997, 76, 1616–1620. [Google Scholar] [CrossRef]
- Gao, J.; Lin, H.; Song, Z.G.; Jiao, H.C. Corticosterone Alters Meat Quality by Changing Pre-and Postslaughter Muscle Metabolism. Poult. Sci. 2008, 87, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Andreotti, M.; Macchia, D.; Spada, M.; Stefano, F. In vivo antiaging effects of alkaline water supplementation. J. Enzym. Inhib. Med. Chem. 2020, 35, 657–664. [Google Scholar] [CrossRef]
- Barbut, S. Microstructure of white and dark turkey meat batters as affected by pH. Poult. Sci. 1997, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Pevzner, I.B.; Isaev, N.K.; Zorov, D.B. Myoglobin causes oxidative stress, increase of.NO production and dysfunction of kidney’s mitochondria. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 796–803. [Google Scholar] [CrossRef]
- Khadijah, N. Physiological Stress Responses and Meat Quality of Rabbits Subjected to Different Road Transportation and Slaughter Methods. Ph.D. Thesis, University Putra, Kembangan, Malaysia, 2014. [Google Scholar]
- Boukhris, H.; Damergi, C.; Najar, T.; Samet, A. Transport stress impact on postmortem metabolisms of turkey meat quality. J. New Sci. 2017, 37, 2049–2054. [Google Scholar]
- Bihan, L.E.; Duval, E. Genetic variability within and between breeds of poultry1technological meat quality. Poult. Sci. J. 2004, 60, 331–340. [Google Scholar] [CrossRef]
- Diaz, O.; Rodriguez, L.; Torres, A.; Cobos, A. Chemical composition and physicochemical properties of meat from capons as affected by breed and age. Span. J. Agri. Res. 2010, 8, 1695–1971. [Google Scholar] [CrossRef]
- Wattanachaut, S.; Benjakul, S.; Ledward, D.A. Composition, Colour and Texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, S.; Xue, Y.; Tao, J.; Li, F.; Chen, Y. Preferential extension of short telomeres induced by low extracellular pH. Nucleic Acid Res. 2016, 44, 8086–8096. [Google Scholar] [CrossRef]
- O’ Callaghan, N.J.; Shusuke, T.; Anthony, R.B.; David, L.T. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin. Nutr. 2011, 31, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rider, O.J.; Cox, P.; Tyler, D.; Clarke, K.; Neubauer, S. Myocardial substrate metabolism in obesity. Int. J. Obes. 2013, 37, 972–979. [Google Scholar] [CrossRef]
- Park, H.J.; Lyons, J.C.; Song, C.W. Acidic environment causes apoptosis by increasing caspase activity. Br. J. Cancer 1999, 80, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Je’zek, P.; Hlavat´a, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Liu, S.; Geng, B.; Zou, L. Development of hypertrophic cardiomyopathy in perilipin-1 null mice with adipose tissue dysfunction. Cardiovasc. Res. 2015, 105, 20–30. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, K.R.; Russell, R.R. Uncoupling Proteins in Heart Failure. Curr. Heart Fail. Rep. 2009, 5, 75–79. [Google Scholar] [CrossRef]
- Fleury, C.; Neverova, M.; Collins, S. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, R.E.; Dembski, M.; Weng, X. Cloning and characterization of an uncoupling protein homolog: A potential molecular mediator of human thermogenesis. Diabetes 1997, 46, 900. [Google Scholar] [CrossRef] [PubMed]
- Hinz, W.; Gruninger, S.; De Pover, A.; Chiesi, M. Properties of the human long and short isoforms of the uncoupling protein-3 expressed in yeast cells. FEBS Lett. 1999, 462, 411. [Google Scholar] [CrossRef]
- Spiers, J.G.; Chen, H.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Anderson, R.E.; Watson, G.C.; Radda, G.K.; Clarke, K. Uncoupling proteins in human heart. Lancet 2004, 364, 1786–1788. [Google Scholar] [CrossRef]
- Sun, L.; Richard, D.Y. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Opdenakker, G.; Struyf, S.; Damme Van, J. Structure and Expression of Different Serum Amyloid A (SAA) Vari- ants and their Concentration-Dependent Functions During Host Insults. Curr. Med. Chem. 2016, 23, 1725–1755. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W.; Mold, C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. J. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Pepys, M.B.; Wood, S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 1999, 7, 169–177. [Google Scholar] [CrossRef]



| Composition (%) | Starter 1 | Finisher 2 |
|---|---|---|
| Crude protein | 23.00 | 19.00 |
| Crude fiber | 5.00 | 5.00 |
| Crude fat | 5.00 | 5.00 |
| Moisture | 13.00 | 13.00 |
| Ash | 8.00 | 8.00 |
| Calcium | 0.80 | 0.80 |
| Phosphorous | 0.40 | 0.40 |
| S/N | Genes | Primer Sequence | Accession Number | Size (bp) |
|---|---|---|---|---|
| 1 | Telomere | F-GGTTTTT-GAGGGTGAGGGTGAGGGTGAGGGTGAGGGT R-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC-CTA | NA | 79 |
| 2 | COX6A1 | F-TTCGACTGGGAGGACCATAG R-TGTTTTTCTGGGACACACCA | NC-006102.5 | 166 |
| 3 | UCP3 | F-AAGGATGGAGGTGTCCACAG R-GTGAGGAATACCCGGACTCA | NC-006088.5 | 161 |
| 4 | SAAL1 | F-GCCACCTCTCAAACTCTTGC R-CCTGCTTGTTTCCAGGAGAG | NC-006092.5 | 240 |
| 5 | CRP | F-CCTAGGAGAACTGGGGAAGG R-CGTAGGAGAAGAGGCTGTGG | NC-006112.4 | 206 |
| 6 | GAPDH | F-ACTATGCGGTTCCCAGTGTC- R-TGCCACCATCAGAAAAATGA | NC-006088.5 | 215 |
| 7 | β-Actin | F-ACTGGATTTCGAGCAGGAGA R-CAGTGGAATGGGACAGACCT | NC-006101.5 | 248 |
| Age | Treatment | Body Weight (g) | Telomere Length (Kb/Diploid Genome) |
|---|---|---|---|
| Week 4 | CTRL | 1509.36 b ± 49.15 | 248.23 ab ± 155.67 |
| CORT | 1054.21 c ± 55.82 | 214.74 b ± 111.17 | |
| Week 6 | CTRL | 2363.55 a ± 100.11 | 466.36 a ± 262.56 |
| CORT | 1479.18 b ± 354.72 | 235.44 ab ± 81.37 | |
| p values | Age | <0.0001 | 0.035 |
| Treatment | <0.0001 | 0.020 | |
| Age × Treatment | <0.0001 | 0.78 |
| Age | Treatment | Duodenum Villi Height (µm) | Jejunum Villi Height (µm) | Ileum Villi Height (µm) | Relative Feed Intake |
|---|---|---|---|---|---|
| Week 4 | CTRL | 508.78 b± 118.67 | 380.95 b ± 81.72 | 302.55 b ± 77.20 | 65.17 b ± 1.29 |
| CORT | 633.16 a ± 134.95 | 441.96 ab ± 125.06 | 319.27 b ± 115.40 | 80.11 a ± 3.81 | |
| Week 6 | CTRL | 457.99 b ± 105.38 | 578.36 a ± 93.50 | 401.54 a ± 84.16 | 44.70 d ± 1.17 |
| CORT | 496.00 b ± 155.49 | 608.22 a ± 130.71 | 388.00 a ± 137.95 | 55.59 c ± 2.14 | |
| p value | Age | 0.033 | <0.0001 | 0.0001 | <0.0001 |
| Treatment | 0.033 | 0.050 | 0.938 | <0.0001 | |
| Age × Treatment | 0.195 | 0.555 | 0.474 | 0.103 |
| Age | Treatment | Duodenum Villi CD (µm) | Jejunum Villi CD (µm) | Ileum Villi CD (µm) |
|---|---|---|---|---|
| Week 4 | CTRL | 68.14 ab ± 18.18 | 68.59 ± 15.49 | 50.83 ab ± 13.08 |
| CORT | 80.17 a ± 18.40 | 59.38 ± 13.60 | 61.20 a ± 23.17 | |
| Week 6 | CTRL | 67.23 ab ± 23.60 | 57.67 ± 7.72 | 56.93 ab ± 9.63 |
| CORT | 54.75 b ± 11.56 | 62.80 ± 20.43 | 48.46 b ± 11.79 | |
| p value | Age | 0.025 | 0.564 | 0.359 |
| Treatment | 0.475 | 0.886 | 0.752 | |
| Age × Treatment | 0.029 | 0.129 | 0.021 |
| Age | Treatment | Myofibril (µm) | Myofibril Bundle (µm) |
|---|---|---|---|
| Week 4 | CTRL | 74.29 a ± 10.88 | 568.20 a ± 272.29 |
| CORT | 52.89 b ± 3.27 | 427.50 b ± 136.89 | |
| Week 6 | CTRL | 88.96 b ± 10.68 | 773.90 a ± 176.68 |
| CORT | 79.61 ab ± 6.35 | 442.60 b ± 153.69 | |
| p value | Age | <0.0001 | 0.134 |
| Treatment | <0.0002 | <0.003 | |
| Age × Treatment | 0.827 | 0.194 |
| Symptoms/Grades | CTRL | CORT |
|---|---|---|
| Week 2 | ||
| Fibrosis | 0 | 2 |
| Ballooning | 0 | 2 |
| Inflammation | 0 | 2 |
| Steatosis | 0 | 3 |
| NAFL | 0 | Severe |
| Week 4 | ||
| Fibrosis | 0 | 2 |
| Ballooning | 0 | 2 |
| Inflammation | 0 | 2 |
| Steatosis | 0 | 3 |
| NAFL | 0 | Severe |
| Age | Treatment | pH | L * | a * | b * | DL% | CL% | SF (Kg) |
|---|---|---|---|---|---|---|---|---|
| Week 4 | CTRL | 5.70 b ± 0.12 | 53.84 a ± 3.07 | 7.39 a ± 1.11 | 21.93 b ± 1.00 | 1.95 c ± 0.47 | 30.83 a ± 9.97 | 1.56 ab ± 0.39 |
| CORT | 5.46 a ± 0.09 | 54.18 a ± 2.21 | 7.60 a ± 1.27 | 23.82 a ± 1.67 | 3.11 b ± 0.81 | 25.47 a ± 3.57 | 1.61 ab ± 0.49 | |
| Week 6 | CTRL | 5.81 b ± 0.20 | 55.01 a ± 3.38 | 5.34 b ± 1.80 | 20.73 b ± 2.78 | 3.57 b ± 0.59 | 31.14 a ± 8.82 | 1.33 b ± 0.16 |
| CORT | 6.13 a ± 0.26 | 50.22 b ± 3.91 | 6.95 a ± 2.06 | 20.73 b ± 2.92 | 5.16 a ± 1.53 | 29.93 a ± 8.21 | 1.75 a ± 0.48 | |
| p value | Age | 0.0001 | 0.046 | 0.0001 | 0.0001 | 0.0001 | 0.514 | 0.674 |
| Treatment | 0.345 | 0.0003 | 0.002 | 0.047 | 0.0001 | 0.110 | 0.043 | |
| Age × Treatment | 0.0001 | 0.0001 | 0.021 | 0.029 | 0.518 | 0.257 | 0.090 |
| Age | Treatment | CP | Energy | EE | MC | DM |
|---|---|---|---|---|---|---|
| Week 4 | CTRL | 19.37 ± 3.58 | 20.67 b ± 0.41 | 5.29 b ± 1.73 | 74.32 a ± 1.50 | 25.68 b ± 1.50 |
| CORT | 18.04 ± 0.94 | 20.90 ab ± 0.52 | 6.81 ab ± 2.22 | 72.89 b ± 1.51 | 27.11 a ± 1.51 | |
| Week 6 | CTRL | 18.80 ± 0.92 | 21.48 a ± 0.24 | 8.57 a ± 3.15 | 74.14 a ± 0.52 | 25.86 b ± 0.52 |
| CORT | 18.50 ± 0.63 | 21.15 ab ± 0.62 | 8.41 a ± 1.33 | 72.84 b ± 1.06 | 27.16 a ± 1.06 | |
| p value | Age | 0.879 | 0.014 | 0.017 | 0.782 | 0.768 |
| Treatment | 0.310 | 0.746 | 0.480 | 0.002 | 0.002 | |
| Age × Treatment | 0.468 | 0.197 | 0.389 | 0.878 | 0.878 |
| Telo | pH | SF | DL | CL | L * | a * b * | |
|---|---|---|---|---|---|---|---|
| Telo | |||||||
| pH | 0.548 * | ||||||
| SF | −0.091 | −0.259 | |||||
| DL | −0.496 * | −0.642 * | −0.387 | ||||
| CL | 0.183 | 0.238 | −0.026 | −0.570 * | |||
| L * | −0.196 | −0.133 | 0.589 | −0.127 | 0.132 | ||
| a * | −0.103 | 0.144 | −0.460 | −0.096 | −0.092 | −0.480 | |
| b * | 0.091 | −0.347 | 0.250 | 0.056 | −0.208 | 0.597 | −0.014 |
| Prediction Model | p Value | |
|---|---|---|
| Telomere and pH | TeloWK4 = 5.426 + 0.00042 pH | 0.028 |
| Telomere and DL | TeloWK4 = 3.392 − 0.0022 DL | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badmus, K.A.; Idrus, Z.; Meng, G.Y.; Mamat-Hamidi, K. Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus). Animals 2021, 11, 3276. https://doi.org/10.3390/ani11113276
Badmus KA, Idrus Z, Meng GY, Mamat-Hamidi K. Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus). Animals. 2021; 11(11):3276. https://doi.org/10.3390/ani11113276
Chicago/Turabian StyleBadmus, Kazeem Ajasa, Zulkifli Idrus, Goh Yong Meng, and Kamalludin Mamat-Hamidi. 2021. "Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus)" Animals 11, no. 11: 3276. https://doi.org/10.3390/ani11113276
APA StyleBadmus, K. A., Idrus, Z., Meng, G. Y., & Mamat-Hamidi, K. (2021). Telomere Length, Apoptotic, and Inflammatory Genes: Novel Biomarkers of Gastrointestinal Tract Pathology and Meat Quality Traits in Chickens under Chronic Stress (Gallus gallus domesticus). Animals, 11(11), 3276. https://doi.org/10.3390/ani11113276






