Age-Dependent Intestinal Repair: Implications for Foals with Severe Colic
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Anatomy of Small Intestinal Colic Injury in Foals
3. Efficient Mucosal Repair Limits Morbidity from Colic
3.1. Evidence of Age-Dependent Barrier Repair: A Comparative Pig Model
3.2. A Complex Mucosal Microenvironment Regulates Epithelial Repair
4. Factors Influencing Postnatal Maturation of Intestinal Repair Mechanisms
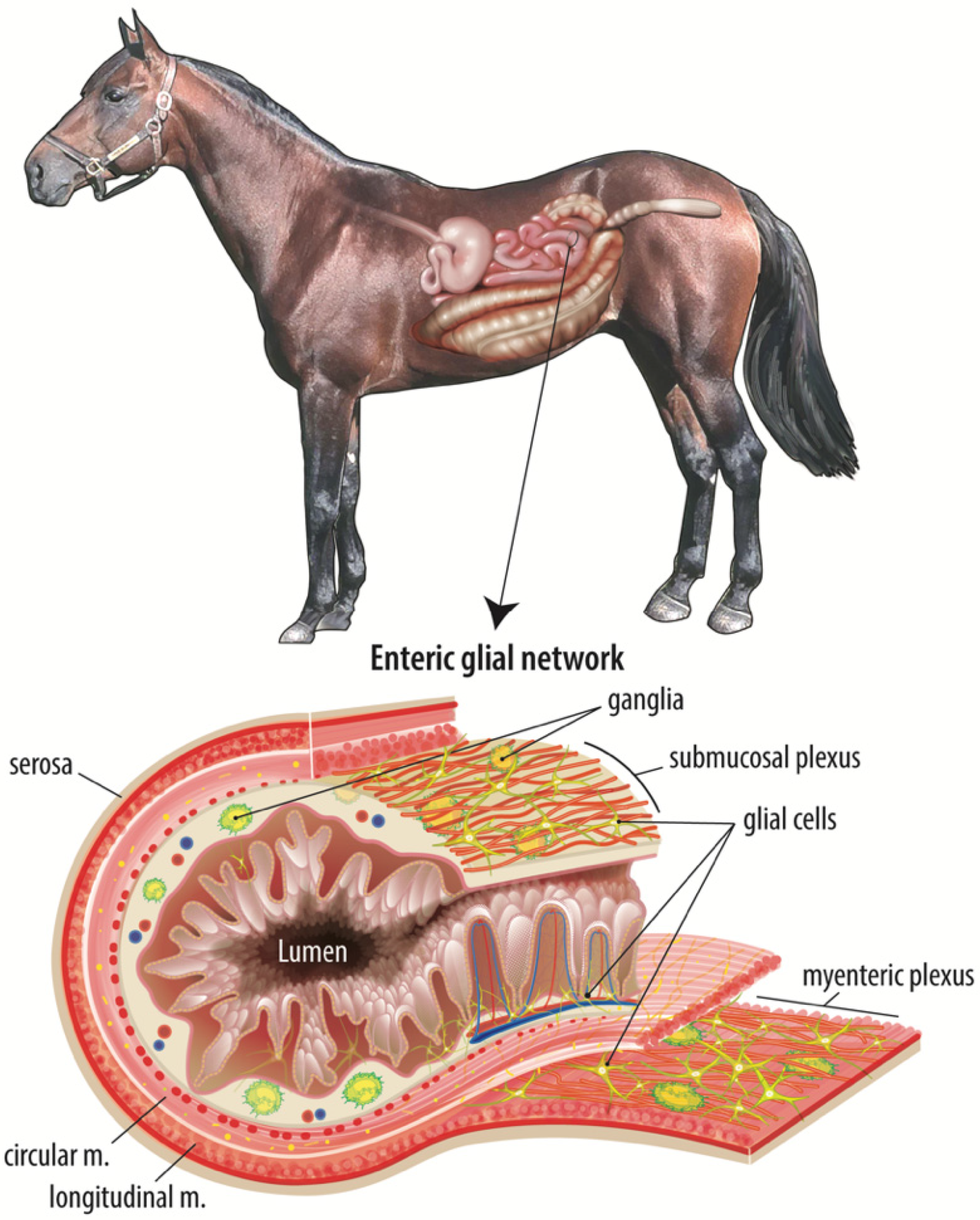
4.1. Enteric Nervous System-Microbiome Interactions
4.2. Microbiota
4.3. Nutrition
4.4. Early Life Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baseline Reference of Equine Health and Management in the United States, 2015. Available online: https://www.aphis.usda.gov/animal_health/nahms/equine/downloads/equine05/Equine05_is_PartI_Highlights.pdf (accessed on 10 May 2020).
- Vatistas, N.J.; Snyder, J.R.; Wilson, W.D.; Drake, C.; Hildebrand, S. Surgical treatment for colic in the foal (67 cases): 1980–1992. Equine Vet. J. 1996, 28, 139–145. [Google Scholar] [CrossRef]
- Ziegler, A.L.; Pridgen, T.A.; Mills, J.K.; Gonzalez, L.M.; Van Landeghem, L.; Odle, J.; Blikslager, A.T. Epithelial restitution defect in neonatal jejunum is rescued by juvenile mucosal homogenate in a pig model of intestinal ischemic injury and repair. PLoS ONE 2018, 13, e0200674. [Google Scholar] [CrossRef] [Green Version]
- Mannoia, K.; Boskovic, D.S.; Slater, L.; Plank, M.S.; Angeles, D.M.; Gollin, G. Necrotizing enterocolitis is associated with neonatal intestinal injury. J. Pediatr. Surg. 2011, 46, 81–85. [Google Scholar] [CrossRef]
- Lim, J.C.; Golden, J.M.; Ford, H.R. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 2015, 31, 509–518. [Google Scholar] [CrossRef]
- Alvarez, J.; Sarradell, J.; Morrison, R.; Perez, A. Impact of Porcine Epidemic Diarrhea on Performance of Growing Pigs. PLoS ONE 2015, 10, e0120532. [Google Scholar] [CrossRef]
- Cavin, J.-B.; Cuddihey, H.; Macnaughton, W.K.; Sharkey, K. Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: The role of the enteric nervous system. Am. J. Physiol. Liver Physiol. 2020, 318, G254–G264. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar]
- McCann, C.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.R.; Rill, B.K.; Carlson, S.L.; Carnes, D.; Kerner, R.; Mrsny, R.J.; Madara, J.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. Content 1997, 273, C1378–C1385. [Google Scholar] [CrossRef]
- White, N.A.; Moore, J.N.; Mair, T.S. Equine Acute Abdomen; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Granger, D.N.; Holm, L.; Kvietys, P. The Gastrointestinal Circulation: Physiology and Pathophysiology. Compr. Physiol. 2015, 5, 1541–1583. [Google Scholar] [CrossRef]
- Chiu, C.-J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Bellamy, J.E.C.; Nielsen, N.O.; Latshaw, W.K. The Vascular Architecture of the Porcine Small Intestine. Can. J. Comp. Med. Rev. Can. Med. Comp. 1973, 37, 56–62. [Google Scholar]
- Aharinejad, S.; Lametschwandtner, A.; Franz, P.; Firbas, W. The vascularization of the digestive tract studied by scanning electron microscopy with special emphasis on the teeth, esophagus, stomach, small and large intestine, pancreas, and liver. Scanning Microsc. 1991, 5, 811–849. [Google Scholar] [PubMed]
- Blikslager, A.T.; Moeser, A.; Gookin, J.; Jones, S.; Odle, J. Restoration of Barrier Function in Injured Intestinal Mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Derikx, J.P.M.; Matthijsen, R.A.; De Bruïne, A.P.; Van Bijnen, A.A.; Heineman, E.; Van Dam, R.M.; DeJong, C.H.C.; Buurman, W.A. Rapid Reversal of Human Intestinal Ischemia-Reperfusion Induced Damage by Shedding of Injured Enterocytes and Reepithelialisation. PLoS ONE 2008, 3, e3428. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, C.; Liu, X.; Qu, L.; Gu, L.; Li, N.; Li, J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J. Cell. Mol. Med. 2009, 13, 4061–4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.M.; Muir, W.W.; Granger, D.N. Mechanisms of Gastrointestinal Ischemia-Reperfusion Injury and Potential Therapeutic Interventions: A Review and Its Implications in the Horse. J. Veter. Intern. Med. 1995, 9, 115–132. [Google Scholar] [CrossRef]
- Blikslager, A.T.; Roberts, M.C.; Gerard, M.P.; A Argenzio, R. How important is intestinal reperfusion injury in horses? J. Am. Veter. Med. Assoc. 1997, 211, 1387–1389. [Google Scholar]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [Green Version]
- Feil, W.; Lacy, E.R.; Wong, Y.-M.M.; Burger, D.; Wenzl, E.; Starlinger, M.; Schiessel, R. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology 1989, 97, 685–701. [Google Scholar] [CrossRef]
- Feil, W.; Wenzl, E.; Vattay, P.; Starlinger, M.; Sogukoglu, T.; Schiessel, R. Repair of rabbit duodenal mucosa after acid injury in vivo and in vitro. Gastroenterology 1987, 92, 1973–1986. [Google Scholar] [CrossRef]
- Nusrat, A.; Delp, C.; Madara, J.L. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J. Clin. Investig. 1992, 89, 1501–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Blikslager, A.T. Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am. J. Physiol. Physiol. 2016, 311, C996–C1004. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Sladojevic, N.; Keep, R.F.; Andjelkovic, A.V. Endocytosis of tight junction proteins and the regulation of degradation and recycling. Ann. N. Y. Acad. Sci. 2017, 1397, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Feier, D.; Birkl, D.; Agarwal, R.; Zhou, D.W.; García, A.J.; Parkos, C.A.; Nusrat, A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc. Natl. Acad. Sci. USA 2020, 117, 9477–9482. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakamura, T.; Sato, T.; Nemoto, Y.; Mizutani, T.; Zheng, X.; Ichinose, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 2012, 18, 618–623. [Google Scholar] [CrossRef]
- Pohl, C.S.; Medland, J.E.; Moeser, A.J. Early-life stress origins of gastrointestinal disease: Animal models, intestinal pathophysiology, and translational implications. Am. J. Physiol. Liver Physiol. 2015, 309, G927–G941. [Google Scholar] [CrossRef] [Green Version]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blisklager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Blikslager, A.T.; Roberts, M.C.; Rhoads, J.; A Argenzio, R. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery 1997, 121, 526–534. [Google Scholar] [CrossRef]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; Dewilde, M.P.; Williams, K.J.; Moeser, A.J. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 2017, 29, e13118. [Google Scholar] [CrossRef] [PubMed]
- Medland, J.E.; Pohl, C.S.; Edwards, L.L.; Frandsen, S.; Bagley, K.; Li, Y.; Moeser, A.J. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol. Motil. 2016, 28, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, W.; Guo, M.; Han, Z.; Wang, Y.; Yang, P.; Xu, C.; Wang, Q.; Du, L.; Li, Q.; Zhao, H.; et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016, 7, e2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sémont, A.; Mouiseddine, M.; François, A.; Demarquay, C.; Mathieu, N.; Chapel, A.; Saché, A.; Thierry, D.; Laloi, P.; Gourmelon, P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2009, 17, 952–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocansey, D.W.; Wang, L.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Mesenchymal stem cell–gut microbiota interaction in the repair of inflammatory bowel disease: An enhanced therapeutic effect. Clin. Transl. Med. 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Swerlick, R.A.; Lawley, T.J. Role of Microvascular Endothelial Cells in Inflammation. J. Investig. Dermatol. 1993, 100, S111–S115. [Google Scholar] [CrossRef] [Green Version]
- Saps, M.; Miranda, A. Gastrointestinal Pharmacology. Mediat. Drugs Gastrointest. Motil. I 2017, 239, 147–176. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, S.T.N.S.J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Caputo, F.J.; Xu, D.-Z.; Deitch, E.A. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock 2008, 29, 372–376. [Google Scholar] [CrossRef]
- Rupani, B.; Caputo, F.J.; Watkins, A.; Vega, D.; Magnotti, L.J.; Lu, Q.; Xu, D.Z.; Deitch, E.A. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery 2007, 141, 481–489. [Google Scholar] [CrossRef]
- Nagy, N.; Goldstein, A.M. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 2017, 66, 94–106. [Google Scholar] [CrossRef]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuckeroth, R.; Pachnis, V. Getting to the guts of enteric nervous system development. Development 2006, 133, 2287–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husso, A.; Jalanka, J.; Alipour, M.J.; Huhti, P.; Kareskoski, M.; Pessa-Morikawa, T.; Iivanainen, A.; Niku, M. The composition of the perinatal intestinal microbiota in horse. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rose, E.; Odle, J.; Blikslager, A.; Ziegler, A. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Li, G.; Goebel, A.; Raju, A.V.; Kong, F.; Lv, Y.; Li, K.; Zhu, Y.; Raja, S.; He, P.; et al. Caspase-11–mediated enteric neuronal pyroptosis underlies Western diet–induced colonic dysmotility. J. Clin. Investig. 2020, 130, 3621–3636. [Google Scholar] [CrossRef]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossais, F.; Durand, T.; Chevalier, J.; Boudaud, M.; Kermarrec, L.; Aubert, P.; Neveu, I.; Naveilhan, P.; Neunlist, M. Postnatal development of the myenteric glial network and its modulation by butyrate. Am. J. Physiol. Liver Physiol. 2016, 310, G941–G951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota Controls the Homeostasis of Glial Cells in the Gut Lamina Propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Kabouridis, P.S.; Pachnis, V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Investig. 2015, 125, 956–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammarota, G.; Ianiro, G.; Cianci, R.; Bibbò, S.; Gasbarrini, A.; Curro’, D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol. Ther. 2015, 149, 191–212. [Google Scholar] [CrossRef]
- Scott, F.W.; Pound, L.D.; Patrick, C.; Eberhard, C.E.; Crookshank, J.A. Where genes meet environment—integrating the role of gut luminal contents, immunity and pancreas in type 1 diabetes. Transl. Res. 2017, 179, 183–198. [Google Scholar] [CrossRef]
- Losurdo, G.; Principi, M.; Iannone, A.; Ierardi, E.; Di Leo, A. The Interaction Between Celiac Disease and Intestinal Microbiota. J. Clin. Gastroenterol. 2016, 50, S145–S147. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Fintl, C.; Lindberg, R.; Press, C.M. Myenteric networks of interstitial cells of Cajal are reduced in horses with inflammatory bowel disease. Equine Veter. J. 2020, 52, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Mullen, K.R.; Yasuda, K.; Divers, T.J.; Weese, J.S. Equine faecal microbiota transplant: Current knowledge, proposed guidelines and future directions. Equine Veter. Educ. 2016, 30, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Hastie, P.; Murray, J.-A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Veter. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef]
- Quercia, S.; Freccero, F.; Castagnetti, C.; Soverini, M.; Turroni, S.; Biagi, E.; Rampelli, S.; Lanci, A.; Mariella, J.; Chinellato, E.; et al. Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals. Equine Veter. J. 2019, 51, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Stampfli, H.R.; Allenvercoe, E.; Weese, J.S. Development of the faecal microbiota in foals. Equine Veter. J. 2016, 48, 681–688. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothoulakis, C.; Lamont, J.T. Microbes and microbial toxins: Paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G178–G183. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Woliński, J.; Słupecka, M.; Weström, B.; Prykhodko, O.; Ochniewicz, P.; Arciszewski, M.; Ekblad, E.; Szwiec, K.; Ushakova; Skibo, G.; et al. Effect of feeding colostrum versus exogenous immunoglobulin G on gastrointestinal structure and enteric nervous system in newborn pigs1. J. Anim. Sci. 2012, 90, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, S.K.; Odle, J. Nutritinal Factors Influencing Intestinal Health of the Neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Moeser, A.; Klok, C.V.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Liver Physiol. 2007, 292, G173–G181. [Google Scholar] [CrossRef] [PubMed]
- Lansade, L.; Foury, A.; Reigner, F.; Vidament, M.; Guettier, E.; Bouvet, G.; Soulet, D.; Parias, C.; Ruet, A.; Mach, N.; et al. Progressive habituation to separation alleviates the negative effects of weaning in the mother and foal. Psychoneuroendocrinology 2018, 97, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.L.; Pridgen, T.A.; Blikslager, A.T. Environmental stressors affect intestinal permeability and repair responses in a pig intestinal ischemia model. Tissue Barriers 2020, 8, 1832421. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erwin, S.J.; Blikslager, A.T.; Ziegler, A.L. Age-Dependent Intestinal Repair: Implications for Foals with Severe Colic. Animals 2021, 11, 3337. https://doi.org/10.3390/ani11123337
Erwin SJ, Blikslager AT, Ziegler AL. Age-Dependent Intestinal Repair: Implications for Foals with Severe Colic. Animals. 2021; 11(12):3337. https://doi.org/10.3390/ani11123337
Chicago/Turabian StyleErwin, Sara J., Anthony T. Blikslager, and Amanda L. Ziegler. 2021. "Age-Dependent Intestinal Repair: Implications for Foals with Severe Colic" Animals 11, no. 12: 3337. https://doi.org/10.3390/ani11123337
APA StyleErwin, S. J., Blikslager, A. T., & Ziegler, A. L. (2021). Age-Dependent Intestinal Repair: Implications for Foals with Severe Colic. Animals, 11(12), 3337. https://doi.org/10.3390/ani11123337





