Simple Summary
Fasciola hepatica is a parasite that affects ruminants. This study evaluated the occurrence of fasciolosis in ovine livestock from central Portugal during a 2-year period. Positive animals were found in most locations and in both years (19.6% and 18.5% seropositive in the first year and second year, respectively). Correct anthelmintic treatment could further reduce egg elimination and pasture contamination.
Abstract
Fasciola hepatica is a parasite that is widespread in Europe, having been reported in ruminants of several European countries and causing an important economic impact. This study ascertained the occurrence and distribution of fasciolosis in Portuguese ovine livestock by assessing F. hepatica IgG antibodies in a cohort of confined sheep from a high-altitude region of central Portugal in a 2-year period. Positive animals were found in most locations and in both years, with 18 of the 92 animals (19.6% [95% confidence interval CI: 12.03–19.15]) and 17 of the same 92 animals (18.5% [95% CI: 11.15–27.93]) showing to be seropositive in the first year and second year, respectively (p = 0.85). Pasture contamination by F. hepatica eggs could be reduced by thorough anthelmintic treatments.
1. Introduction
Zoonotic foodborne trematodiases (ZFTs) are caused by species of the genera Clonorchis, Opisthorchis, Paragonimus and Fasciola, known to cause up to 7000 deaths and 200,000 morbidity cases each year, with an estimated 2 million disability-adjusted life-years worldwide [1]. Among these ZFTs, Fasciola spp. parasites pose an important impact in herbivores but also occur in humans. The worldwide prevalence of Fasciola infection in humans is calculated to range between 2.4 and 17 million and is considered under-reported and under-diagnosed [2,3]. Fasciola hepatica is endemic to Asia and Europe, occurring in lower numbers in South and Central America, Middle East and Northern Africa, with sporadic cases occurring in the United States of America and the Caribbean [2]. Fasciola gigantica occurs in the Pacific Islands, Asia and Northern Africa [2]. Domestic ruminants are the most common definitive hosts and humans living in close proximity to cattle and sheep industries and who consume raw aquatic vegetation are particularly at risk [4], overall causing serious public health concerns and considerable economic losses [5,6]. However, studies have shown that in some regions fascioliasis in humans does not necessarily occur in areas where fascioliasis is a major veterinary problem. This typically occurs in human hyperendemic zones such as the Bolivian Altiplano, where human prevalences are sufficient and maintained over time; egg fecal shedding in humans is sufficiently high and shed eggs are proved to be viable [7,8,9].
In domestic livestock fasciolosis is an important disease [10] and both immature and mature stages of the parasite in the final host result in a 15% decrease in milk yield [11], an average reduction of 1.5 kg [12] or 0.7 kg milk/cow per day [13]. Annual losses have been estimated to be around EUR 2.5 billion to the livestock and food industry worldwide [14].
Fasciola hepatica is a parasite that is widespread in Europe and has been reported in ruminants of several European countries such as Belgium, Denmark, England, Germany, Ireland, Italy, Poland, Spain, Switzerland, and Wales [15].
To the best of our knowledge no study has been performed serologically ascertaining the occurrence and distribution of fasciolosis in Portuguese ovine livestock. As such we assessed F. hepatica IgG antibodies in a cohort of confined sheep from a high-altitude region of central Portugal in a 2-year period.
2. Materials and Methods
This study considered sheep from the Serra da Estrela breed, a local autochthonous breed, to best mirror the circulation of F. hepatica. This sheep breed is located in the Serra da Estrela mountain plateau, with vast local pastures where sheep graze. The average annual temperature is ~7 °C, with an average precipitation from 1000 mm to 2500 mm per year. This sheep breed is managed by the “National Association of Serra da Estrela Sheep breed” (ANCOSE) that assures geographical restraint to this region to maintain the breed status. As these animals are confined to this region, the assessment of their serological status can be a valuable tool to reflect the local circulation of diseases [16,17].
Sera collection was performed in a previous study [17] preserved at −80 °C. The sampling scheme consisted of sera from a sheep cohort (n = 92), initially collected in January/February 2015, and again in January/February 2016 from the same animals (in a total of 184 sera samples = 92 paired). As herd size averages 40 animals, a total of four animals (~10% of herd size) aging 6 months and older, was randomly selected from 23 herds of Serra da Estrela sheep, located on 21 parishes of 4 municipalities of the region (Carregal do Sal, Celorico da Beira, Gouveia, and Seia). All 184 sera were screened for F. hepatica IgG antibodies, using a commercially available enzyme-linked immunosorbent assay (IDEXX Fasciola hepatica antibody test kit, Hoofddorp, the Netherlands), an assay based on the coating of microwells by f2 antigen of F. hepatica that has been shown to present 100% sensitivity and specificity [18]. Procedures were performed according to the manufacturer’s instructions, with samples being tested in duplicates. Optical densities (OD) were measured at 450 nm. Sample to positive ratio (S/P%) were calculated and considered negative if S/P% ≤ 30% and positive if S/P% > 30%, as described by the manufacturer. To assess differences in the seropositivity obtained in each year, a Chi-square test was used (GraphPad Prism version 5.04, GraphPad Software, La Jolla California), considering p values < 0.05 as statistically significant.
3. Results
Screening for F. hepatica IgG antibodies revealed that positive animals were found in most locations and in both years. In particular, in the year 2015, a total of 18 of the 92 animals showed to be seropositive, while in 2016, 17 of the same 92 animals were positive. This corresponds to an occurrence of 19.6% (95% confidence interval [CI] 12.03–19.15) in 2015 and 18.5% (95% CI: 11.15–27.93) in 2016. These proportions were found to be not statistically different (p = 0.85), and only 1 animal seroreverted from the 1st to the 2nd year. The occurrence of anti-F. hepatica IgG seropositive animals in 2015 and 2016 is depicted in Table 1.

Table 1.
Occurrence of F. hepatica IgG seropositive Serra da Estrela sheep sampled in the years 2015 and 2016.
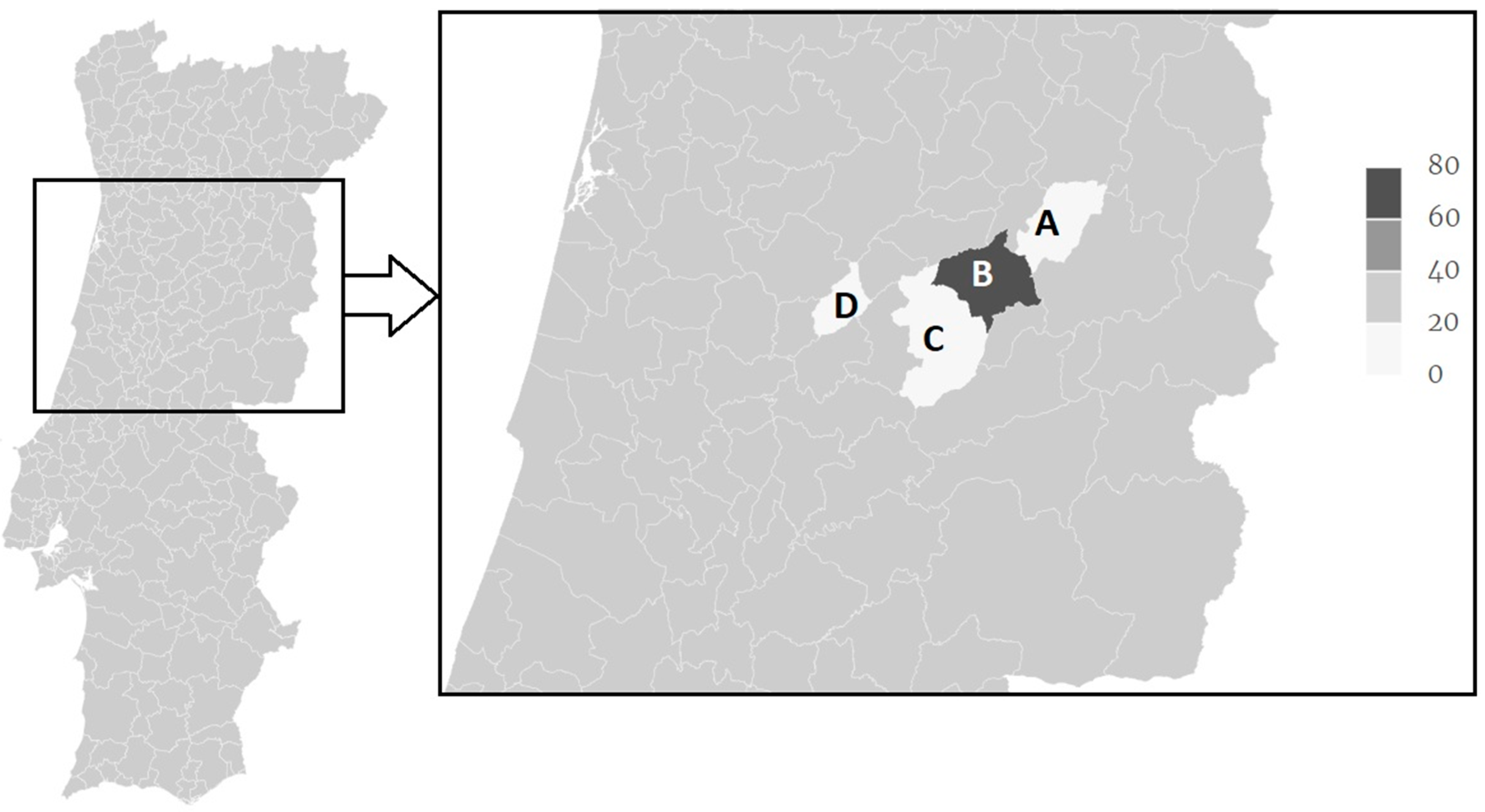
A distinction between high versus low endemicity regions can also be observed (Figure 1) as the Gouveia municipality presented an occurrence of F. hepatica IgG seropositive animals of 62.5% in both years, while all others where below 20%. In particular, Carregal do Sal presented 0% (95% CI: 0–26.5), Celorico da Beira 3.6% (95% CI: 0.1–18.4) in both years, Seia presented 19.4% (95% CI: 6.5–32.4) and 16.7% (95% CI: 6.4–32.8) in 2015 and 2016, respectively (Table 1).
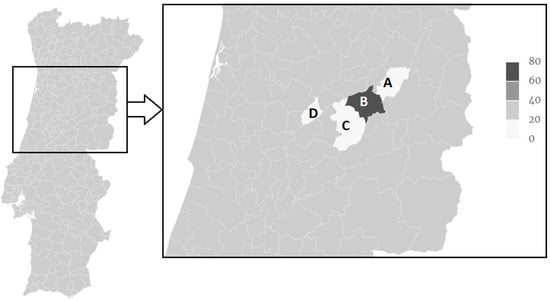
Figure 1.
Distribution of F. hepatica IgG seropositivity Serra da Estrela sheep sampled in the years 2015 and 2016 in Celorico da Beira (A), Gouveia (B), Seia (C) and Carregal do Sal (D) municipalities.
4. Discussion
Subacute fasciolosis in sheep is a worthy cause of poor reproductive performance and is associated with high rates of non-pregnancy, reduced twinning rates and prolonged lambing periods [19]. In cases of chronic fasciolosis, a reduction of the growth rate can be seen in young animals, while weight loss, reduction of milk production and wool quality is observed in adults [20]. Fasciolosis undermines animal health status, leading to a high morbidity and mortality [21] whereby it is necessary to prevent and to improve the diagnostic capacity.
Data on the occurrence and distribution of fasciolosis in sheep in Europe are usually focused on specific areas and management regimens, using different diagnostic methods, making it difficult to compare results. Information regarding the detection of eggs in feces by coprological techniques shows a highly variable prevalence of F. hepatica in sheep throughout Europe. In Spain, a prevalence of 59.3% has been reported [22], in Ireland 50.4% [23], in Poland 10.9% [24], in Italy 7.9%, in Switzerland 4.0% [25] and in Portugal 1.8% [26]. Coprological methods are only sensitive from 8–12 weeks post-infection (wpi), so the presence of the parasite cannot be determined in the acute phase and during pre-patent period of fasciolosis, due to the lack of egg output in feces [27].
As such, antibody detection by ELISA is commonly used to diagnose F. hepatica infection due to its high sensitivity on diagnosis and detection of pre-patent infections, when compared with coprological methods [28]. The ELISA method can detect antibodies to F. hepatica in serum of experimentally infected sheep from the first wpi [29]. The highest values are obtained between the 4th and 12th wpi, then slowly decreasing until the 32nd wpi [30]. In this study the presence of F. hepatica IgG antibodies in sheep was evaluated in two consecutive years. From a total of 92 animals, 18 (19.6%) were seropositive in 2015 and 17 (18.5%) were seropositive in 2016. Nonetheless, we alert that antibody repertoire in naturally chronically infected sheep is not yet well characterized. Natural infections in sheep are typically chronic and last for years, being also associated with repeated exposure [31]. As such, the approach of screening the same animals (prospectively) is likely to reflect a change in the circulation of the parasite. As no seroconversions were observed in this study, one can assume that at least no new infections have occurred in seronegative animals.
Data about F. hepatica seroprevalence in grazing sheep in Europe is limited. However, higher results were found in Spain (77.6% in Leon, 56% in Galicia and 42.6% in Pyrenees) [32,33,34], in Sweden (67%) [35] and in Greece (47.3%) [36].
Despite the low seroprevalence values found in the present study, there was one municipality, Gouveia, with a high percentage (62.5%) of seropositive animals in both consecutive years. Interestingly, herds from the Gouveia municipality do not strictly follow the sanitary management defined guidelines regarding deworming for the central region of Portugal. The defined guidelines consider a combination of closantel and mebendazole or ivermectin with clorsulon, known to be effective on adult flukes [20]. Additionally, triclabendazole is also used for effectiveness against young immature liver fluke [37]. No flukicides were ever used in Gouveia municipality, unlike in the rest of the region.
Nonetheless, the resistance of flukes to triclabendazole treatment is becoming a problem [20,37]. The wrong choice of antiparasitic agents, associated with over/underdosage practices at the time of application, may result in the development of resistance. Flukicide resistance was first noted when reporting resistance to triclabendazole in Australia in 1995 [38], later being described in other regions of the world such as Europe [39,40] and South America [41]. Rotational use of triclabendazole, closantel or nitroxynil should be considered where flukicides are used strategically in order to prevent the development of resistance [42]. The F. hepatica life cycle is dependent on eco-climatic factors. The extent of rainfall season combined with high average temperature have an influence on the development of F. hepatica eggs and larval stages, as well as in the development of its intermediated host [43,44]. Ecological factors such as presence of vegetation [45], poorly drained soils [46], presence of water bodies [47], and management factors as high herd/flock density and absence of flukicide treatment [48], are also important factors that increase the levels of F. hepatica infection.
5. Conclusions
Albeit a low circulation of F. hepatica was found, authors suggest improving practical guidelines for management of fasciolosis in grazing sheep. The correct flukicide treatment is an important strategy to minimize parasite circulation.
Author Contributions
Conceptualization, J.R.M.; methodology, J.R.M. and I.A.; investigation, C.C. and R.C.; writing—original draft preparation, J.R.M., C.C., R.C. and C.N.; writing—review and editing, M.A.P., C.N., H.V. and F.E.; funding acquisition, H.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Funds by FCT—Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020 and UID/04413/202.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fürst, T.; Keiser, J.; Utzinger, J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 210–221. [Google Scholar] [CrossRef]
- Good, R.; Scherbak, D. Fascioliasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nyindo, M.; Lukambagire, A.H. Fascioliasis: An ongoing zoonotic trematode infection. Biomed. Res. Int. 2015, 2015, 786195. [Google Scholar] [CrossRef] [Green Version]
- Biu, A.A.; Ahmed, M.I.; Mshelia, S.S. Economic assessment of losses due to parasitic diseases common at the Maiduguri abattoir, Nigeria. Afr. Sci. 2006, 7, 143–145. [Google Scholar]
- Chen, J.X.; Chen, M.X.; Ai, L.; Xu, X.N.; Jiao, J.M.; Zhu, T.J.; Su, H.Y.; Zang, W.; Luo, J.J.; Guo, Y.H.; et al. An outbreak of human fascioliasis gigantica in southwest China. PLoS ONE 2013, 8, e71520. [Google Scholar] [CrossRef] [Green Version]
- Piedrafita, D.; Spithill, T.W.; Smith, R.E.; Raadsma, H.W. Improving animal and human health through understanding liver fluke immunology. Parasite Immunol. 2010, 32, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.B.; Flores, A.; Aguirre, C.; Strauss, W.; Angles, R.; Mas-Coma, S. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the Northern Bolivian Altiplano. Acta Trop. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Esteban, J.B.; Flores, A.; Angles, R.; Strauss, W.; Aguirre, C.; Mas-Coma, S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop. Med. Int. Health 1997, 2, 695–699. [Google Scholar] [CrossRef]
- Mas-Coma, M.S.; Esteban, J.G.; Bargues, M.D. Epidemiology of human fascioliasis: A review and proposed new classification. Bull. World Health Organ. 1999, 77, 340–346. [Google Scholar]
- Olah, S.; van Wyk, J.A.; Wall, R.; Morgan, E.R. FAMACHA ©: A potential tool for targeted selective treatment of chronic fasciolosis in sheep. Vet. Parasitol. 2015, 212, 188–192. [Google Scholar] [CrossRef]
- Howell, A.; Baylis, M.; Smith, R.; Pinchbeck, G.; Williams, D. Epidemiology and impact of Fasciola hepatica exposure in high-yielding dairy herds. Prev. Vet. Med. 2015, 121, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Mezo, M.; González-Warleta, M.; Castro-Hermida, J.A.; Muiño, L.; Ubeira, F.M. Association between anti-F. hepatica antibody levels in milk and production losses in dairy cows. Vet. Parasitol. 2011, 180, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Duchateau, L.; Claerebout, E.; Williams, D.; Vercruysse, J. Associations between anti-Fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev. Vet. Med. 2007, 78, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Animal Health Ireland. Liver Fluke-The Facts. 2011. Available online: https://animalhealthireland.ie/assets/uploads/2021/06/PC-Liver-Fluke-2021.pdf?dl=1 (accessed on 18 October 2021).
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Saleem, M.H.; Rehman, M.U.; Iqbal, M.K.; Wang, Y.; et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Costa, J.; Esteves, F.; Santos, S. Sheep Grazing Management in the Mountain Region: Serra da Estrela, Portugal. In Sheep Farming: An Approach to Feed, Growth and Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Esteves, F.; Cruz, R.; Vasconcelos-Nóbrega, C.; Santos, C.; Ferreira, A.S.; Petrovic, T.; Cardoso, L.; Coelho, A.C.; Vala, H.; Nascimento, M.S.J.; et al. Serologic evidence for Schmallenberg virus circulation at high altitude, Central Portugal, 2015–2016. Transbound. Emerg. Dis. 2018, 65, 1553–1556. [Google Scholar] [CrossRef]
- Munita, M.P.; Rea, R.; Martinez-Ibeas, A.M.; Byrne, N.; Kennedy, A.; Sekiya, M.; Mulcahy, G.; Sayers, R. Comparison of four commercially available ELISA kits for diagnosis of Fasciola hepatica in Irish cattle. BMC Vet. Res. 2019, 15, 414. [Google Scholar] [CrossRef] [Green Version]
- Sargison, N.D. Fluke diseases of UK ruminant livestock Part 1: Life cycles, economic consequences and diagnosis. UK Vet. Livest. 2008, 13, 59–67. [Google Scholar] [CrossRef]
- Sargison, N.D.; Scott, P. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet. Rec. 2011, 168, 159–164. [Google Scholar] [CrossRef]
- Bosco, A.; Rinaldi, L.; Musella, V.; Amadesi, A.; Cringoli, G. Outbreak of acute fasciolosis in sheep farms in a Mediterranean area arising as a possible consequence of climate change. Geospat. Health 2015, 9, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Valladares, M.; Robles-Pérez, D.; Martínez-Pérez, J.M.; Cordero-Pérez, C.; del Rosario Famularo, M.; Fernández-Pato, N.; González-Lanza, C.; Castañón-Ordóñez, L.; Rojo-Vázquez, F.A. Prevalence of gastrointestinal nematodes and Fasciola hepatica in sheep in the northwest of Spain: Relation to climatic conditions and/or man-made environmental modifications. Parasites Vectors 2013, 6, 282. [Google Scholar] [CrossRef] [Green Version]
- Munita, M.P.; Rea, R.; Martinez-Ibeas, A.M.; Byrne, N.; McGrath, G.; Munita-Corbalan, L.E.; Sekiya, M.; Mulcahy, G.; Sayers, R.G. Liver fluke in Irish sheep: Prevalence and associations with management practices and co-infection with rumen fluke. Parasites Vectors 2019, 12, 525. [Google Scholar] [CrossRef]
- Gorski, P.; Niznikowski, R.; Strzelec, E.; Popielarczyk, D.; Gajewska, A.; Wedrychowicz, H. Prevalence of protozoan and helminth internal parasite infections in goat and sheep flocks in Poland. Arch. Tieraucht Dummerstorf 2004, 47, 43–49. [Google Scholar]
- Rinaldi, L.; Biggeri, A.; Musella, V.; De Waal, T.; Hertzberg, H.; Mavrot, F.; Torgerson, P.R.; Selemetas, N.; Coll, T.; Bosco, A.; et al. Sheep and Fasciola hepatica in Europe: The GLOWORM experience. Geospat. Health 2015, 9, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Ruano, Z.; Cortinhas, A.; Carolino, N.; Gomes, J.; Costa, M.; Mateus, T. Gastrointestinal parasites as a possible threat to an endangered autochthonous Portuguese sheep breed. J. Helminthol. 2020, 94, E103. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.E.D.; George, S.D.; Windsor, P.A.; Bush, R.D.; Šlapeta, J. Comparison of early detection of Fasciola hepatica in experimentally infected Merino sheep by real-time PCR, coproantigen ELISA and sedimentation. Vet. Parasitol. 2018, 15, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Meulemeester, L.; Claerebout, E.; Williams, D.; Vercruysse, J. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet. Parasitol. 2008, 153, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Avila, G.; Quiroz, H.; Ibarra, F.; Ochoa, P. Effect of parasite burden on the detection of Fasciola hepatica antigens in sera and feces of experimentally infected sheep. Vet. Parasitol. 2001, 97, 101–112. [Google Scholar] [CrossRef]
- Valero, M.A.; Ubeira, F.M.; Khoubbane, M.; Artigas, P.; Muiño, L.; Mezo, M.; Pérez-Crespo, I.; Periago, M.V.; Mas-Coma, S. MM3-ELISA evaluation of coproantigen release and serum antibody production in sheep experimentally infected with Fasciola hepatica and F. gigantica. Vet. Parasitol. 2009, 159, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Ainsworth, S.; Armstrong, S.; Hodgkinson, J.; Williams, D. Differences in the antibody response to adult Fasciola hepatica excretory/secretory products in experimentally and naturally infected cattle and sheep. Vet. Parasitol. 2021, 289, 109321. [Google Scholar] [CrossRef]
- Ferre, I.; López, P.; Gonzalo-Orden, M.; Julian, M.D.; Rojo-Vázquez, F.A.; González-Gallego, J. The effects of subclinical fasciolosis on hepatic secretory function in sheep. Parasitol. Res. 1995, 81, 127–131. [Google Scholar] [CrossRef]
- Paz-Silva, A.; Sánchez-Andrade, R.; Suárez, J.L.; Pedreira, J.; Arias, M.; López, C.; Panadero, R.; Díaz, P.; Díez-Baños, P.; Morrondo, P. Prevalence of natural ovine fasciolosis shown by demonstrating the presence of serum circulating antigens. Parasitol. Res. 2003, 91, 328–331. [Google Scholar] [CrossRef]
- Roldán, C.; Begovoeva, M.; López-Olvera, J.R.; Velarde, R.; Cabezón, Ó.; Molinar Min, A.R.; Pizzato, F.; Pasquetti, M.; Fernandez Aguilar, X.; Mentaberre, G.; et al. Endemic occurrence of Fasciola hepatica in an alpine ecosystem, Pyrenees, Northeastern Spain. Transbound. Emerg. Dis. 2020, 58, 2589–2594. [Google Scholar] [CrossRef]
- Novobilský, A.; Averpil, H.B.; Höglund, J. The field evaluation of albendazole and triclabendazole efficacy against Fasciola hepatica by coproantigen ELISA in naturally infected sheep. Vet. Parasitol. 2012, 190, 272–276. [Google Scholar] [CrossRef]
- Kantzoura, V.; Kouam, M.; Demiris, N.; Feidas, H.; Theodoropoulos, G. Risk factors and geospatial modelling for the presence of Fasciola hepatica infection in sheep and goat farms in the Greek temperate Mediterranean environment. Parasitology 2011, 138, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.; Anderson, F.; Wilson, K.; McCormick, I.; Skuce, P.; O’Roarke, J. Triclabendazole-resistant liver fluke: Issues and strategies. Livestock 2018, 23 (Suppl. S5), 4–14. [Google Scholar] [CrossRef]
- Overend, D.J.; Bowen, F.L. Resistance of Fasciola hepatica to triclabendazole. Aust. Vet. J. 1995, 72, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; van Dijk, J.; Jenkins, T.; Akca, A.; Mearns, R.; Williams, D.J.L. A composite faecal egg count reduction test to detect resistance to triclabendazole in Fasciola hepatica. Vet. Rec. 2012, 171, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.E.; McMahon, C.; Ellison, S.; Edgar, H.W.; Kajugu, P.E.; Gordon, A.; Irwin, D.; Barley, J.P.; Malone, F.E.; Brennan, G.P.; et al. Fasciola hepatica: A comparative survey of adult fluke resistance to triclabendazole, nitroxynil and closantel on selected upland and lowland sheep farms in Northern Ireland using faecal egg counting, coproantigen ELISA testing and fluke histology. Vet. Parasitol. 2015, 207, 34–43. [Google Scholar] [CrossRef]
- Olaechea, F.; Lovera, V.; Larroza, M.; Raffo, F.; Cabrera, R. Resistance of Fasciola hepatica against triclabendazole in cattle in Patagonia (Argentina). Vet. Parasitol. 2011, 178, 364–366. [Google Scholar] [CrossRef]
- Stubbings, L.; Bartley, D.; Busin, V.; Lovatt, F.; Page, P.; Rose Vineer, H.; Skuce, P. SCOPS Technical Manual. 2020. Available online: https://osf.io/sqa4e/ (accessed on 23 November 2021).
- Caminade, C.; van Dijk, J.; Baylis, M.; Williams, D. Modelling recent and future climatic suitability for fasciolosis in Europe. Geospat. Health 2015, 9, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Beesley, N.J.; Caminade, C.; Charlier, J.; Flynn, R.J.; Hodgkinson, J.E.; Martinez-Moreno, A.; Martinez-Valladares, M.; Perez, J.; Rinaldi, L.; Williams, D.J.L. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 199–216. [Google Scholar] [CrossRef]
- Bennema, S.C.; Ducheyne, E.; Vercruysse, J.; Claerebout, E.; Hendrickx, G.; Charlier, J. Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. Int. J. Parasitol. 2011, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Bennema, S.C.; Caron, Y.; Counotte, M.; Ducheyne, E.; Hendrickx, G.; Vercruysse, J. Towards assessing fine-scale indicators for the spatial transmission risk of Fasciola hepatica in cattle. Geospat. Health 2011, 5, 239–245. [Google Scholar] [CrossRef] [PubMed]
- De Roeck, E.; Van Coillie, F.; De Wulf, R.; Soenen, K.; Charlier, J.; Vercruysse, J.; Hantson, W.; Ducheyne, E.; Hendrickx, G. Fine-scale mapping of vector habitats using very high resolution satellite imagery: A liver fluke case-study. Geospat. Health 2014, 8, S671–S683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Mancera, A.; Reynoso-Palomar, A. Bulk tank milk ELISA to detect IgG1 prevalence and clustering to determine spatial distribution and risk factors of Fasciola hepatica-infected herds in Mexico. J. Helminthol. 2019, 93, 704–710. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).