Maternal Dietary Nitrate Supplementation Lowers Incidence of Stillbirth in Hyper Prolific Sows under Commercial Circumstances
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Animal Housing and Management
2.3. Measurements
2.4. Placenta Analysis
- 0 = No score possible or placenta was brown, because of deteriorating tissue.
- 1 = Placenta color was pale pink.
- 2 = Placenta color was light red or bright pink.
- 3 = Placenta color was bright red.
- 4 = Placenta color was deep red.
2.5. Statistical Analysis
3. Results
3.1. Sow Performance
3.2. Piglet Weights and Average Daily Gain (ADG)
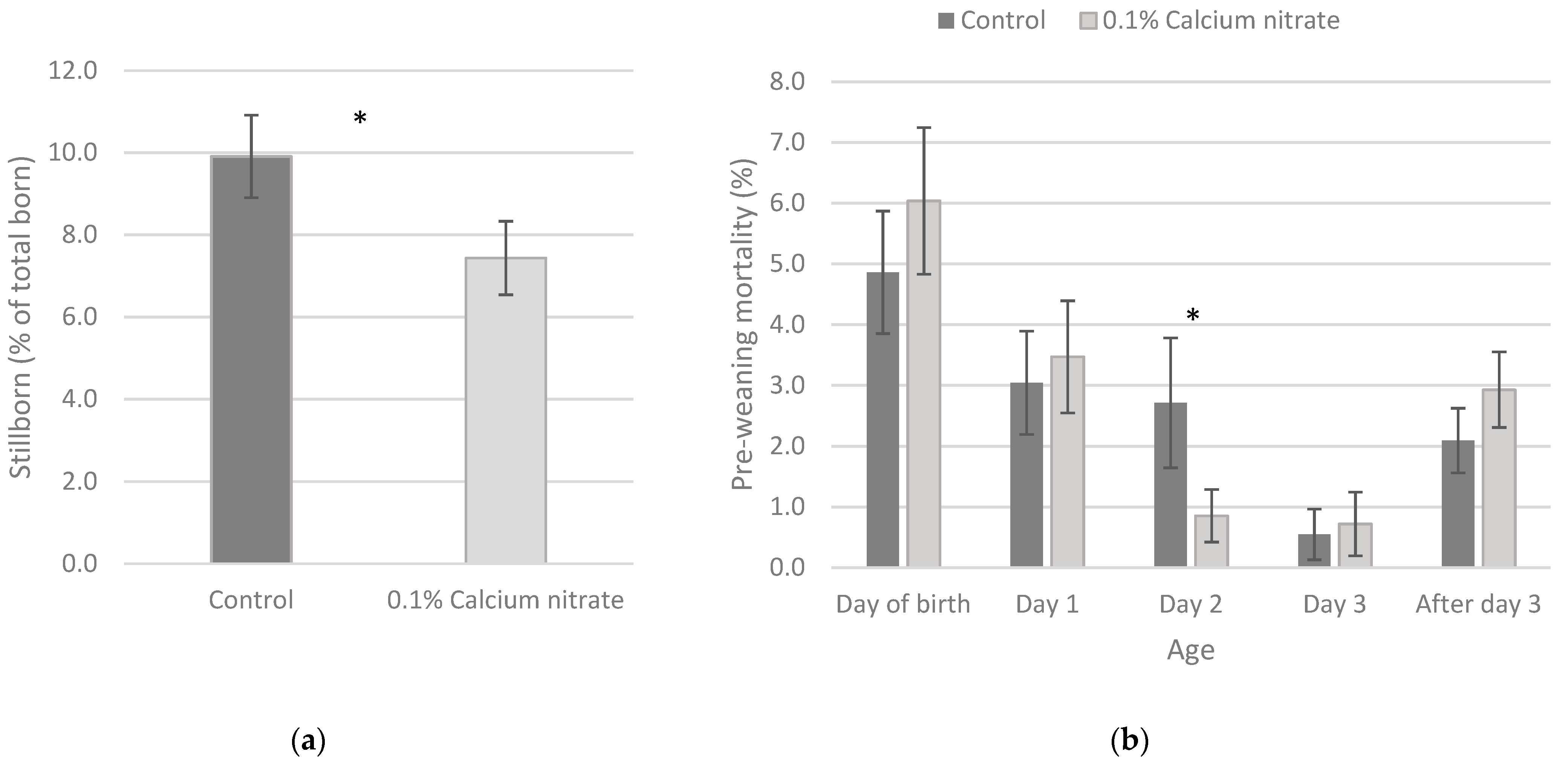
3.3. Piglet Survival
3.4. Placental Color Score
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanderhaeghe, C.; Dewulf, J.; de Kruif, A.; Maes, D. Non-infectious factors associated with stillbirth in pigs: A review. Anim. Reprod. Sci. 2013, 139, 76–88. [Google Scholar] [CrossRef]
- Hansen, H. Landgennemsnit for Produktivitet i Produktionen af Grise i 2019; SEGES Svineproduktion: Copenhagen, Denmark, 2020; p. 8. (In German) [Google Scholar]
- Sprecher, D.J.; Leman, A.D.; Dziuk, P.D.; Cropper, M.; DeDecker, M. Causes and control of swine stillbirths. J. Am. Vet. Med. Assoc. 1974, 165, 698–701. [Google Scholar]
- Curtis, S.E. Responses of the piglet to perinatal stressors. J. Anim. Sci. 1974, 38, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Christianson, W.T. Stillbirths, mummies, abortions, and early embryonic death. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 623–639. [Google Scholar] [CrossRef]
- Randall, G.C. The relationship of arterial blood pH and pCO2 to the viability of the newborn piglet. Can. J. Comp. Med. 1971, 35, 141–146. [Google Scholar]
- Borges, V.F.; Bernardi, M.L.; Bortolozzo, F.P.; Wentz, I. Risk factors for stillbirth and foetal mummification in four Brazilian swine herds. Prev. Vet. Med. 2005, 70, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Canario, L.; Cantoni, E.; Le Bihan, E.; Caritez, J.C.; Billon, Y.; Bidanel, J.P.; Foulley, J.L. Between-breed variability of stillbirth and its relationship with sow and piglet characteristics. J. Anim. Sci. 2006, 84, 3185–3196. [Google Scholar] [CrossRef]
- van Dijk, A.J.; van Rens, B.T.T.M.; van der Lende, T.; Taverne, M.A.M. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 2005, 64, 1573–1590. [Google Scholar] [CrossRef]
- Langendijk, P.; Fleuren, M.; van Kempen, T.A. Birth interval or duration of parturition: Which is relevant to risk of stillbirth and intervention? In Proceedings of the 69th Annual Meeting of the European Federation of Animal Science, Dubrovnik, Croatia, 27–31 August 2018; p. 112. [Google Scholar]
- Feyera, T.; Pedersen, T.F.; Krogh, U.; Foldager, L.; Theil, P.K. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 2018, 96, 2320–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bosch, M.; Wijnen, H.J.; van de Linde, I.B.; van Wesel, A.A.M.; Melchior, D.; Kemp, B.; Clouard, C.; van den Brand, H. Effects of maternal dietary nitrate supplementation during the perinatal period on piglet survival, body weight, and litter uniformity. Transl. Anim. Sci. 2019, 3, 464–472. [Google Scholar] [CrossRef] [Green Version]
- van den Bosch, M.; Wijnen, H.J.; van der Linde, I.B.; van Wesel, A.A.M.; Melchior, D.; Kemp, B.; van den Brand, H.; Clouard, C.M. Effects of maternal dietary nitrate supplementation on farrowing and placental characteristics, level of asphyxiation at birth and piglet vitality. Theriogenology 2019, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gourley, K.M.; Swanson, A.J.; Royall, R.Q.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Hastad, C.W.; Woodworth, J.C. Effects of timing and size of meals prior to farrowing on sow and litter performance. Transl. Anim. Sci. 2020, 4, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Neves, J.S.; Castro, D.S.; Lopes, S.O.; Santos, S.L.; Silva, S.V.C.; Araújo, V.O.; Vieira, M.F.A.; Muro, B.B.D.; Leal, D.F.; et al. Supplying sows energy on the expected day of farrowing improves farrowing kinetics and newborn piglet performance in the first 24 h after birth. Animal 2020, 14, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Feyera, T.; Skovmose, S.J.W.; Nielsen, S.E.; Vodolazska, D.; Bruun, T.S.; Theil, P.K. Optimal feed level during the transition period to achieve faster farrowing and high colostrum yield in sows. J. Anim. Sci. 2021, 99, skab040. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012, 12, 309–320. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlstörm, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. NO generation from nitrite and its role in vascular control. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Cermak, N.M.; Gibala, M.J.; Loon, L.J.C.V. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.; Bailey, S.; Vanhatalo, A.; Wilkerson, D.; Blackwell, J.; Gilchrist, M.; Benjamin, N.; Jones, A. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Benjamin, N. Dietary nitrate—Good or bad? Nitric Oxide 2010, 22, 104–109. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.; Ekblom, B.; Sahlin, K.; Weitzberg, E.; Lundberg, J. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 28, 2792–2793. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl–Kraupp, B.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; et al. Scientific Opinion on the risk assessment of nitrate and nitrite in feed. EFSA J. 2020, 18, 6290. [Google Scholar] [CrossRef]
- Baxter, E.M.; Jarvis, S.; D’Eath, R.B.; Ross, D.W.; Robson, S.K.; Farish, M.; Nevison, I.M.; Lawrence, A.B.; Edwards, S.A. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 2008, 69, 773–783. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Govoni, M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free. Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef]
- Miller, G.D.; Marsh, A.P.; Dove, R.W.; Beavers, D.; Presley, T.; Helms, C.; Bechtold, E.; King, S.B.; Kim-Shapiro, D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr. Res. 2012, 32, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free. Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Che, L.; Yang, P.; Fang, Z.; Lin, Y.; Wu, D. Effects of dietary arginine supplementation on reproductive performance and immunity of sows. Czech. J. Anim. Sci. 2013, 58, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Mateo, R.D.; Wu, G.; Bazer, F.W.; Park, J.C.; Shinzato, I.; Sung, W.K. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 2007, 137, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Jiang, Z.; Lin, Y.; Zheng, C.; Zhou, G.; Chen, F.; Yang, L.; Wu, G. Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 2012, 42, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.G. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid. Redox Signal. 2015, 23, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Koketsu, Y. Variability and repeatability in gestation length related to litter performance in female pigs on commercial farms. Theriogenology 2007, 68, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Hanenberg, E.H.A.T.; Knol, E.F.; Merks, J.W.M. Estimates of genetic parameters for reproduction traits at different parities in Dutch Landrace pigs. Livest. Prod. Sci. 2001, 69, 179–186. [Google Scholar] [CrossRef]
- Senger, P.L. Placentation, the endocrinology of the gestation and parturition. In Pathways to Pregnancy and Parturition; Current Conceptions Inc.: Pullman, WA, USA, 2003; pp. 304–325. [Google Scholar]
- Rydhmer, L.; Lundeheim, N.; Canario, L. Genetic correlations between gestation length, piglet survival and early growth. Livstock Sci. 2008, 115, 287–293. [Google Scholar] [CrossRef]
- Vanderhaeghe, C.; Dewulf, J.; Jourquin, J.; De Kruif, A.; Maes, D. Incidence and prevention of early parturition in sows. Reprod. Domest Anim. 2011, 46, 428–433. [Google Scholar] [CrossRef]
- Bird, I.M.; Zhang, L.; Magness, R.R. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R245–R258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexopoulos, J.G.; Lines, D.S.; Hallett, S.; Plush, K.J. A review of success factors for piglet fostering in lactation. Animals 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.P.; Redmer, D.A. Utero-placental vascular development and placental function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Pallares, P.; Garcia-Fernandez, R.A.; Criado, L.M.; Letelier, C.A.; Esteban, D.; Fernandez-Toro, J.M.; Flores, J.M.; Gonzalez-Bulnes, A. Disruption of the endothelial nitric oxide synthase gene affects ovulation, fertilization and early embryo survival in a knockout mouse model. Reproduction 2008, 136, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ingredients (%) | Control | 0.1% CaNO3 | Nutrient Levels | Control | 0.1% CaNO3 |
|---|---|---|---|---|---|
| Provisoy | 50.000 | 50.000 | Dry Matter (%) | 94.64 | 94.50 |
| Chicory Pulp | 10.000 | 10.000 | NE (MJ/kg) | 7.011 | 7.095 |
| Calcium Carbonate | 7.351 | 6.856 | Crude protein (%) | 28.50 | 29.95 |
| Monocalcium Phosphate | 6.888 | 6.884 | AID Lys (%) | 1.933 | 1.933 |
| Wheat | 5.797 | 6.602 | AID Met + Cys (%) | 1.141 | 1.146 |
| Salt | 3.757 | 3.454 | Calcium (%) | 4.61 | 4.61 |
| Potassium Chloride | 3.358 | 3.449 | Phosphorus (%) | 1.90 | 1.90 |
| Choline Chloride 60% | - | 2.500 | Sodium (%) | 2.20 | 1.68 |
| Choline Chloride 70% | 2.143 | - | Potassium (%) | 2.89 | 2.89 |
| Sodium Bicarbonate | 2.479 | 1.056 | Magnesium (%) | 1.26 | 1.26 |
| Bolifor CNF 1 | - | 1.000 | |||
| Soya Oil | 2.000 | 2.000 | |||
| L-Lysine HCL | 0.439 | 0.417 | |||
| DL-Methionine | 0.396 | 0.391 | |||
| Commercial Premix 2 | 5.393 | 5.393 | |||
| Ingredients (%) | Control | 0.1% CaNO3 |
|---|---|---|
| Barley | 48.50 | 48.50 |
| Wheat | 25.00 | 25.00 |
| Beet pulp, sugar 5.9% | 8.80 | 8.80 |
| Soybean meal, dehulled | 7.00 | 7.00 |
| Soybean oil | 0.70 | 0.70 |
| Concentrate Control | 10.00 | - |
| Concentrate CaNO3 1 | - | 10.00 |
| Variable | Control | 0.1% Calcium Nitrate 1 | p-Value |
|---|---|---|---|
| N (number of sows/litters) | 63 | 57 | - |
| Parity before farrowing | 3.3 ± 0.2 | 3.5 ± 0.3 | 0.70 |
| Gestation length (days) | 117.0 b ± 0.5 | 117.4 a ± 0.5 | 0.05 |
| Number of days on feed before farrowing (days) | 4.5 b ± 0.5 | 5.0 a ± 0.5 | 0.03 |
| Sow backfat thickness | |||
| At approximately day 112 of gestation (mm) | 15.0 ± 0.8 | 15.5 ± 0.8 | 0.37 |
| At weaning (mm) 2 | 12.6 ± 0.6 | 12.7 ± 0.6 | 0.88 |
| Backfat loss during lactation (mm) 2 | 2.8 ± 0.5 | 3.2 ± 0.5 | 0.14 |
| Reproductive performance | |||
| Total born | 18.7 ± 0.5 | 17.9 ± 0.5 | 0.25 |
| Number of piglets after cross-fostering | 14.7 ± 0.6 | 15.0 ± 0.6 | 0.59 |
| Number of piglets weaned | 12.7 ± 0.2 | 12.6 ± 0.2 | 0.38 |
| Piglet weights and ADG | |||
| Birth weight live-born piglets (kg) 3 | 1.34 ± 0.09 | 1.33 ± 0.09 | 0.76 |
| Birth weight stillborn piglets (kg) 3 | 1.08 ± 0.07 | 1.08 ± 0.07 | 0.97 |
| Average weight after cross-fostering (kg) | 1.23 ± 0.09 | 1.25 ± 0.10 | 0.65 |
| Average weaning weight (kg) 4 | 6.06 ± 0.62 | 6.21 ± 0.63 | 0.48 |
| ADG cross-fostering until 24 h after cross-fostering (g/piglet/day) | 87 ± 13 | 92 ± 13 | 0.65 |
| ADG from 24 h after cross-fostering until 3 days of age (g/piglet/day) | 119 ± 19 | 121 ± 19 | 0.79 |
| Score 1 | Control | 0.1% Calcium Nitrate | Pooled SEM | p-Value | ||
|---|---|---|---|---|---|---|
| n 2 | % | n | % | |||
| 3.2 | 0.19 | |||||
| 1 | 27 | 7.8 | 41 | 12.1 | ||
| 2 | 145 | 41.9 | 135 | 39.8 | ||
| 3 | 146 | 42.2 | 144 | 42.5 | ||
| 4 | 28 | 8.1 | 19 | 5.6 | ||
| Total | 346 | 100.0 | 339 | 100.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Bosch, M.; Bronsvoort, B.; Kemp, B.; van den Brand, H. Maternal Dietary Nitrate Supplementation Lowers Incidence of Stillbirth in Hyper Prolific Sows under Commercial Circumstances. Animals 2021, 11, 3364. https://doi.org/10.3390/ani11123364
van den Bosch M, Bronsvoort B, Kemp B, van den Brand H. Maternal Dietary Nitrate Supplementation Lowers Incidence of Stillbirth in Hyper Prolific Sows under Commercial Circumstances. Animals. 2021; 11(12):3364. https://doi.org/10.3390/ani11123364
Chicago/Turabian Stylevan den Bosch, Moniek, Bram Bronsvoort, Bas Kemp, and Henry van den Brand. 2021. "Maternal Dietary Nitrate Supplementation Lowers Incidence of Stillbirth in Hyper Prolific Sows under Commercial Circumstances" Animals 11, no. 12: 3364. https://doi.org/10.3390/ani11123364
APA Stylevan den Bosch, M., Bronsvoort, B., Kemp, B., & van den Brand, H. (2021). Maternal Dietary Nitrate Supplementation Lowers Incidence of Stillbirth in Hyper Prolific Sows under Commercial Circumstances. Animals, 11(12), 3364. https://doi.org/10.3390/ani11123364






