Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Risberg, A.; Spadavecchia, C.; Ranheim, B.; Krontveit, R.; Haga, H.A. Antinociceptive Effects of Three Escalating Dexmedetomidine and Lignocaine Constant Rate Infusions in Conscious Horses. Vet. J. 2014, 202, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, C.; Levionnois, O.; Kronen, P.; Andersen, O.K. The Effects of Isoflurane Minimum Alveolar Concentration on Withdrawal Reflex Activity Evoked by Repeated Transcutaneous Electrical Stimulation in Ponies. Vet. J. 2010, 183, 337–344. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Arendt-Nielsen, L.; Spadavecchia, L.; Mosing, M.; Auer, U.; Van Den Hoven, R. Effects of Butorphanol on the Withdrawal Reflex Using Threshold, Suprathreshold and Repeated Subthreshold Electrical Stimuli in Conscious Horses. Vet. Anaesth. Analg. 2007, 34, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, C.; Levionnois, O.; Kronen, P.W.; Leandri, M.; Spadavecchia, L.; Schatzmann, U. Evaluation of Administration of Isoflurane at Approximately the Minimum Alveolar Concentration on Depression of a Nociceptive Withdrawal Reflex Evoked by Transcutaneous Electrical Stimulation in Ponies. Am. J. Vet. Res. 2006, 67, 762–769. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Arendt-Nielsen, L.; Andersen, O.K.; Spadavecchia, L.; Schatzmann, U. Effect of Romifidine on the Nociceptive Withdrawal Reflex and Temporal Summation in Conscious Horses. Am. J. Vet. Res. 2005, 66, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, C.; Andersen, O.K.; Arendt-Nielsen, L.; Spadavecchia, L.; Doherr, M.; Schatzmann, U. Investigation of the Facilitation of the Nociceptive Withdrawal Reflex Evoked by Repeated Transcutaneous Electrical Stimulations as a Measure of Temporal Summation in Conscious Horses. Am. J. Vet. Res. 2004, 65, 901–908. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Arendt-Nielsen, L.; Andersen, O.K.; Spadavecchia, L.; Doherr, M.; Schatzmann, U. Comparison of Nociceptive Withdrawal Reflexes and Recruitment Curves between the Forelimbs and Hind Limbs in Conscious Horses. Am. J. Vet. Res. 2003, 64, 700–707. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Spadavecchia, L.; Andersen, O.K.; Arendt-Nielsen, L.; Leandri, M.; Schatzmann, U. Quantitative Assessment of Nociception in Horses by Use of the Nociceptive Withdrawal Reflex Evoked by Transcutaneous Electrical Stimulation. Am. J. Vet. Res. 2002, 63, 1551–1556. [Google Scholar] [CrossRef]
- Veres-Nyéki, K.O.; Leandri, M.; Spadavecchia, C. Nociceptive Trigeminal Reflexes in Non-Sedated Horses. Vet. J. 2012, 191, 101–107. [Google Scholar] [CrossRef]
- Haussler, K.K.; Erb, H.N. Mechanical Nociceptive Thresholds in the Axial Skeleton of Horses. Equine Vet. J. 2006, 38, 70–75. [Google Scholar] [CrossRef]
- Long, K.; McGowan, C.M.; Hyytiäinen, H.K. Effect of Caudal Traction on Mechanical Nociceptive Thresholds of Epaxial and Pelvic Musculature on a Group of Horses With Signs of Back Pain. J. Equine Vet. Sci. 2020, 93, 103197. [Google Scholar] [CrossRef] [PubMed]
- Luna, S.P.L.; Lopes, C.; Rosa, A.C.; Oliveira, F.A.; Crosignani, N.; Taylor, P.M.; Pantoja, J.C. Validation of Mechanical, Electrical and Thermal Nociceptive Stimulation Methods in Horses: Validation of Nociceptive Methods in Horses. Equine. Vet. J. 2015, 47, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.S. Flexion-Reflex of the Limb, Crossed Extension-Reflex, and Reflex Stepping and Standing. J. Physiol. 1910, 40, 28–121. [Google Scholar] [CrossRef] [PubMed]
- Hagbarth, K.E. Spinal Withdrawal Reflexes in the Human Lower Limbs. J. Neurol. Neurosurg. Psychiatry 1960, 23, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugelberg, E.; Eklund, K.; Grimby, L. An Electromyographic Study of the Nociceptive Reflexes of the Lower Limb. Mechanism of the Plantar Responses. Brain 1960, 83, 394–410. [Google Scholar] [CrossRef]
- Kugelberg, E. Demonstration of A and C Fibre Components in the Babinski Plantar Response and the Pathological Flexion Reflex. Brain 1948, 71, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Willer, J.C. Comparative Study of Perceived Pain and Nociceptive Flexion Reflex in Man. Pain 1977, 3, 69–80. [Google Scholar] [CrossRef]
- Chan, C.W.Y.; Dallaire, M. Subjective Pain Sensation Is Linearly Correlated with the Flexion Reflex in Man. Brain Res. 1989, 479, 145–150. [Google Scholar] [CrossRef]
- Sandrini, G.; Arrigo, A.; Bono, G.; Nappi, G. The Nociceptive Flexion Reflex as a Tool for Exploring Pain Control Systems in Headache and Other Pain Syndromes. Cephalalgia 1993, 13, 21–27. [Google Scholar] [CrossRef]
- Sandrini, G.; Ruiz, L.; Capararo, M.; Garofoli, F.; Beretta, A.; Nappi, G. Central Analgesic Activity of Ibuprofen. A Neurophysiological Study in Humans. Int. J. Clin. Pharmacol. Res. 1992, 12, 197–204. [Google Scholar]
- Willer, J.C.; Bathien, N. Pharmacological Modulations on the Nociceptive Flexion Reflex in Man. Pain 1977, 3, 111–119. [Google Scholar] [CrossRef]
- Rohrbach, H.; Korpivaara, T.; Schatzmann, U.; Spadavecchia, C. Comparison of the Effects of the Alpha-2 Agonists Detomidine, Romifidine and Xylazine on Nociceptive Withdrawal Reflex and Temporal Summation in Horses. Vet. Anaesth. Analg. 2009, 36, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.P.A.M.; Menke, E.S.; Doornenbal, A.; Back, W.; Hellebrekers, L.J. Antinociceptive Effects of Low Dose Lumbosacral Epidural Ropivacaine in Healthy Ponies. Vet. J. 2012, 193, 240–245. [Google Scholar] [CrossRef]
- Skljarevski, V.; Ramadan, N.M. The Nociceptive Flexion Reflex in Humans—Review Article. Pain 2002, 96, 3–8. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Petersen-Felix, S. Wind-up and Neuroplasticity: Is There a Correlation to Clinical Pain? Eur. J. Anaesthesiol. Suppl. 1995, 10, 1–7. [Google Scholar]
- Arendt-Nielsen, L.; Yarnitsky, D. Experimental and Clinical Applications of Quantitative Sensory Testing Applied to Skin, Muscles and Viscera. J. Pain 2009, 10, 556–572. [Google Scholar] [CrossRef]
- Price, D.D.; Mao, J.; Frenk, H.; Mayer, D.J. The Symbol Receptor Antagonist Dextromethorphan Selectively Reduces Temporal Summation of Second Pain in Man. Pain 1994, 59, 165–174. [Google Scholar] [CrossRef]
- Spadavecchia, C.; Rohrbach, H.; Levionnois, O.; Leandri, M. The model of the Nociceptive Withdrawal Reflex in horses. PHK 2016, 32, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Sandrini, G.; Serrao, M.; Rossi, P.; Romaniello, A.; Cruccu, G.; Willer, J.C. The Lower Limb Flexion Reflex in Humans. Prog. Neurobiol. 2005, 77, 353–395. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, M.R.; Faganel, J.; Gregorić, M.; Nathan, P.W.; Trontelj, J.K. Habituation: Effects of Regular and Stochastic Stimulation. J. Neurol. Neurosurg. Psychiatry 1972, 35, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.K. Physiological and Pharmacological Modulation of the Human Nociceptive Withdrawal Reflex. Ph.D Thesis, Aalborg University, Aalborg, Denmark, 1996. [Google Scholar]
- NCSS. Version 10.0.19; Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2015. [Google Scholar]
- SigmaPlot. Version 12.5.0.38; Statistical Software; Systat Software Inc.: San José, CA, USA, 2013. [Google Scholar]
- Bergadano, A.; Andersen, O.K.; Arendt-Nielsen, L.; Schatzmann, U.; Spadavecchia, C. Quantitative Assessment of Nociceptive Processes in Conscious Dogs by Use of the Nociceptive Withdrawal Reflex. Am. J. Vet. Res. 2006, 67, 882–889. [Google Scholar] [CrossRef]
- Rohrbach, H.; Zeiter, S.; Andersen, O.K.; Wieling, R.; Spadavecchia, C. Quantitative Assessment of the Nociceptive Withdrawal Reflex in Healthy, Non-Medicated Experimental Sheep. Physiol. Behav. 2014, 129, 181–185. [Google Scholar] [CrossRef]
- Andersen, O.K.; Sonnenborg, F.A.; Arendt-Nielsen, L. Modular Organization of Human Leg Withdrawal Reflexes Elicited by Electrical Stimulation of the Foot Sole. Muscle Nerve 1999, 22, 1520–1530. [Google Scholar] [CrossRef]
- Blythe, L.L.; Engel, H.N.; Rowe, K.E. Comparison of Sensory Nerve Conduction Velocities in Horses versus Ponies. Am. J. Vet. Res. 1988, 49, 2138–2142. [Google Scholar]
- Burgess, P.R.; Perl, E.R. Myelinated Afferent Fibres Responding Specifically to Noxious Stimulation of the Skin. J. Physiol. 1967, 190, 541–562. [Google Scholar] [CrossRef]
- Erlanger, J.; Gasser, H.S. The Action Potential in Fibers of Slow Conduction in Spinal Roots and Somatic Nerves. Am. J. Physiol.-Leg. Content 1930, 92, 43–82. [Google Scholar] [CrossRef] [Green Version]
- Le Bars, D.; Willer, J.C.; De Broucker, T. Morphine Blocks Descending Pain Inhibitory Controls in Humans. Pain 1992, 48, 13–20. [Google Scholar] [CrossRef]
- Añor, S.; Espadaler, J.M.; Monreal, L.; Mayhew, I.G. Electrically Induced Blink Reflex in Horses. Vet. Rec. 1996, 139, 621–624. [Google Scholar]
- Shahani, B.T.; Young, R.R. Human Flexor Reflexes. J. Neurol. Neurosurg. Psychiatry 1971, 34, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, A.; Schieppati, M. Medium-Latency Response to Muscle Stretch in Human Lower Limb: Estimation of Conduction Velocity of Group II Fibres and Central Delay. Neurosci. Lett. 1998, 249, 29–32. [Google Scholar] [CrossRef]
- More, H.L.; Donelan, J.M. Scaling of Sensorimotor Delays in Terrestrial Mammals. Proc. R. Soc. B 2018, 285, 20180613. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.K.; Graven-Nielsen, T.; Matre, D.; Arendt-Nielsen, L.; Schomburg, E.D. Interaction between Cutaneous and Muscle Afferent Activity in Polysynaptic Reflex Pathways: A Human Experimental Study. Pain 2000, 84, 29–36. [Google Scholar] [CrossRef]
- Inaba, A.; Yokota, T.; Komori, T.; Hirose, K. Proximal and Segmental Motor Nerve Conduction in the Sciatic Nerve Produced by Percutaneous High Voltage Electrical Stimulation. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1996, 101, 100–104. [Google Scholar] [CrossRef]
- Djouhri, L.; Lawson, S.N. Abeta-Fiber Nociceptive Primary Afferent Neurons: A Review of Incidence and Properties in Relation to Other Afferent A-Fiber Neurons in Mammals. Brain Res. Rev. 2004, 46, 131–145. [Google Scholar] [CrossRef]
- Nagi, S.S.; Marshall, A.G.; Makdani, A.; Jarocka, E.; Liljencrantz, J.; Ridderström, M.; Shaikh, S.; O’Neill, F.; Saade, D.; Donkervoort, S.; et al. An Ultrafast System for Signaling Mechanical Pain in Human Skin. Sci. Adv. 2019, 5, eaaw1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambier, J.; Dehen, H.; Bathien, N. Upper Limb Cutaneous Polysynaptic Reflexes. J. Neurol. Sci. 1974, 22, 39–49. [Google Scholar] [CrossRef]

| Score | Behavioral Reaction |
|---|---|
| 0 | No reaction |
| 1 | Slight brisk whole-body reaction |
| 2 | Moderate brisk whole-body reaction |
| 3 | Prompt whole-body reaction and delayed lifting of the stimulated limb |
| 4 | Immediate lifting of the stimulated limb |
| 5 | Vigorous whole-body reaction with immediate lifting of the stimulated limb |
| Stimulation Intensity 1 | ||||||
|---|---|---|---|---|---|---|
| Variable | 0.9 | 1.0 (TH) | 1.1 | 1.2 | 1.3 | P2 |
| Reaction Score | 1 (1–2) | 2 (2–3) | 2 (2–3) | 3 (3–4) | 4 (4–5) | |
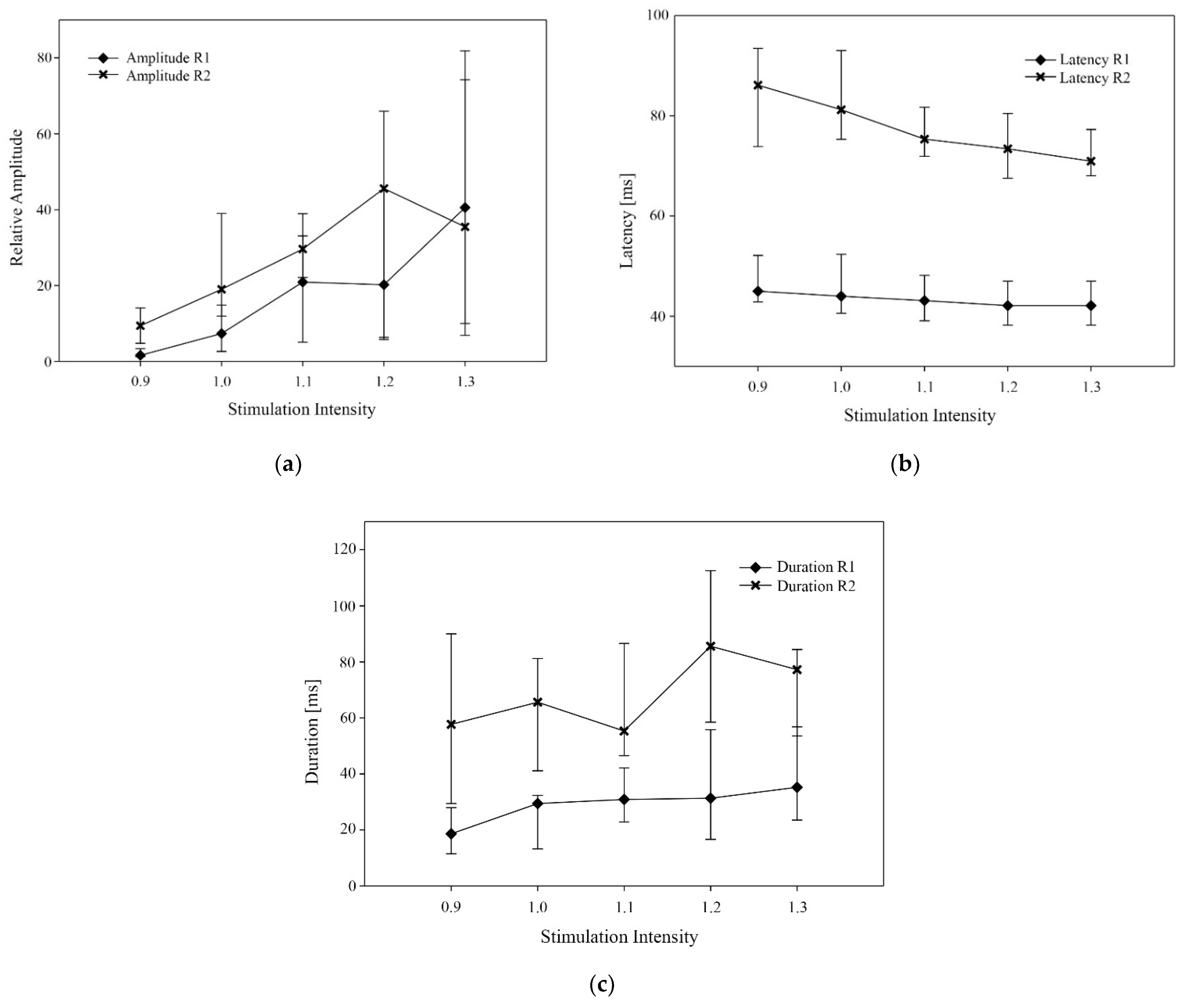
| Relative Amplitude R1 | 1.6 (1.2–3.4) a | 7.3 (2.6–14.8) a,b | 20.9 (5.1–33.1) b,c | 20.2 (6.3–45.1) b,c | 40.6 (10–74.3) c | <0.001 |
| Relative Amplitude R2 | 9.4 (4.8–14-1) a | 19 (11.9–39) a,b | 29.6 (22.1–39) a,b | 45.6 (5.8–65.9) b | 35.5 (6.9–81.9) b | <0.01 |
| Latency R1 (ms) | 45 (43–52) a | 44 (41–52) a,b | 43 (39–48) b,c | 42 (38–47) c | 42 (38–47) c | <0.001 |
| Latency R2 (ms) | 86 (74–94) a | 81 (75–93) a | 75 (72–82) a,b | 73 (68–81) a,b | 71 (68–77) b | <0.001 |
| Duration R1 (ms) | 19 (12–28) a | 29 (13–32) a,b | 31 (23–42) a,b | 31 (17–56) b | 35 (24–57) b | <0.001 |
| Duration R2 (ms) | 58 (29–90) a | 66 (41–81) a | 55 (47–87) a | 86 (59–113) a,b | 77 (54–84) b | <0.001 |
| Variable | TH | TO | P 1 |
|---|---|---|---|
| Reaction Score | 2 (2–3) | 5 (5–5) | |
| Relative Amplitude R1 | 7.3 (2.6–14.8) | 66.5 (47.7–84.8) | <0.002 |
| Relative Amplitude R2 | 19 (11.9–39) | 78.2 (49.2–85) | <0.02 |
| Latency R1 (ms) | 44 (41–52) | 39 (38–41) | <0.02 |
| Latency R2 (ms) | 81 (75–93) | 70 (64–74) | <0.01 |
| Duration R1 (ms) | 29 (13–32) | 58 (29–71) | <0.002 |
| Duration R2 (ms) | 66 (41–81) | 69 (58–112) | <0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mühlemann, S.; Leandri, M.; Risberg, Å.I.; Spadavecchia, C. Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses. Animals 2021, 11, 3380. https://doi.org/10.3390/ani11123380
Mühlemann S, Leandri M, Risberg ÅI, Spadavecchia C. Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses. Animals. 2021; 11(12):3380. https://doi.org/10.3390/ani11123380
Chicago/Turabian StyleMühlemann, Selina, Massimo Leandri, Åse Ingvild Risberg, and Claudia Spadavecchia. 2021. "Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses" Animals 11, no. 12: 3380. https://doi.org/10.3390/ani11123380
APA StyleMühlemann, S., Leandri, M., Risberg, Å. I., & Spadavecchia, C. (2021). Comparison of Threshold and Tolerance Nociceptive Withdrawal Reflexes in Horses. Animals, 11(12), 3380. https://doi.org/10.3390/ani11123380






