An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain
Abstract
:Simple Summary
Abstract
1. Introduction
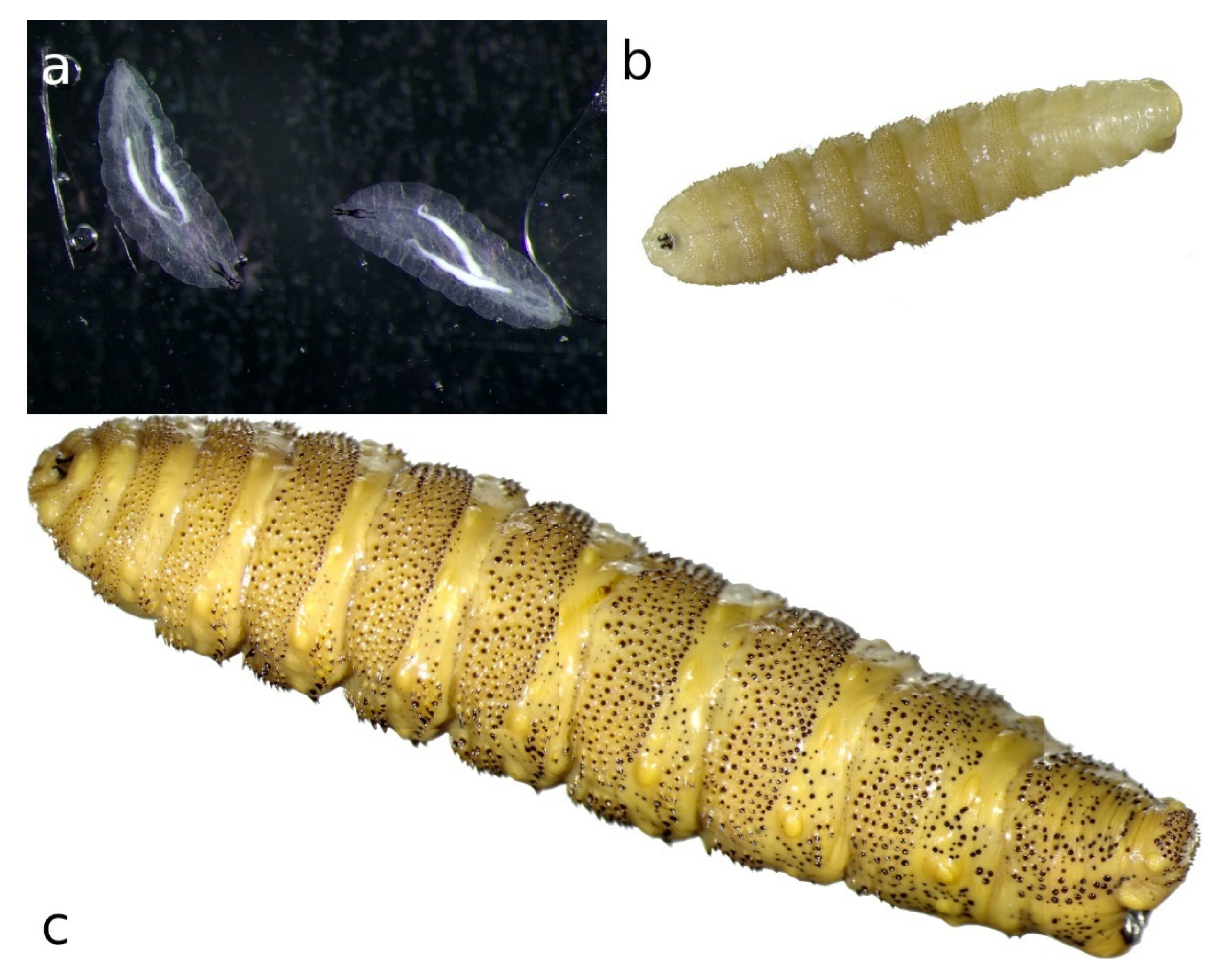
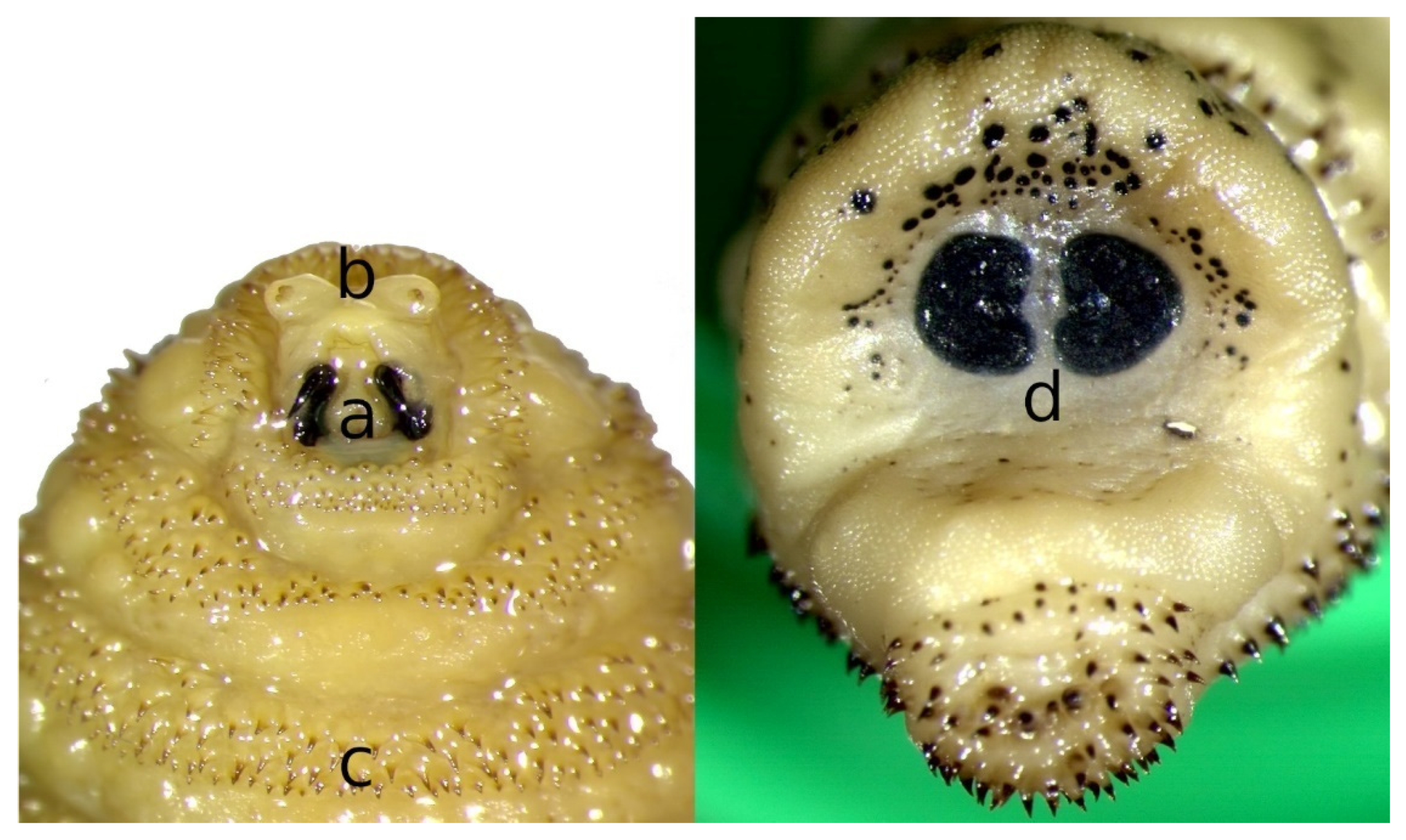
2. Morphology of Flies and Larvae
3. Life Cycle and Chronobiology
4. Epidemiology
4.1. Environment and Climate
4.2. Gender
4.3. Age
4.4. Body Condition
5. Clinical Signs
6. Diagnosis
7. Prevalence and Infestation Intensity
8. Treatment and Control
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo-Valadez, C.E.; Scholl, P.J.; Cepeda-Palacios, R.; Jacquiet, P.; Dorchies, P. Nasal bots… a fascinating world! Vet. Parasitol. 2010, 174, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Colwell, D.D.; Hall, M.J.R.; Scholl, P.J. A Synopsis of the Biology, Hosts, Distribution, Disease Significance and Management of the Genera. In The Oestrid Flies. Biology, Host-Parasite Relationships, Impact and Management; Colwell, D.D., Hall, M.J.R., Scholl, P.J., Eds.; CAB Int.: Oxfordshire, UK, 2006; p. 359. [Google Scholar]
- Leitner, N.; Schwarzmann, L.; Zittra, C.; Palmieri, N.; Eigner, B.; Otranto, D.; Glawischnig, W.; Fuehrer, H.-P. Morphological and molecular identification of nasopharyngeal bot fly larvae infesting red deer (Cervus elaphus) in Austria. Parasitol. Res. 2016, 115, 4417–4422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, L.; Szappanos, A. Bagócslegyek Gasterophilidae, Oestridae, Hypodermatidae; Magyar Természettudományi Múzeum: Budapest, Hungary, 1992. [Google Scholar]
- Zumpt, F. Myiasis in Man and Animals in the Old World; Butterworths: London, UK, 1965; pp. 146–153, 217–229. [Google Scholar]
- Pajares, G. Estudio Sobre la Infestación por Larvas de Cephenemyia Stimulator (Diptera: Oestridae) en Corzos (Capreolus capreolus) del Norte de España. Ph.D. Thesis, Facultad de Veterinaria de Lugo, Universidade de Santiago de Compostela, Lugo, Spain, 2016. [Google Scholar]
- Calero-Bernal, R.; Habela, M. First report of Cephenemyia stimulator (Diptera, Oestridae) parasitizing Roe deer (Capreolus capreolus) in Extremadura (Spain). Galemys Span. J. Mammal. 2013, 25, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Minář, J. Family Oestridae. In Contributions to a Manual Palaearctic Diptera; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; pp. 467–478. [Google Scholar]
- McMahon, D.C.; Bunch, T.D. Bot Fly Larvae (Cephenemyia spp. Oestridae) in Mule Deer (Odocoileus hemionus) from Utah. J. Wildl. Dis. 1989, 25, 636–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintela, L. Estudio Morfológico de las Larvas Nasofaríngeas (Diptera: Oestridae) Encontradas en Corzos de la Provincia de Lugo. Trabajo Fin de Grado; Facultad de Veterinaria de Lugo. Universidad de Santiago de Compostela: Lugo, Spain, 2021. [Google Scholar]
- Ullrich, H. Untersuchungen uber die Biologie der Rachenbremse (genus Cephenemyia Latreille), uber die Pathogenen Einflusse der Rachenbremsenllarven auf ihre Wirtstiere und uber Bekampfungsmoglishkeiten der Rachenbremsenplage. Ph.D. Dissertation, Friedrich Wilhelm University, Berlin, Germany, 1936. [Google Scholar]
- Colwell, D.D.; Scholl, P.J. Cuticular sensilla on newly hatched larvae of Gasterophilus intestinalis and Oestrus ovis. Med. Veter-Èntomol. 1995, 9, 85–93. [Google Scholar] [CrossRef]
- Martínez Calabuig, N. Prevalencia y Desarrollo Larvario de Cephenemyia sp. en Corzos del Norte de España. Trabajo Fin de Grado; Facultad de Veterinaria de Lugo. Universidade de Santiago de Compostela: Lugo, Spain, 2020. [Google Scholar]
- Martín-Vega, D.; Clark, B.; Ferrer, L.M.; López, S.; Panadero, R.; Cepeda-Palacios, R.; Colwell, D.D.; Hall, M.J.R. Major di-versity in the larval anatomy of the digestive and excretory systems of three Oestridae species revealed by micro-CT. Med. Vet. Entomol. 2021, 35, 106–210. [Google Scholar] [CrossRef]
- Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A.; Suárez, J.L.; Cazapal-Monteiro, C.; Prieto, J.M.; Casais, R.; Díez-Baños, P.; Morrondo, P. Assessment of Cephenemyia stimulator infection in roe deer (Capreolus capreolus) from Asturias (North Spain) by ELISA. Mappe Parassitol. 2012, 18, 129. [Google Scholar]
- Pajares, G.; Arias, M.S.; Pérez-Creo, A.; Prieto, A.; Callejo, A.; Díez-Baños, P.; Morrondo, P. Epidemiología de la cefenemiosis en corzos del noroeste de España. Boletín de la Asociación del Corzo Español. IV Simp. Sobre El Corzo En La Península Ibérica 2017, 15, 129–134. [Google Scholar]
- Anderson, J.R. Adult Biology. In The Oestrid Flies: Biology, Host-Parasite Relationships, Impact and Management; Colwell, D.D., Hall, M.J.R., Scholl, P.J., Eds.; CAB Int.: Wallingford, UK, 2006; pp. 140–164. [Google Scholar]
- Vaca, D. Biology of Nasopharyngeal Bot Fly Cephenemyia Stimulator (Diptera, Oestridae) and Its Distribution in the Czech Republic; COST Action 833: Brussels, Belgium, 2000; pp. 189–194. [Google Scholar]
- Cepelak and Macicka. Ksezönnemu vyskytu an ekológii streckov raticovej zveri na lesnej správe v Kamenici nad Hronom. Folia Venatoria 1979, 9, 293–299. [Google Scholar]
- Király, I.; Egri, B. Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larvae infestation in European deer (Capreolus capreolus) in Hungary. Acta Zool. Acad. Sci. Hung. 2007, 53, 271–279. [Google Scholar]
- Dudziňski, W. Studies on Cephenemyia stimulator (Clark, 1815) (Diptera, Oestridae), the parasite of European roe deer, Capreolus capreolus (L.). I. Biology. Acta Parasitol. Pol. 1970, 18, 555–572. [Google Scholar]
- Arias, M.S.; Pajares, G.; Paz-Silva, A.; Díez-Baños, N.; Suárez, J.L.; Díez-Baños, P.; Sánchez-Andrade, R.; Morrondo, P. Antigen characterization from second instar larvae of Oestridae flies for the detection of anti-Cephenemyia stimulator antibodies by ELISA in roe deer (Capreolus capreolus). Med. Vet. Entomol. 2014, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Rolandsen, C.M.; Madslien, K.; Ytrehus, B.; Hamnes, I.S.; Solberg, E.J.; Mysterud, A.; Vikøren, T.; Våge, J.; Hanssen, O.; Miller, A.L. Distribution, prevalence and intensity of moose nose bot fly (Cephenemyia ulrichii) larvae in moose (Alces alces) from Norway. Int. J. Parasitol. Parasites Wildl. 2021, 15, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.R.; Hoberg, E.P. How will global climate change affect parasite-host assemblages? Trends Parasitol. 2007, 23, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Ellse, L.S. Climate change and livestock parasites: Integrated management of sheep blowfly strike in a warmer envi-ronment. Global Chang. Biol. 2011, 17, 1770–1777. [Google Scholar] [CrossRef]
- Notario, A.; Castresana, L. Contribution to the knowledge of Cephenemyia stimulator Clark, 1815 (Diptera, Oestridae) in Spain. Folia Venatoria 2001, 30–31, 325–326. [Google Scholar]
- Pajares, G. Apuntes de Biología. Primera cita en España de Cephenemyia stimulator en corzos. Boletín De La Asoc. Del Corzo Español 2009, 11, 36. [Google Scholar]
- Morrondo, M.P.; Pérez-Creo, A.; Prieto, A.; Cabanelas, E.; Díaz-Cao, J.M.; Arias, M.S.; Fernández, P.D.; Pajares, G.; Remesar, S.; López-Sández, C.M.; et al. Prevalence and distribution of infectious and parasitic agents in roe deer from Spain and their possible role as reservoirs. Ital. J. Anim. Sci. 2016, 16, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Molander, M. A first Swedish record of the roe deer botfly Cephenemyia stimulator (Diptera: Oestridae). Entomologisk Tidskrift 2013, 134, 69–75. [Google Scholar]
- Fidalgo, L.E.; López, J.M.; Gonzalo, J.M.; González, A. El Corzo: Aspectos Biológicos y Aprovechamiento Cinegenético, 1st ed.; FEDENCA-Escuela Española de Caza: Madrid, Spain, 2009. (In Italic) [Google Scholar]
- Arias, M.S.; Pajares, G.; Díez-Baños, N.; Pérez-Creo, A.; Prieto, A.; Díez-Baños, P.; Morrondo, P. Cephenemyiosis, an emergent myiasis in roe deer (Capreolus capreolus) from northwestern Spain. Parasitol. Res. 2016, 115, 4605–4610. [Google Scholar]
- Panadero, R.; Dacal, V.; López, C.; Vázquez, L.; Cienfuegos, S.; Díaz, P.; Morrondo, P.; Díez-Baños, P. Immunomodulatory effect of Hypoderma lineatum antigens: In vitro effect on bovine lymphocyte proliferation and cytokine production. Parasite Immunol. 2009, 31, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, E.D. Dutch roe deer (Capreolus capreolus), Review of Cases Presented at the Dutch Wildlife Health Centre. Doctoral Thesis, Facultad de Veterinaria, Utrecht, The Netherlands, 2013. [Google Scholar]
- Kołodziej-Sobocińska, M. Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mammal Res. 2019, 64, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.; Gaillard, J.-M.; Linnell, J.D.C.; Duncan, P. Factors affecting maternal care in an income breeder, the European roe deer. J. Anim. Ecol. 2000, 69, 672–682. [Google Scholar] [CrossRef]
- Gilot-Fromont, E.; Jégo, M.; Bonenfant, C.; Gibert, P.; Rannou, B. Immune phenotype and body condition in roe deer: Indi-viduals with high body condition have different, not stronger immunity. PLoS ONE 2012, 7, e45576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, J.; Albon, S.D.; Irvine, R.J.; Woodin, S. The cost of parasites to caribou. Parasitology 2009, 136, 253–265. [Google Scholar] [CrossRef]
- Salaba, O.; Vadlejch, J.; Petrtyl, M.; Valek, P.; Kudrnacova, M.; Jankovska, I.; Bartak, M.; Sulakova, H.; Langrova, I. Cephen-emyia stimulator and Hypoderma diana infection of roe deer in the Czech Republic over an 8-year period. Parasitol. Res. 2013, 112, 1661–1666. [Google Scholar] [CrossRef]
- Witter, L.A.; Johnson, C.J.; Croft, B.; Gunn, A.; Gillingham, M.P. Behavioural trade-offs in response to external stimuli: Time allocation of an Arctic ungulate during varying intensities of harassment by parasitic flies. J. Anim. Ecol. 2011, 81, 284–295. [Google Scholar] [CrossRef]
- Blank, D.; Yang, E. Behavioral responses of Goitered gazelle (Gazella subguturosa) to parasitic activity of botflies. J. Parasitol. 2014, 100, 66–72. [Google Scholar] [CrossRef]
- Tabouret, G.; Vouldoukis, I.; Duranton, C.; Prevot, F.; Bergeaud, J.P.; Dorchies, P.; Mazier, D.; Jacquiet, P. Oestrus ovis (Diptera: Oestridae): Effects of larval excretory/secretory products on nitric oxide production by murine RAW 264·7 macrophages. Parasite Immunol. 2001, 23, 111–119. [Google Scholar] [CrossRef]
- Cogley, T.P. EFFECTS OF CEPHENEMYIA SPP. (DIPTERA: OESTRIDAE) ON THE NASOPHARYNX OF BLACK-TAILED DEER (ODOCOILEUS HEMIONUS COLUMBIANUS). J. Wildl. Dis. 1987, 23, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Jagannath, M.S.; Cozab, N.; Vijayasarathi, S.K. Histopatological changes in the nasal passages of sheep and goats infested with Oestrus ovis (Diptera: Oestridade). Indian J. An. Sci. 1989, 59, 87–91. [Google Scholar]
- Farina, G.; Giovannini, R. Principale Patologie Evidenziate Nella Fauna Selvática dal 2001 a la 2011 in Provincia di Trento; Foreste, S., Ed.; Fauna della Provincia Autonoma di Trento: Trento, Italy, 2013. [Google Scholar]
- Ahaduzzaman, M.; Islam, M.S.; Akter, S.; Uddin, M.J.; Sharif, S.M.O.; Mannan, A. Asphyxial death by Oestrus ovis in a pneu-monic goat. J. Adv. Parasitol. 2015, 2, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, L.E.; Lopez-Beceiro, A.M.; Vila-Pastor, M.; Ínez-Carrasco, C.M.; Barreiro-Vázquez, J.D.; Perez, J.M. Use of computed tomography as a non-invasive method for diagnosing cephenemyiosis in roe deer (Capreolus capreolus). Med. Veter.-EÈntomol. 2014, 29, 110–113. [Google Scholar] [CrossRef]
- Mattoon, J.S.; Gerros, T.C.; Parker, J.E.; Carter, C.A.; Lamarche, R.M. UPPER AIRWAY OBSTRUCTION IN A LLAMA CAUSED BY ABERRANT NASOPHARYNGEAL BOTS (CEPHENEMYIA SP.). Veter.-Radiol. Ultrasound 1997, 38, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Maes, S. Etude Sero-Epidemiologique de L’Hypodermose et des Myiases Naso-Pharyngees (Cephenemyiose et Pharyngomyiose) des Cervides en France. Doctoral Thesis, University of Paris-Est, Creteil, France, 2000. [Google Scholar]
- Maes, S.; Boulard, C. Deer myiasis in France. In Proceedings of the COST Action 83, Mange and myiasis of livestock, Brussels, Belgium, 2001. [Google Scholar]
- Sugár, L. Data on the Parasitic Infections of Cervidae in Hungary. In Big Game Management; Izrael, Ed.; Mémvadászati és Vadgazdálkodási Főosztály: Budapest, Hungary, 1975; pp. 85–102. [Google Scholar]
- Sugár, L. A Vadon élő Kérődzők Orr-Garat (torok) Bagócs-Fertőzöttsége (oestridosis) (Nasopharyngeal bot infestation of wild ruminants (oestridosis). In A Vadon Elő Allatok Betegségei (Diseases of Wildl.); Hőnich, M., Sugár, L., Kemenes, F., Eds.; Mezőgazdasági Kiadó: Budapest, Hungary, 1978; pp. 156–158. [Google Scholar]
- Király, I.; Egri, B. Data on the nasopharyngeal bot infestation of roe deer in Tolna county in 2003. Vadbiológia 2003, 10, 55–60. [Google Scholar]
- Pinnyey, S. Examination of nasal botfly (Cephenemyia stimulator, Clark, 1815) of roe deer. Fac. Manag. Agric. 2013, 15, 40–42. [Google Scholar]
- Pinnyey, S.; Majzinger, I.; Barta, T.; Huber, J. Examination of the Nasal Botfly (Cephenemyia stimulator, Clark, 1815) in the Roe Deer (Capreolus capreolus, Linnaeus 1758), in Hungary. Sci. Pap. Anim. Sci. Biotechnol. 2017, 50, 143–146. [Google Scholar]
- Lamka, J.; Suchy, J.; Staud, F. Efficacy of Orally Administered Ivermectin Against Larval Stages of Bot Fly (Cephenemyia stimulator C.) in Roe Deer. Acta Veter-Brno 1997, 66, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Urlík, J.; Letková, V.; Ciberej, J.; Lazar, P.; Goldová, M.; Kočišová, A.; Košuthová, L.; Trávniček, M.; Bhide, M.; Lazar, G.; et al. The occurrence of the genera Hypoderma, Cephenemyia and Pharyngomyia in deer in the Slovak Republic. Folia Veterinaria 2004, 48, 92–94. [Google Scholar]
- Kornaś, S.; Kowal, J.; Wajdzik, M.; Nosal, P.; Wojtaszek, M.; Basiaga, M. Cephenemyia stimulator (Diptera) infection in roe deer (Capreolus capreolus) from Kraków area, southern Poland. Ann. Parasitol. 2016, 6, 115–118. [Google Scholar]
- Barth, D.; Kudlich, H.; Schaich, K. Occurrence and Significance of Nasal Bot Infestation in Roe Bucks (Capreolus capreolus). In Wildlife Diseases; Springer: Boston, MA, USA, 1976; pp. 609–613. [Google Scholar] [CrossRef]
- Kusak, J.S.; Spicic, V.; Slijepcevic, S.; Bosnic, R.R.; Janje, S.; Duvnjak, M.; Sindicic, D.; Majnaric, Z.; Cvetnic, D.; Huber, D. Health status of red deer and roe deer in Gorski kotar, Croatia. Veterinarski Arhiv. 2012, 82, 59–73. [Google Scholar]
- Díaz Carrasco, M.S.; Espuny, A.; Escudero, E.; Cárceles, C.M. Farmacología de los endectocidas: Aplicaciones. An. De Vet. De Murcia 1998, 13, 3–22. [Google Scholar]
- Díaz Carrasco, M.S.; Espuny, A.; Escudero, E.; Cárceles, C.M. Farmacología de los endectocidas: Aplicaciones terapéuticas II. An. De Vet. De Murcia 2000, 16, 15–40. [Google Scholar]
- European Agency for the Evaluation of Medicinal Products (E.A.E.M.P.). Ivermectin (extension to deer). Sum. Rep. 1998, 4, 1–3. [Google Scholar]
- Lo, P.-K.A.; Fink, D.W.; Williams, J.B.; Blodinger, J. Pharmacokinetic studies of ivermectin: Effects of formulation. Veter-Res. Commun. 1985, 9, 251–268. [Google Scholar] [CrossRef]
- Prichard, R.K.; Steel, J.W.; Lacey, E.; Hennessy, D.R. Pharmacokinetics of ivermectin in sheep following intravenous, intra-abomasal or intraruminal administration. J. Veter-Pharmacol. Ther. 1985, 8, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kutzer, E. Treatment of oestrinosis and hypodermosis in Cervidae (red deer, roe deer) by ivermectin (IVOMEC).). Berl. Und Münchener Tierärztliche Wochenschr. 2000, 113, 149–151. [Google Scholar]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. Methods for the detection of an-thelmintic resistance in nematodes of veterinary importance. World Association for the Advancement of Veterinary Parasit-ology (W.A.A.V.P.). Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Scott, J.G.; Roush, R.T.; Liu, N. Selection of high-level abamectin resistance from field-collected house flies, Musca domestica. Cell. Mol. Life Sci. 1991, 47, 288–291. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Miller, R.J.; Ojeda-Chi, M.M.; Rosado-Aguilar, J.A.; Trinidad-Martínez, I.C.; Pérez de León, A.A. Aca-ricide and ivermectin resistance in a field population of Rhipicephalus microplus (Acari: Ixodidae) collected from red deer (Cervus elaphus) in the Mexican tropics. Vet. Parasitol. 2014, 200, 179–188. [Google Scholar] [CrossRef]
- Oksanen, A. Antti Oksanen; Endectocide treatment of the reindeer. Rangifer 1999, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Suarez, V.H. Helminthic control on grazing ruminants and environmental risks in South America. Veter.-Res. 2002, 33, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herd, R.P.; Sams, R.A.; Ashcraft, S.M. Persistence of ivermectin in plasma and faeces following treatment of cows with iver-mectin sustained-release, pour-on or injectable formulations. Int. J. Parasitol. 1996, 26, 1087–1093. [Google Scholar] [CrossRef]
- Maublanc, M.-L.; Bideau, E.; Picot, D.; Rames, J.-L.; Dubois, M.; Ferté, H.; Gérard, J.-F. Demographic crash associated with high parasite load in an experimental roe deer (Capreolus capreolus) population. Eur. J. Wildl. Res. 2009, 55, 621–625. [Google Scholar] [CrossRef]



| Species | Distribution | Hosts |
|---|---|---|
| C. trompe (Modeer, 1786) | Neartic/Paleartic | deer, moose, reindeer/caribou |
| C. ulrichii (Brauer, 1863) | Paleartic | moose |
| C. auribarbis (Meigen, 1824) | Paleartic | red deer |
| C. stimulator (Hunter, 1916) | Paleartic | roe deer |
| C. phobifer (Clark, 1815) | Neartic | white-tailed deer |
| C. apicata (Bennett & Sabrosky, 1962) | Neartic | mule deer |
| C. jellisoni (Townsend, 1941) | Neartic | mule deer, white-tailed deer, moose, elk |
| C. pratti (Hunter, 1916) | Neartic | mule deer, white-tailed deer |
| Country | Prevalence | Intensity | Method | References |
|---|---|---|---|---|
| Hungary | 70.8 | 12 | Necropsy | [50,51] |
| 34.8–35.2 | 9.8–8.8 | Necropsy | [52] | |
| 11.1–76.9 | 3.9–19 | Necropsy | [20] | |
| 17.3–22.9 | 15.3–25.4 | Necropsy | [53,54] | |
| Czech Republic | 60–90 | - | Necropsy | [55] |
| 11.1–25.6 | 7.7 | Necropsy | [18] | |
| 44 | 13 | Necropsy | [56] | |
| 16.1–42.9 | 6–11 | Necropsy | [37] | |
| Poland | 13 | 1–10 | Necropsy | [57] |
| Germany | 49 | 3–11 | Necropsy | [58] |
| Croatia | 27 | - | Necropsy | [59] |
| France | 32–43.2 | - | iELISA | [49] |
| Spain | 40 | 11–49 | Necropsy | [46] |
| 31.6 | 19.7 ± 21 | Necropsy | [31] | |
| 36–60 | - | iELISA | [31] | |
| 23.8 | - | iELISA | [28] | |
| 43.2 | 16.9 ± 22.47 | Necropsy | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrondo, P.; Pajares, G.; Arias, M.S.; Martínez-Calabuig, N.; Remesar, S.; García-Dios, D.; Díaz, P.; López, C.M.; Panadero, R.; Díez-Baños, P. An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain. Animals 2021, 11, 3382. https://doi.org/10.3390/ani11123382
Morrondo P, Pajares G, Arias MS, Martínez-Calabuig N, Remesar S, García-Dios D, Díaz P, López CM, Panadero R, Díez-Baños P. An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain. Animals. 2021; 11(12):3382. https://doi.org/10.3390/ani11123382
Chicago/Turabian StyleMorrondo, Patrocinio, Gerardo Pajares, María Sol Arias, Néstor Martínez-Calabuig, Susana Remesar, David García-Dios, Pablo Díaz, Ceferino Manuel López, Rosario Panadero, and Pablo Díez-Baños. 2021. "An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain" Animals 11, no. 12: 3382. https://doi.org/10.3390/ani11123382
APA StyleMorrondo, P., Pajares, G., Arias, M. S., Martínez-Calabuig, N., Remesar, S., García-Dios, D., Díaz, P., López, C. M., Panadero, R., & Díez-Baños, P. (2021). An Update on Cephenemyiosis in the European Roe Deer: Emergent Myiasis in Spain. Animals, 11(12), 3382. https://doi.org/10.3390/ani11123382







