Changes in the Spectrum of Free Fatty Acids in Blood Serum of Dairy Cows during a Prolonged Summer Heat Wave
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Keeping and Feeding Animals
2.3. Weather Conditions
2.4. Collection of Blood Samples and Analysis of Serum
2.5. Equipment and Instrument Operation Mode during Research
2.6. KOH-Methylation of Lipids in Biological Substrates Containing Water (Blood Serum)
2.7. Statistical Analysis
3. Results
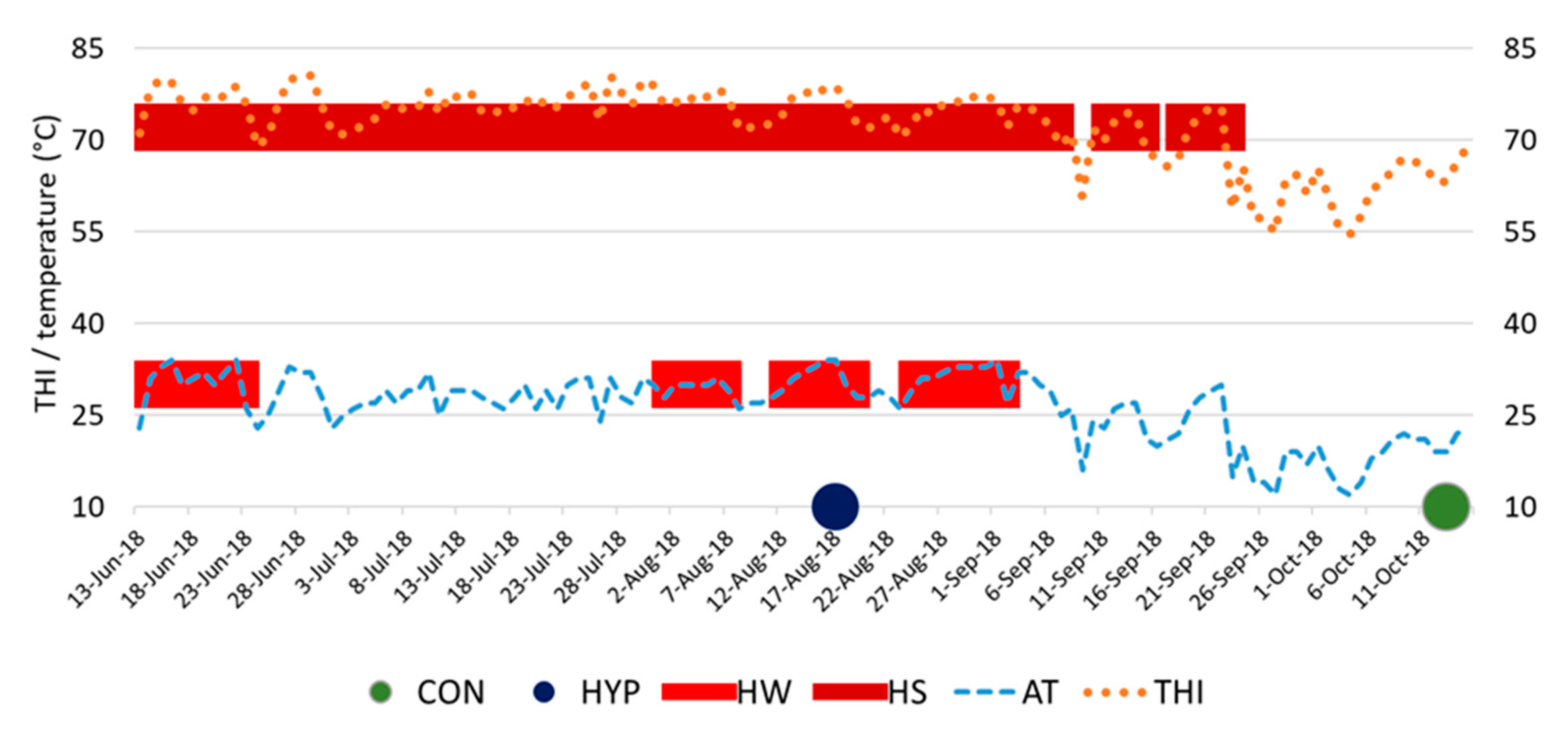
3.1. Barn Climate Conditions
3.2. The Spectrum of Serum Fatty Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovalchuk, I.M.; Gzhegotsky, M.R.; Rivis, Y.F.; Kovalchuk, S.M. Modification of the fatty acid composition of phospholipids in liver, myocardium and plasma tissues under the influence of ionizing radiation and with the prior application of a hydrogen sulfide donor. Bull. Probl. Biol. Med. 2018, 2, 130–137. [Google Scholar] [CrossRef]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Docosahexaenoic acid-derived oxidized lipid metabolites modulate the inflammatory response of lipolysaccharide-stimulated macrophages. Prostaglandins Other Lipid Mediat. 2018, 136, 76–83. [Google Scholar] [CrossRef]
- Didenko, V.I.; Klenina, I.A.; Babii, S.O.; Karachynova, V.A. Topicality of identification of free fatty acids pattern in biologic substrates in the diagnosis of gastroenterological diseases. Gastroenterology 2017, 51, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Hammami, H.; Vandenplas, J.; Vanrobays, M.L.; Rekik, B.; Bastin, C.; Gengler, N. Genetic analysis of heat stress effects on yield traits, udder health, and fatty acids of walloon holstein cows. J. Dairy Sci. 2015, 98, 4956–4968. [Google Scholar] [CrossRef] [Green Version]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.Y.; Zhu, M.J. Free radical oxidation of cardiolipin: Chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases. Free Radic. Res. 2012, 46, 959–974. [Google Scholar] [CrossRef]
- Brzozowska, A.M.; Lukaszewicz, M.; Oprazadek, J.M. Energy-protein supplementation and lactation affect fatty acid profile of liver and adipose tissue of dairy cows. Molecules 2018, 23, 618. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.B.; Bayat, A.R.; Trevisi, E.; Minuti, A.; Kairenius, P.; Viitala, S.; Mutikainen, M.; Leskinen, H.; Elo, K.; Kokkonen, T.; et al. Dietary supplement of conjugated linoleic acids or polyunsaturated fatty acids suppressed the mobilization of body fat reserves in dairy cows at early lactation through different pathways. J. Dairy Sci. 2018, 101, 7954–7970. [Google Scholar] [CrossRef]
- Ryman, V.E.; Packiriswamy, N.; Norby, B.; Schmidt, S.E.; Lock, A.L.; Sordillo, L.M. Supplementation of linoleic acid (c18:2n-6) or a-linolenic acid (c18:3n-3) changes microbial agonist-induced oxylipid biosynthesis. J. Dairy Sci. 2017, 100, 1870–1887. [Google Scholar] [CrossRef] [Green Version]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.J.; Wang, C.; Hao, Y.; Gu, X.H.; Wang, H.F. Chronic heat stress induces acute phase responses and serum metabolome changes in finishing pigs. Animals 2019, 9, 395. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Wang, W.Y.; Zheng, N.; Cheng, J.B.; Li, S.L.; Zhang, Y.D.; Wang, J.Q. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Hanuš, O.; Samkova, E.; Krizova, L.; Hasonova, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability-a review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- Mylostyvyi, R.; Izhboldina, O.; Chernenko, O.; Khramkova, O.; Kapshuk, N.; Hoffmann, G. Microclimate modeling in naturally ventilated dairy barns during the hot season: Checking the accuracy of forecasts. J. Therm. Biol. 2020, 93, 102720. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.M.; Bednorz, E.; Półrolniczak, M. The occurrence of heat waves in europe and their circulation conditions. Geografie 2019, 124, 1–17. [Google Scholar] [CrossRef]
- Mylostyvyi, R.; Chernenko, O. Correlations between environmental factors and milk production of holstein cows. Data 2019, 4, 103. [Google Scholar] [CrossRef] [Green Version]
- Kibler, H.H. Environmental physiology and shelter engineering with special reference to domestic animals. In LXVII, Thermal Effects of Various Temperature-Humidity Combinations on Holstein Cattle as Measured by Eight Physiological Responses; University of Missouri, College of Agriculture, Agricultural Experiment Station: Columbia, MO, USA, 1964; Volume 862, pp. 1–42. [Google Scholar]
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental parameters to assessing of heat stress in dairy cattle—A review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, K.; Yamaguchi, C.; Araya, Y.; Sakamoto, A.; Yoneda, K. Preparation of fatty acid methyl esters by selective methanolysis of polar glycerolipids. Lipids 2010, 45, 367–374. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Hoffmann, G.; Herbut, P.; Pinto, S.; Heinicke, J.; Kuhla, B.; Amon, T. Animal-related, non-invasive indicators for determining heat stress in dairy cows. Biosyst. Eng. 2020, 199, 83–96. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; de Souza, J.; Gandy, J.; Mavangira, V.; Lock, A.L.; Sordillo, L.M. Periparturient lipolysis and oxylipid biosynthesis in bovine adipose tissues. PLoS ONE 2017, 12, e0188621. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. Lipid Metabolism in Ruminant Animals, 1st ed.; Pergamon Press: Oxford, UK, 1981; p. 460. [Google Scholar]
- Caccamo, M.; Veerkamp, R.F.; Licitra, G.; Petriglieri, R.; La Terra, F.; Pozzebon, A.; Ferguson, J.D. Association of total-mixed-ration chemical composition with milk, fat, and protein yield lactation curves at the individual level. J. Dairy Sci. 2012, 95, 6171–6183. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.; Salavati, M.; Grelet, C.; Crowe, M.A.; Matthews, E.; O’Flaherty, R.; Opsomer, G.; Foldager, L.; Hostens, M.; McLoughlin, N.; et al. Prediction of metabolic clusters in early-lactation dairy cows using models based on milk biomarkers. J. Dairy Sci. 2019, 102, 2631–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, V.A.R.; Oliveira, H.R.; Brito, L.F.; Fleming, A.; Larmer, S.; Miglior, F.; Schenkel, F.S. Genome-wide association study for milk fatty acids in holstein cattle accounting for the dgat1 gene effect. Animals 2019, 9, 997. [Google Scholar] [CrossRef] [Green Version]
- Revskij, D.; Haubold, S.; Viergutz, T.; Kroger-Koch, C.; Tuchscherer, A.; Kienberger, H.; Rychlik, M.; Troscher, A.; Hammon, H.M.; Schuberth, H.J.; et al. Dietary fatty acids affect red blood cell membrane composition and red blood cell atp release in dairy cows. Int. J. Mol. Sci. 2019, 20, 2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Fernandes, E.A.; Cano, A.E.P.; Vinitwatanakhun, J.; Boeren, S.; van Hooijdonk, T.; van Knegsel, A.; Vervoort, J.; Hettinga, K.A. Changes in milk proteome and metabolome associated with dry period length, energy balance, and lactation stage in postparturient dairy cows. J. Proteome Res. 2013, 12, 3288–3296. [Google Scholar] [CrossRef]
- Markiewicz-Keszycka, M.; Czyzak-Runowska, G.; Lipinska, P.; Wojtowski, J. Fatty acid profile of milk—A review. Bull. Vet. Inst. Pulawy 2013, 57, 135–139. [Google Scholar] [CrossRef] [Green Version]
| Indicator | HYP | CON | ||||
|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | |
| Microclimatic parameters during blood sampling 1 | ||||||
| Temperature, °C | 34.0 | 33.0 | 34.0 | 19.0 | 19.0 | 19.0 |
| Relative humidity, % | 26.0 | 25.0 | 28.0 | 35.0 | 30.0 | 35.0 |
| THI | 78.4 | 77.9 | 78.6 | 63.1 | 62.9 | 63.1 |
| Microclimatic parameters on the day of blood sampling | ||||||
| Temperature, °C | 25.5 | 19.0 | 34.0 | 9.0 | 4.0 | 19.0 |
| Relative humidity, % | 46.0 | 25.0 | 83.0 | 58.5 | 30.0 | 81.0 |
| THI | 71.8 | 65.4 | 79.1 | 50.0 | 41.2 | 63.1 |
| Microclimatic parameters on the day before blood sampling | ||||||
| Temperature, °C | 25.5 | 21.0 | 34.0 | 10.5 | 8.0 | 19.0 |
| Relative humidity, % | 41.0 | 23.0 | 65.0 | 57.5 | 32.0 | 82.0 |
| THI | 71.6 | 66.4 | 78.0 | 52.2 | 47.6 | 63.2 |
| Microclimatic parameters during the week before blood sampling | ||||||
| Temperature, °C | 24.0 | 14.0 | 34.0 | 11.0 | 3.0 | 22.0 |
| Relative humidity, % | 47.0 | 23.0 | 83.0 | 63.0 | 25.0 | 93.0 |
| THI | 70.0 | 57.3 | 78.7 | 52.6 | 38.9 | 67.1 |
| Fatty Acid | Group of Animals | |
|---|---|---|
| HYP (n = 8) | CON (n = 10) | |
| Saturated fatty acids (SFA) | ||
| Butyric acid (C4:0) | 0.083 (0.054; 2.945) a | 0.001 (0.001; 0.001) b |
| Caproic acid (C6:0) | 0.939 (0.056; 1.163) a | 0.049 (0.049; 0.049) a |
| Caprylic acid (C8:0) | 0.897 (0.464; 1.124) | BDL |
| Capric acid (C10:0) | 0.008 (0.005; 0.014) | BDL |
| Undecanoic acid (C11:0) | 0.007 (0.001; 0.007) | BDL |
| Lauric acid (C12:0) | 0.008 (0.004; 0.015) a | 0.006 (0.004; 0.027) a |
| Tridecanoic acid (13:0) | 0.002 (0.001; 0.003) a | 0.002 (0.001; 0.002) a |
| Myristic acid (C14:0) | 0.002 (0.001; 0.003) a | 0.007 (0.005; 0.010) b |
| Pentadecanoic acid (C15:0) | 0.010 (0.005; 0.182) a | 0.15 (0.058; 0.178) a |
| Palmitic acid (C16:0) | 0.013 (0.008; 0.019) a | 0.01 (0.007; 0.015) a |
| Margarinic acid (C17:0) | 0.203 (0.033; 0.555) a | 0.799 (0.195; 1.068) a |
| Stearic acid (C18:0) | 0.039 (0.027; 0.092) a | 0.036 (0.018; 0.078) a |
| Arachinic acid (C20:0) | 0.019 (0.016; 0.038) a | 0.043 (0.032; 0.061) a |
| Geneicosanoic acid (C21:0) | 0.054 (0.032; 0.070) a | 0.222 (0.101; 0.347) b |
| Monounsaturated fatty acids (MUFA) | ||
| Myristoleic acid (cis-9 C14:1) | 0.001 (0.001; 0.001) a | 0.002 (0.002; 0.004) a |
| Pentadecenoic acid (cis-10 C15:1) | 0.002 (0.001; 0.003) a | 0.001 (0.001; 0.001) a |
| Palmitoleic acid (cis-9 C16:1) | 0.003 (0.002; 0.005) a | 0.013 (0.012; 0.014) a |
| Heptadecenoic acid (cis-10 C17:1) | 0.002 (0.002; 0.002) a | 0.004 (0.002; 0.013) a |
| Elaidic acid (trans-9 C18:1) | 0.001 (0.001; 0.005) a | 0.002 (0.002; 0.004) a |
| Oleic acid (cis-9 C18:1) | 0.015 (0.010; 0.019) a | 0.028 (0.013; 0.038) a |
| Eicosanoic acid (cis-11 C20:1) | BDL | 0.036 (0.025; 0.043) |
| Polyunsaturated fatty acids (PUFA) | ||
| Linoleic acid (trans-9,12, cis-9,12 C18:2n-6) | 0.048 (0.016; 0.069) a | 0.009 (0.003; 0.023) a |
| γ-linolenic acid (cis-6,9,12 C18:3n-6) | 0.006 (0.003; 0.028) a | 0.014 (0.013; 0.018) a |
| α-linolenic acid (cis-9,12,15 C18:3n-3) | 0.257 (0.101; 0.616) a | 0.169 (0.126; 0.175) a |
| Indicator | HYP | CON | p-Value between Groups | ||
|---|---|---|---|---|---|
| μg/μL | % of Total Fatty Acids | μg/μL | % of Total Fatty Acids | ||
| Free fatty acids (FFA) | 17.749 | 100.0 | 11.946 | 100.0 | 0.2447 |
| Σ saturated fatty acids (SFA) | 12.490 | 70.37 | 10.158 | 85.03 | 0.2291 |
| Σ unsaturated fatty acids (UFA) | 5.259 | 29.63 | 1.788 | 14.97 | 0.1105 |
| Σ monounsaturated fatty acids (MUFA) | 0.931 | 5.25 | 0.399 | 3.34 | 0.0059 |
| Σ polyunsaturated fatty acids (PUFA) | 4.328 | 24.38 | 1.389 | 11.63 | 0.1559 |
| including, Σ n-3 PUFA | 0.037 | 0.2 | 0.073 | 0.6 | 0.1381 |
| Σ n-6 PUFA | 2.894 | 16.3 | 0.146 | 1.2 | 0.2354 |
| UFA/SFA ratio | 0.42 | 0.18 | 0.0525 | ||
| n-6/n-3 PUFA ratio | 78.2 | 2.0 | 0.1663 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylostyvyi, R.; Sejian, V.; Izhboldina, O.; Kalinichenko, O.; Karlova, L.; Lesnovskay, O.; Begma, N.; Marenkov, O.; Lykhach, V.; Midyk, S.; et al. Changes in the Spectrum of Free Fatty Acids in Blood Serum of Dairy Cows during a Prolonged Summer Heat Wave. Animals 2021, 11, 3391. https://doi.org/10.3390/ani11123391
Mylostyvyi R, Sejian V, Izhboldina O, Kalinichenko O, Karlova L, Lesnovskay O, Begma N, Marenkov O, Lykhach V, Midyk S, et al. Changes in the Spectrum of Free Fatty Acids in Blood Serum of Dairy Cows during a Prolonged Summer Heat Wave. Animals. 2021; 11(12):3391. https://doi.org/10.3390/ani11123391
Chicago/Turabian StyleMylostyvyi, Roman, Veerasamy Sejian, Olena Izhboldina, Olena Kalinichenko, Lina Karlova, Olena Lesnovskay, Natalia Begma, Oleh Marenkov, Vadym Lykhach, Svitlana Midyk, and et al. 2021. "Changes in the Spectrum of Free Fatty Acids in Blood Serum of Dairy Cows during a Prolonged Summer Heat Wave" Animals 11, no. 12: 3391. https://doi.org/10.3390/ani11123391
APA StyleMylostyvyi, R., Sejian, V., Izhboldina, O., Kalinichenko, O., Karlova, L., Lesnovskay, O., Begma, N., Marenkov, O., Lykhach, V., Midyk, S., Cherniy, N., Gutyj, B., & Hoffmann, G. (2021). Changes in the Spectrum of Free Fatty Acids in Blood Serum of Dairy Cows during a Prolonged Summer Heat Wave. Animals, 11(12), 3391. https://doi.org/10.3390/ani11123391









