Effect of Essential Oils on the Oxyntopeptic Cells and Somatostatin and Ghrelin Immunoreactive Cells in the European Sea Bass (Dicentrarchus labrax) Gastric Mucosa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Conditions and Tissue Sampling
2.2. Immunohistochemistry
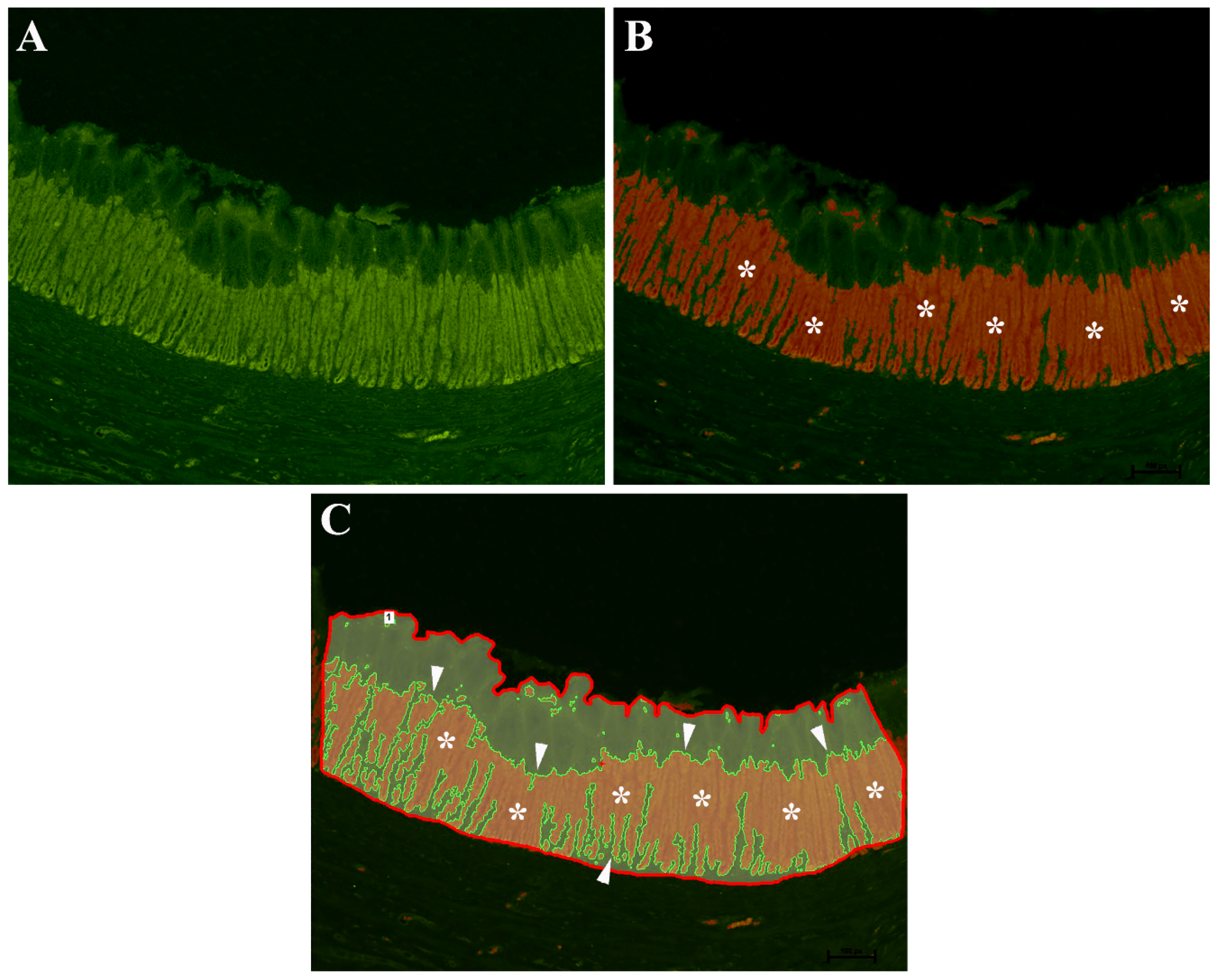
2.3. Threshold Binarization Method
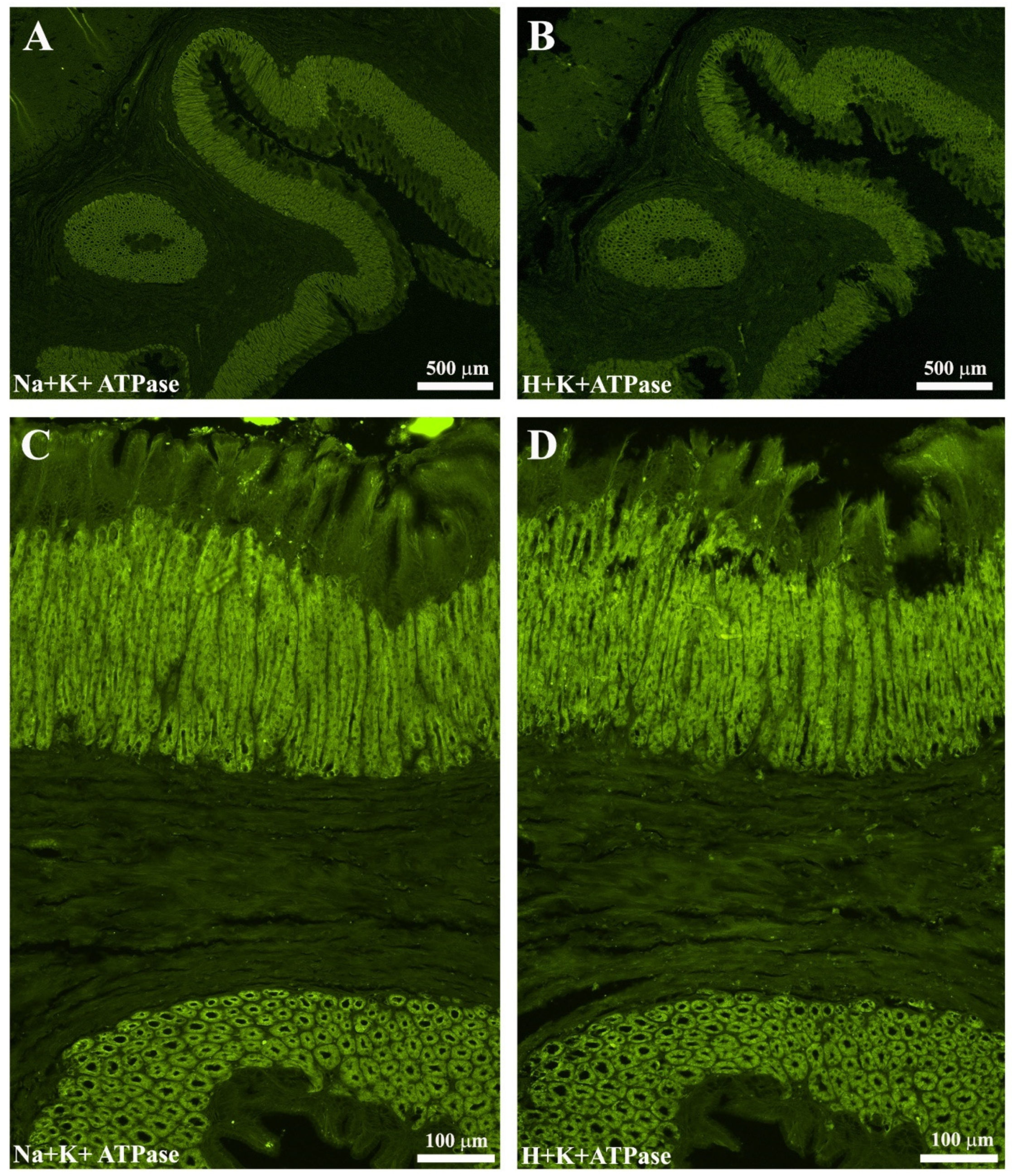
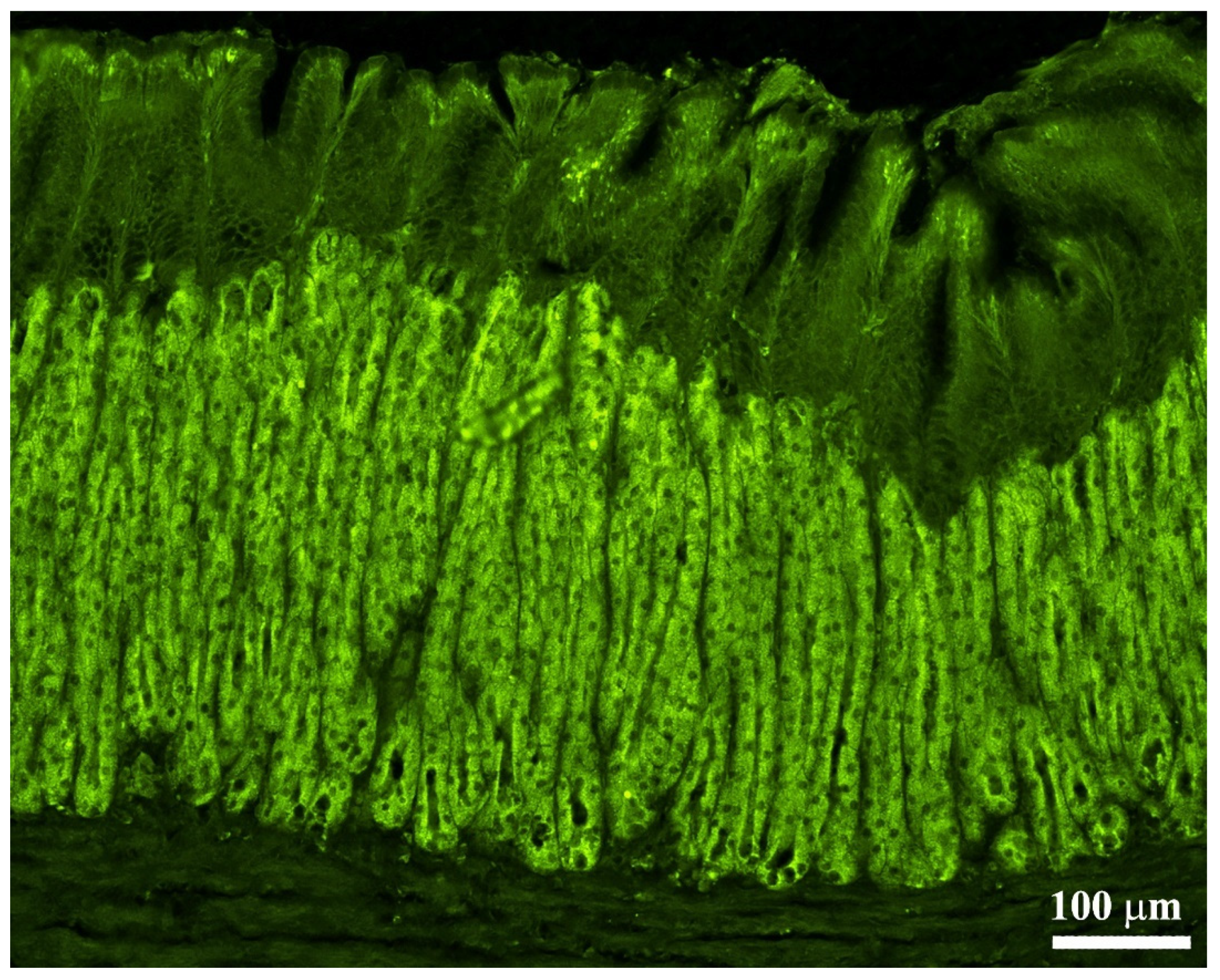
2.4. Antibody Specificity
2.5. Validation of the Na+K+-ATPase/H+K+-ATPase Antibodies as a Marker of Oxyntopeptic Cells (OPs)
2.6. Morphometric Evaluations
2.7. Statistical Analysis
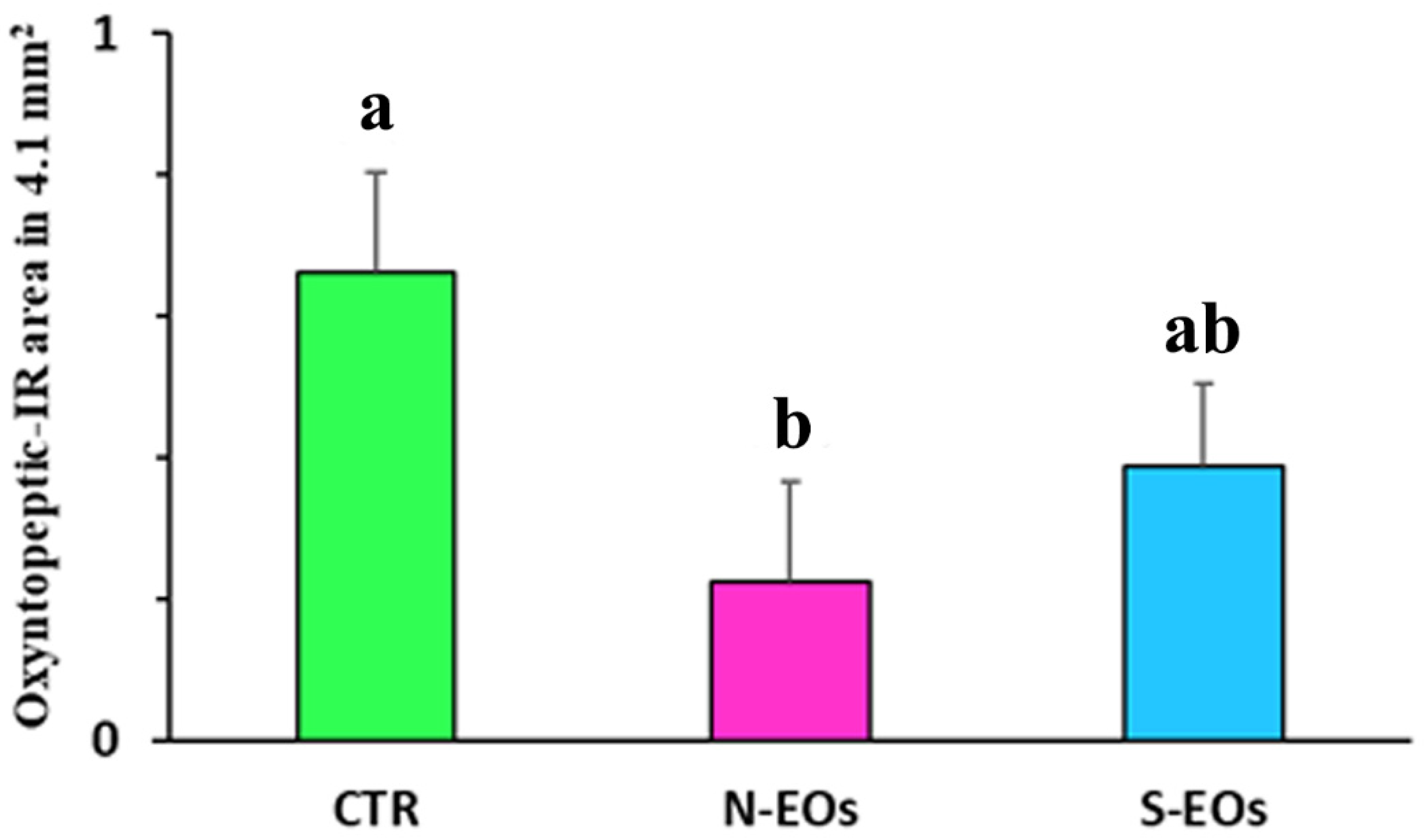
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutili, F.J.; Gatlin, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant Essential Oils as Fish Diet Additives: Benefits on Fish Health and Stability in Feed. Rev. Aquac. 2018, 10, 716–726. [Google Scholar] [CrossRef]
- Parma, L.; Pelusio, N.F.; Gisbert, E.; Esteban, M.A.; D’Amico, F.; Soverini, M.; Candela, M.; Dondi, F.; Gatta, P.P.; Bonaldo, A. Effects of Rearing Density on Growth, Digestive Conditions, Welfare Indicators and Gut Bacterial Community of Gilthead Sea Bream (Sparus Aurata L. 1758) Fed Different Fishmeal and Fish Oil Dietary Levels. Aquaculture 2020, 518, 734854. [Google Scholar] [CrossRef]
- De Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef] [Green Version]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Baydar, H.; Sağdiçb, O.; Özkanc, G.; Karadoğana, T. Antibacterial activity composition of essential oils Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Sökmen, M.; Serkedjieva, J.; Daferera, D.; Gulluce, M.; Polissiou, M.; Tepe, B.; Akpulat, H.A.; Sahin, F.; Sokmen, A. In Vitro Antioxidant, Antimicrobial, and Antiviral Activities of the Essential Oil and Various Extracts from Herbal Parts and Callus Cultures of Origanum Acutidens. J. Agric. Food Chem. 2004, 52, 3309–3312. [Google Scholar] [CrossRef] [PubMed]
- Barrington, W.E.J. Gastric digestion in the lower vertebrates. Biol. Rev. 1942, 17, 1–27. [Google Scholar] [CrossRef]
- Bomgren, P.; Einarsson, S. Similarities and Differences in Oxynticopeptic Cell Ultrastructure of One Marine Teleost, Gadus Morhua and One Freshwater Teleost, Oncorhynchus Mykiss, during Basal and Histamine-Stimulated Phases of Acid Secretion. Fish Physiol. Biochem. 1998, 18, 285–296. [Google Scholar] [CrossRef]
- Douglas, S.E.; Gawlicka, A.; Mandla, S.; Gallant, J.W. Ontogeny of the Stomach in Winter Flounder: Characterization and Expression of the Pepsinogen and Proton Pump Genes and Determination of Pepsin Activity. J. Fish Biol. 1999, 55, 897–915. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Roy, P.K.; Ferraris, R.P. Dietary Acidification Enhances Phosphorus Digestibility but Decreases H+/K+-ATPase Expression in Rainbow Trout. J. Exp. Biol. 2006, 209, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Elhassan, M.M.O.; Ali, A.M.; Blanch, A.; Kehlet, A.B.; Madekurozwa, M.-C. Morphological Responses of the Small Intestine of Broiler Chicks to Dietary Supplementation with a Probiotic, Acidifiers, and Their Combination. J. Appl. Poult. Res. 2019, 28, 108–117. [Google Scholar] [CrossRef]
- Darias, M.J.; Murray, H.M.; Gallant, J.W.; Douglas, S.E.; Yúfera, M.; Martínez-Rodríguez, G. Ontogeny of Pepsinogen and Gastric Proton Pump Expression in Red Porgy (Pagrus Pagrus): Determination of Stomach Functionality. Aquaculture 2007, 270, 369–378. [Google Scholar] [CrossRef]
- Hersey, S.J.; Sachs, G. Gastric Acid Secretion. Physiol. Rev. 1995, 75, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Tokhtaeva, E.; Jimenez, J.L.; Wen, Y.; Naini, B.V.; Heard, A.N.; Kim, S.; Capri, J.; Cohn, W.; Whitelegge, J.P.; et al. Helicobacter Pylori Infection Impairs Chaperone-Assisted Maturation of Na-K-ATPase in Gastric Epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G931–G945. [Google Scholar] [CrossRef]
- Wong, M.K.-S.; Pipil, S.; Ozaki, H.; Suzuki, Y.; Iwasaki, W.; Takei, Y. Flexible Selection of Diversified Na+/K+-ATPase α-Subunit Isoforms for Osmoregulation in Teleosts. Zool. Lett. 2016, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Pouyet, B.; Piloquet, P.; Vo, N.H.; Pradal, G.; Lefranc, G. Ultrastructural and Cytochemical Analysis of Na+, K+, ATPase and H+, K+, ATPase in Parietal Cells of Gastric Mucosa in the Rabbit. Histochemistry 1992, 97, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Kobayashi, M.; Sugimoto, T.; Araki, K. An Immunocytochemical Study of Regeneration of Gastric Epithelia in Rat Experimental Ulcers. Med. Mol. Morphol. 2005, 38, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, K.L.; Yang, W.K.; Lee, T.H.; Lo, W.Y.; Lee, J.D. Expression and Potential Roles of Sodium-Potassium ATPase and E-Cadherin in Human Gastric Adenocarcinoma. PLoS ONE 2017, 12, e0183692. [Google Scholar] [CrossRef]
- Helmstetter, C.; Reix, N.; T’Flachebba, M.; Pope, R.K.; Secor, S.M.; Le Maho, Y.; Lignot, J.-H. Functional Changes with Feeding in the Gastro-Intestinal Epithelia of the Burmese Python (Python Molurus). Zool. Sci. 2009, 26, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Sakata, I.; Nakamura, K.; Yamazaki, M.; Matsubara, M.; Hayashi, Y.; Kangawa, K.; Sakai, T. Ghrelin-Producing Cells Exist as Two Types of Cells, Closed-and Opened-Type Cells, in the Rat Gastrointestinal Tract. Peptides 2002, 23, 531–536. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L. The Distribution and Mechanism of Action of Ghrelin in the CNS Demonstrates a Novel Hypothalamic Circuit Regulating Energy Homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Horvath, T.L.; Castañeda, T.; Tang-Christensen, M.; Pagotto, U.; Tschop, M.H. Ghrelin as a Potential Anti-Obesity Target. Curr. Pharm. Des. 2003, 9, 1383–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A Role for Ghrelin in the Central Regulation of Feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin Enhances Appetite and Increases Food Intake in Humans. Clin. Endocrinol. Metab. 2001, 12, 5992. [Google Scholar] [CrossRef] [PubMed]
- Unniappan, S.; Canosa, L.F.; Peter, R.E. Orexigenic Actions of Ghrelin in Goldfish: Feeding-Induced Changes in Brain and Gut MRNA Expression and Serum Levels, and Responses to Central and Peripheral Injections. Neuroendocrinology 2004, 79, 100–108. [Google Scholar] [CrossRef]
- Amole, N.; Unniappan, S. Fasting Induces Preproghrelin MRNA Expression in the Brain and Gut of Zebrafish, Danio Rerio. Gen. Comp. Endocrinol. 2009, 161, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kaiya, H.; Kojima, M.; Hosoda, H.; Riley, L.G.; Hirano, T.; Grau, E.G.; Kangawa, K. Identification of Tilapia Ghrelin and Its Effects on Growth Hormone and Prolactin Release in the Tilapia, Oreochromis Mossambicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 421–429. [Google Scholar] [CrossRef]
- Kaiya, H.; Kojima, M.; Hosoda, H.; Moriyama, S.; Takahashi, A.; Kawauchi, H.; Kangawa, K. Peptide Purification, Complementary Deoxyribonucleic Acid (DNA) and Genomic DNA Cloning, and Functional Characterization of Ghrelin in Rainbow Trout. Endocrinology 2003, 144, 5215–5226. [Google Scholar] [CrossRef]
- Unniappan, S.; Lin, X.; Cervini, L.; Rivier, J.; Kaiya, H.; Kangawa, K.; Peter, R.E. Goldfish Ghrelin: Molecular Characterization of the Complementary Deoxyribonucleic Acid, Partial Gene Structure and Evidence for Its Stimulatory Role in Food Intake. Endocrinology 2002, 143, 4143–4146. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Harvey, S. Ghrelin: A Hypothalamic GH-Releasing Factor in Domestic Fowl (Gallus Domesticus). J. Endocrinol. 2002, 172, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Wada, R.; Sakata, I.; Kaiya, H.; Nakamura, K.; Hayashi, Y.; Kangawa, K.; Sakai, T. Existence of Ghrelin-Immunopositive and-Expressing Cells in the Proventriculus of the Hatching and Adult Chicken. Regul. Pept. 2003, 111, 123–128. [Google Scholar] [CrossRef]
- Neglia, S.; Arcamone, N.; Esposito, V.; Gargiulo, G.; de Girolamo, P. Presence and Distribution of Ghrelin-Immunopositive Cells in the Chicken Gastrointestinal Tract. Acta Histochem. 2005, 107, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kaiya, H.; Sakata, I.; Kojima, M.; Hosoda, H.; Sakai, T.; Kangawa, K. Structural Determination and Histochemical Localization of Ghrelin in the Red-Eared Slider Turtle, Trachemys Scripta Elegans. Gen. Comp. Endocrinol. 2004, 138, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Galas, L.; Chartrel, N.; Kojima, M.; Kangawa, K.; Vaudry, H. Immunohistochemical Localization and Biochemical Characterization of Ghrelin in the Brain and Stomach of the Frog Rana Esculenta. J. Comp. Neurol. 2002, 450, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Sakata, I.; Mori, T.; Kaiya, H.; Yamazaki, M.; Kangawa, K.; Inoue, K.; Sakai, T. Localization of Ghrelin-Producing Cells in the Stomach of the Rainbow Trout (Oncorhynchus Mykiss). Zoolog. Sci. 2004, 21, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Olsson, C.; Holbrook, J.D.; Bompadre, G.; Jönsson, E.; Hoyle, C.H.; Sanger, G.J.; Holmgren, S.; Andrews, P.L. Identification of genes for the ghrelin and motilin receptors and a novel related gene in fish, and stimulation of intestinal motility in zebrafish (Danio rerio) by ghrelin and motilin. Gen. Comp. Endocrinol. 2008, 155, 217–226. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Kittilson, J.D. The Role of Somatostatin in the Regulation of Metabolism in Fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 323–330. [Google Scholar] [CrossRef]
- Volkoff, H.; Canosa, L.F.; Unniappan, S.; Cerdá-Reverter, J.M.; Bernier, N.J.; Kelly, S.P.; Peter, R.E. Neuropeptides and the Control of Food Intake in Fish. Gen. Comp. Endocrinol. 2005, 142, 3–19. [Google Scholar] [CrossRef]
- Gahete, M.D.; Cordoba-Chacón, J.; Duran-Prado, M.; Malagón, M.M.; Martinez-Fuentes, A.J.; Gracia-Navarro, F.; Luque, R.M.; Castaño, J.P. Somatostatin and Its Receptors from Fish to Mammals: Gahete et Al. Ann. N. Y. Acad. Sci. 2010, 1200, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Bosi, G.; Lorenzoni, M.; Carosi, A.; Sayyaf Dezfuli, B. Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox Lucius (Linnaeus). Animals 2020, 10, 1479. [Google Scholar] [CrossRef]
- Lin, X.; Wang, P.; Ou, Y.; Li, J.; Wen, J. An Immunohistochemical Study on Endocrine Cells in the Neuroendocrine System of the Digestive Tract of Milkfish Chanos Chanos (Forsskal, 1775). Aquac. Res. 2017, 48, 1439–1449. [Google Scholar] [CrossRef]
- Groff, K.E.; Youson, J.H. An Immunohistochemical Study of the Endocrine Cells within the Pancreas, Intestine, and Stomach of the Gar (Lepisosteus Osseus L.). Gen. Comp. Endocrinol. 1997, 106, 1–16. [Google Scholar] [CrossRef]
- Krogdahl, A.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of Graded Levels of Standard Soybean Meal on Intestinal Structure, Mucosal Enzyme Activities, and Pancreatic Response in Atlantic Salmon (Salmo Salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Bonaldo, A.; Roem, A.J.; Fagioli, P.; Pecchini, A.; Cipollini, I.; Gatta, P.P. Influence of Dietary Levels of Soybean Meal on the Performance and Gut Histology of Gilthead Sea Bream (Sparus Aurata L.) and European Sea Bass (Dicentrarchus Labrax L.): Soybean Meal in Bass and Bream. Aquac. Res. 2008, 39, 970–978. [Google Scholar] [CrossRef]
- Bonvini, E.; Bonaldo, A.; Mandrioli, L.; Sirri, R.; Dondi, F.; Bianco, C.; Fontanillas, R.; Mongile, F.; Gatta, P.P.; Parma, L. Effects of Feeding Low Fishmeal Diets with Increasing Soybean Meal Levels on Growth, Gut Histology and Plasma Biochemistry of Sea Bass. Animal 2018, 12, 923–930. [Google Scholar] [CrossRef]
- Bonvini, E.; Bonaldo, A.; Parma, L.; Mandrioli, L.; Sirri, R.; Grandi, M.; Fontanillas, R.; Viroli, C.; Gatta, P.P. Feeding European Sea Bass with Increasing Dietary Fibre Levels: Impact on Growth, Blood Biochemistry, Gut Histology, Gut Evacuation. Aquaculture 2018, 494, 1–9. [Google Scholar] [CrossRef]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Improved Glucose and Lipid Metabolism in European Sea Bass (Dicentrarchus Labrax) Fed Short-Chain Fructooligosaccharides and Xylooligosaccharides. Aquaculture 2015, 441, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M.Á. Enrichment of Gilthead Seabream (Sparus Aurata L.) Diet with Microalgae: Effects on the Immune System. Fish Physiol. Biochem. 2012, 38, 1729–1739. [Google Scholar] [CrossRef]
- Busti, S.; Rossi, B.; Volpe, E.; Ciulli, S.; Piva, A.; D’Amico, F.; Soverini, M.; Candela, M.; Gatta, P.P.; Bonaldo, A.; et al. Effects of Dietary Organic Acids and Nature Identical Compounds on Growth, Immune Parameters and Gut Microbiota of European Sea Bass. Sci. Rep. 2020, 10, 21321. [Google Scholar] [CrossRef]
- Ramudu, K.R.; Dash, G. A Review on Herbal Drugs against Harmful Pathogens in Aquaculture. Am. J. Drug Discov. Dev. 2013, 3, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Shakya, S.R. Effect of Herbs and Herbal Products Feed Supplements on Growth in Fishes: A Review. Nepal J. Biotechnol. 2017, 5, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Serradell, A.; Torrecillas, S.; Makol, A.; Valdenegro, V.; Fernández-Montero, A.; Acosta, F.; Izquierdo, M.S.; Montero, D. Prebiotics and Phytogenics Functional Additives in Low Fish Meal and Fish Oil Based Diets for European Sea Bass (Dicentrarchus Labrax): Effects on Stress and Immune Responses. Fish. Shellfish Immunol. 2020, 100, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of Phytochemicals as Growth-Promoters and Endocrine Modulators in Fish Culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Cunha, J.A.; Heinzmann, B.M.; Baldisserotto, B. The Effects of Essential Oils and Their Major Compounds on Fish Bacterial Pathogens—A Review. J. Appl. Microbiol. 2018, 125, 328–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorothy, M.S.; Raman, S.; Nautiyal, V.; Singh, K.; Yogananda, T.; Kamei, M. Use of Potential Plant Leaves as Ingredient in Fish Feed-a Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 112–125. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential Oil and Aromatic Plants as Feed Additives in Non-Ruminant Nutrition: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Pirgozliev, V.; Mansbridge, S.; Rose, S.; Mackenzie, A.; Beccaccia, A.; Karadas, F.; Ivanova, S.; Staykova, G.; Olowatosin, O.; Bravo, D. Dietary Essential Oils Improve Feed Efficiency and Hepatic Antioxidant Content of Broiler Chickens. Animal 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Watts, J.; Schreier, H.; Lanska, L.; Hale, M. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Wannes, D.; Jridi, M.; Marzouki, L.; Sebai, H. The Potential of Thymus Vulgaris Aqueous Extract to Protect against Delayed Gastric Emptying and Colonic Constipation in Rats. RSC Adv. 2019, 9, 20593–20602. [Google Scholar] [CrossRef] [Green Version]
- Guesmi, F.; Ben Ali, M.; Barkaoui, T.; Tahri, W.; Mejri, M.; Ben-Attia, M.; Bellamine, H.; Landoulsi, A. Effects of Thymus Hirtus sp. Algeriensis Boiss. et Reut. (Lamiaceae) Essential Oil on Healing Gastric Ulcers According to Sex. Lipids Health Dis. 2014, 13, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Macedo, L.M.; dos Santos, É.M.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus Officinalis L., Syn Salvia Rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Sandhya, S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus Officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A Novel Insight on an Ancient Aromatic Plant: The Rosemary (Rosmarinus Officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New Insights into Antibacterial and Antioxidant Activities of Rosemary Essential Oils and Their Main Components. Food Control. 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Pereira, P.; Tysca, D.; Oliveira, P.; da Silva Brum, L.F.; Picada, J.N.; Ardenghi, P. Neurobehavioral and Genotoxic Aspects of Rosmarinic Acid. Pharmacol. Res. 2005, 52, 199–203. [Google Scholar] [CrossRef]
- Pérez-Fons, L.; Aranda, F.J.; Guillén, J.; Villalaín, J.; Micol, V. Rosemary (Rosmarinus Officinalis) Diterpenes Affect Lipid Polymorphism and Fluidity in Phospholipid Membranes. Arch. Biochem. Biophys. 2006, 453, 224–236. [Google Scholar] [CrossRef]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; de Portella, R.L.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.M.; da Luz, S.C.A.; Boligon, A.A.; et al. Protective Action of Ethanolic Extract of Rosmarinus officinalis L. in Gastric Ulcer Prevention Induced by Ethanol in Rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Dias, P.C.; Foglio, M.A.; Possenti, A.; de Carvalho, J.E. Antiulcerogenic Activity of Crude Hydroalcoholic Extract of Rosmarinus officinalis L. J. Ethnopharmacol. 2000, 69, 57–62. [Google Scholar] [CrossRef]
- Sindi, H.A.; Basaprain, R. Protective Effect of Ginger and Cinnamon Aqueous Extracts Against Aspirin-Induced Peptic Ulcer. World Appl. Sci. J. 2016, 34, 1436–1448. [Google Scholar] [CrossRef]
- Ozbayer, C.; Kurt, H.; Ozdemir, Z.; Tuncel, T.; Moheb Saadat, S.; Burukoglu, D.; Senturk, H.; Degirmenci, I.; Gunes, H.V. Gastroprotective, Cytoprotective and Antioxidant Effects of Oleum Cinnamomi on Ethanol Induced Damage. Cytotechnology 2014, 66, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Rafsanjani, F.N.; Shahrani, M.; Vahedian, J. Garlic effects on gastric acid and pepsin secretions in rat. Pak. J. Med. Sci. 2006, 22, 265–268. [Google Scholar]
- Lee, D.H.; Lim, S.R.; Han, J.J.; Lee, S.W.; Ra, C.S.; Kim, J.D. Effects of Dietary Garlic Powder on Growth, Feed Utilization and Whole-Body Composition Changes in Fingerling Sterlet Sturgeon, Acipenser ruthenus. Asian-Australas. J. Anim. Sci. 2014, 27, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Gastroprotective Effect of Garlic in Indomethacin Induced Gastric Ulcer in Rats. Nutrition 2016, 32, 849–854. [Google Scholar] [CrossRef]
- Koelz, H.R. Gastric Acid in Vertebrates. Scand. J. Gastroenterol. 1992, 27, 2–6. [Google Scholar] [CrossRef]
- Vial, J.D.; Garrido, J. Comparative Cytology of Hydrochloric Acid Secreting Cells. Arch. Biol. Med. Exp. 1979, 12, 39–48. [Google Scholar] [PubMed]
- Kil, D.Y.; Kwon, W.B.; Kim, B.G. Dietary Acidifiers in Weanling Pig Diets: A Review. Rev. Colomb. Cienc. Pecu. 2011, 24, 231–247. [Google Scholar]
- Bosi, P.; Mazzoni, M.; De Filippi, S.; Trevisi, P.; Casini, L.; Petrosino, G.; Lalatta-Costerbosa, G. A Continuous Dietary Supply of Free Calcium Formate Negatively Affects the Parietal Cell Population and Gastric RNA Expression for H+/K+-ATPase in Weaning Pigs. J. Nutr. 2006, 136, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, M.; Le Gall, M.; De Filippi, S.; Minieri, L.; Trevisi, P.; Wolinski, J.; Lalatta-Costerbosa, G.; Lallès, J.-P.; Guilloteau, P.; Bosi, P. Supplemental Sodium Butyrate Stimulates Different Gastric Cells in Weaned Pigs. J. Nutr. 2008, 138, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Jun-Sheng, L.; Jian-Lin, L.; Ting-Ting, W. Ontogeny of Protease, Amylase and Lipase in the Alimentary Tract of Hybrid Juvenile Tilapia (Oreochromis niloticus x Oreochromis aureus). Fish Physiol. Biochem. 2006, 32, 295–303. [Google Scholar] [CrossRef]
- Lückstädt, C. Effect of Dietary Potassium Diformate on the Growth and Digestibility of Atlantic Salmon (Salmo salar). In Proceedings of the 13th International Symposium on Fish Nutrition & Feeding, Florianopolis, Brazil, 28 August–1 September 2008; p. 279. [Google Scholar]
- Nikolopoulou, D.; Moutou, K.A.; Fountoulaki, E.; Venou, B.; Adamidou, S.; Alexis, M.N. Patterns of Gastric Evacuation, Digesta Characteristics and PH Changes along the Gastrointestinal Tract of Gilthead Sea Bream (Sparus Aurata L.) and European Sea Bass (Dicentrarchus Labrax L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 406–414. [Google Scholar] [CrossRef]
- Parma, L.; Yúfera, M.; Navarro-Guillén, C.; Moyano, F.J.; Soverini, M.; D’Amico, F.; Candela, M.; Fontanillas, R.; Gatta, P.P.; Bonaldo, A. Effects of Calcium Carbonate Inclusion in Low Fishmeal Diets on Growth, Gastrointestinal PH, Digestive Enzyme Activity and Gut Bacterial Community of European Sea Bass (Dicentrarchus labrax L.) Juveniles. Aquaculture 2019, 510, 283–292. [Google Scholar] [CrossRef]
- Friis-Hansen, L. Gastric Functions in Gastrin Gene Knock-Out Mice. Pharmacol. Toxicol. 2002, 91, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.-M.; Wang, X.; Friis-Hansen, L.; Waldum, H.L.; Halgunset, J.; Wadström, T.; Chen, D. Chronic Helicobacter Pylori Infection Results in Gastric Hypoacidity and Hypergastrinemia in Wild-Type Mice but Vagally Induced Hypersecretion in Gastrin-Deficient Mice. Regul. Pept. 2003, 115, 161–170. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, C.-M.; Hakanson, R.; Samuelson, L.C.; Rehfeld, J.F.; Friis-Hansen, L. Altered Control of Gastric Acid Secretion in Gastrin-Cholecystokinin Double Mutant Mice. Gastroenterology 2004, 126, 476–487. [Google Scholar] [CrossRef]
- Samuelson, L.C.; Hinkle, K.L. Insights into the Regulation of Gastric Acid Secretion Through Analysis of Genetically Engineered Mice. Annu. Rev. Physiol. 2003, 65, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.N.; Berne, R.M.; Koeppen, B.M.; Stanton, B.A. Berne & Levy Principles of Physiology; Elsevier Mosby: Philadelphia, PA, USA, 2006; ISBN 0-323-03195-1. [Google Scholar]
- Bakir, B.; Karadag Sari, E.; Elis Yildiz, S.; Asker, H. Effects of Thymoquinone Supplementation on Somatostatin Secretion in Pancreas Tissue of Rats. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 409–413. [Google Scholar] [CrossRef]
- Colombo, M.; Priori, D.; Gandolfi, G.; Boatto, G.; Nieddu, M.; Bosi, P.; Trevisi, P. Effect of Free Thymol on Differential Gene Expression in Gastric Mucosa of the Young Pig. Animal 2014, 8, 786–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [Green Version]
- Bossus, M.; Charmantier, G.; Lorin-Nebel, C. Transient Receptor Potential Vanilloid 4 in the European Sea Bass Dicentrarchus Labrax: A Candidate Protein for Osmosensing. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2011, 160, 43–51. [Google Scholar] [CrossRef]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The Zebrafish Ortholog of TRPV1 Is Required for Heat-Induced Locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef] [Green Version]
- Nisembaum, L.G.; Besseau, L.; Paulin, C.-H.; Charpantier, A.; Martin, P.; Magnanou, E.; Fuentès, M.; Delgado, M.-J.; Falcón, J. In the Heat of the Night: Thermo-TRPV Channels in the Salmonid Pineal Photoreceptors and Modulation of Melatonin Secretion. Endocrinology 2015, 156, 4629–4638. [Google Scholar] [CrossRef] [Green Version]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient Receptor Potential Channels: Targeting Pain at the Source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faussone-Pellegrini, M.S.; Taddei, A.; Bizzoco, E.; Lazzeri, M.; Vannucchi, M.G.; Bechi, P. Distribution of the Vanilloid (Capsaicin) Receptor Type 1 in the Human Stomach. Histochem. Cell Biol. 2005, 124, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 Receptor Differs in Quiescent Inflammatory Bowel Disease with or without Abdominal Pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.; Szitter, I.; Kemény, Á.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, Á.; Gódi, S.; Szabó, I.; Szolcsányi, J.; et al. Upregulation of the Transient Receptor Potential Ankyrin 1 Ion Channel in the Inflamed Human and Mouse Colon and Its Protective Roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef]
- Csekő, K.; Pécsi, D.; Kajtár, B.; Hegedűs, I.; Bollenbach, A.; Tsikas, D.; Szabó, I.L.; Szabó, S.; Helyes, Z. Upregulation of the TRPA1 Ion Channel in the Gastric Mucosa after Iodoacetamide-Induced Gastritis in Rats: A Potential New Therapeutic Target. Int. J. Mol. Sci. 2020, 21, 5591. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Viana, F. TRPA1 Channels: Molecular Sentinels of Cellular Stress and Tissue Damage: TRPA1 Channels and Cellular Stress. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef] [Green Version]
- Ellis, A.E. Innate Host Defense Mechanisms of Fish against Viruses and Bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A Review on the Interactions between Gut Microbiota and Innate Immunity of Fish: Table 1. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Múzquiz, J.L. The Role of Probiotics in Aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; le Coutre, J. Anti-Obesity and Anti-Hyperglycemic Effects of Cinnamaldehyde via Altered Ghrelin Secretion and Functional Impact on Food Intake and Gastric Emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef] [Green Version]
- Okamura, H.; Yasuhara, J.C.; Fambrough, D.M.; Takeyasu, K. P-Type ATPases in Caenorhabditis and Drosophila: Implications for Evolution of the P-Type ATPase Subunit Families with Special Reference to the Na,K-ATPase and H,K-ATPase Subgroup. J. Membr. Biol. 2003, 191, 13–24. [Google Scholar] [CrossRef]
- Corradi, N.; Sanders, I.R. Evolution of the P-Type II ATPase Gene Family in the Fungi and Presence of Structural Genomic Changes among Isolates of Glomus Intraradices. BMC Evol. Biol. 2006, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, O.; Castro, L.F.C.; Smolka, A.J.; Fontainhas, A.; Wilson, J.M. The Gastric Phenotype in the Cypriniform Loaches: A Case of Reinvention? PLoS ONE 2016, 11, e0163696. [Google Scholar] [CrossRef]
- Hevans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]



| Fatty Acid Composition (g/100 g) | CTR | N-EOs | S-EOs |
|---|---|---|---|
| Caprinic acid (10:0) | 0.006 ± 0.001 | 0.006 ± 0.001 | |
| Lauric acid (12:0) | 0.027 ± 0.006 | 0.031 ± 0.007 | 0.028 ± 0.006 |
| Myristic acid (14:0) | 0.163 ± 0.035 | 0.146 ± 0.031 | 0.150 ± 0.032 |
| Pentadecanoic acid (15:0) | 0.020 ± 0.004 | 0.019 ± 0.004 | 0.019 ± 0.004 |
| Palmitic acid (16:0) | 1.570 ± 0.190 | 1.550 ± 0.180 | 1.540 ± 0.180 |
| Isoheptadecanoic acid (17:0 iso) | 0.013 ± 0.003 | 0.011 ± 0.002 | |
| Hexadecenoic acid (16:1) | 0.220 ± 0.630 | 0.193 ± 0.041 | 0.210 ± 0.630 |
| 14-Methylhexadecanoic acid (17:0 anteiso) | 0.016 ± 0.003 | 0.015 ± 0.003 | 0.014 ± 0.003 |
| Margaric acid (17:0) | 0.026 ± 0.005 | 0.025 ± 0.005 | 0.024 ± 0.005 |
| Heptadecenoic acid (17:1) | 0.028 ± 0.004 | 0.029 ± 0.005 | 0.029 ± 0.004 |
| Stearic acid (18:0) | 0.425 ± 0.060 | 0.422 ± 0.061 | 0.418 ± 0.060 |
| Octadecenoic acid (18:1) | 8.250 ± 0.680 | 9.090 ± 0.760 | 8.930 ± 0.750 |
| Octadecadienoic acid (18:2) | 3.900 ± 0.370 | 4.070 ± 0.380 | 3.960 ± 0.380 |
| Arachidic acid (20:0) | 0.090 ± 0.019 | 0.091 ± 0.020 | 0.090 ± 0.019 |
| Octadecatrienoic acid (18:3) | 1.550 ± 0.180 | 1.440 ± 0.170 | 1.480 ± 0.170 |
| Eicosenoic acid (20:1) | 0.446 ± 0.063 | 0.374 ± 0.056 | 0.395 ± 0.058 |
| Stearidonic acid (18:4 n-3) | 0.050 ± 0.011 | 0.043 ± 0.009 | 0.044 ± 0.009 |
| Behenic acid (22:0) | 0.047 ± 0.010 | 0.050 ± 0.011 | 0.048 ± 0.010 |
| Docosanoic acid (22:1) | 0.267 ± 0.042 | 0.229 ± 0.036 | 0.234 ± 0.038 |
| Lignoceric acid (24:0) | 0.062 ± 0.013 | 0.061 ± 0.013 | 0.037 ± 0.008 |
| Polyunsaturated fatty acids (>C20) | 0.386 ± 0.049 | 0.342 ± 0.046 | 0.337 ± 0.046 |
| Polyunsaturated fatty acids | 6.210 ± 0.420 | 6.160 ± 0.420 | 6.100 ± 0.420 |
| Monounsaturated fatty acids | 9.260 ± 0.800 | 9.960 ± 0.760 | 9.840 ± 0.850 |
| Saturated fatty acids | 2.470 ± 0.210 | 2.420 ± 0.200 | 2.400 ± 0.200 |
| Fatty acids ratios | |||
| Polyunsaturated fatty acids/monounsaturated fatty acids | 0.671 ± 0.074 | 0.618 ± 0.064 | 0.620 ± 0.069 |
| Polyunsaturated fats/saturated fatty acids | 2.510 ± 0.280 | 2.550 ± 0.280 | 2.540 ± 0.280 |
| Volatile organic acids (mg/kg) | |||
| Acetic acid | 627 ± 94 | 700 ± 110 | 700 ± 110 |
| Butyric acid | 67 ± 22 | 62 ± 22 | 66 ± 22 |
| Experimental Diet | CTR | N-EOs | S-EOs | p Value |
|---|---|---|---|---|
| IBW (g) | 75.3 ± 2.88 | 74.9 ± 1.54 | 74.9 ± 2.42 | 0.835 |
| FBW (g) | 274.2 ± 7.81 | 267.9 ± 3.49 | 263.6 ± 4.19 | 0.137 |
| SGR | 1.10 ± 0.05 | 1.09 ± 0.03 | 1.07 ± 0.02 | 0.529 |
| FI | 1.47 ± 0.05 | 1.41 ± 0.02 | 1.42 ± 0.03 | 0.158 |
| FCR | 1.52 ± 0.08 | 1.54 ± 0.06 | 1.52 ± 0.02 | 0.894 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzoni, M.; Lattanzio, G.; Bonaldo, A.; Tagliavia, C.; Parma, L.; Busti, S.; Gatta, P.P.; Bernardi, N.; Clavenzani, P. Effect of Essential Oils on the Oxyntopeptic Cells and Somatostatin and Ghrelin Immunoreactive Cells in the European Sea Bass (Dicentrarchus labrax) Gastric Mucosa. Animals 2021, 11, 3401. https://doi.org/10.3390/ani11123401
Mazzoni M, Lattanzio G, Bonaldo A, Tagliavia C, Parma L, Busti S, Gatta PP, Bernardi N, Clavenzani P. Effect of Essential Oils on the Oxyntopeptic Cells and Somatostatin and Ghrelin Immunoreactive Cells in the European Sea Bass (Dicentrarchus labrax) Gastric Mucosa. Animals. 2021; 11(12):3401. https://doi.org/10.3390/ani11123401
Chicago/Turabian StyleMazzoni, Maurizio, Giulia Lattanzio, Alessio Bonaldo, Claudio Tagliavia, Luca Parma, Serena Busti, Pier Paolo Gatta, Nadia Bernardi, and Paolo Clavenzani. 2021. "Effect of Essential Oils on the Oxyntopeptic Cells and Somatostatin and Ghrelin Immunoreactive Cells in the European Sea Bass (Dicentrarchus labrax) Gastric Mucosa" Animals 11, no. 12: 3401. https://doi.org/10.3390/ani11123401







