Risk and Predictive Factors of Leptospirosis in Dogs Diagnosed with Kidney and/or Liver Disease in Selangor, Malaysia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Inclusion Criteria
2.2. Molecular Detection Using Polymerase Chain Reaction (PCR)
2.3. Isolation and Identification of Leptospira spp.
2.4. Risk Factors Analysis
2.5. Statistical Analysis
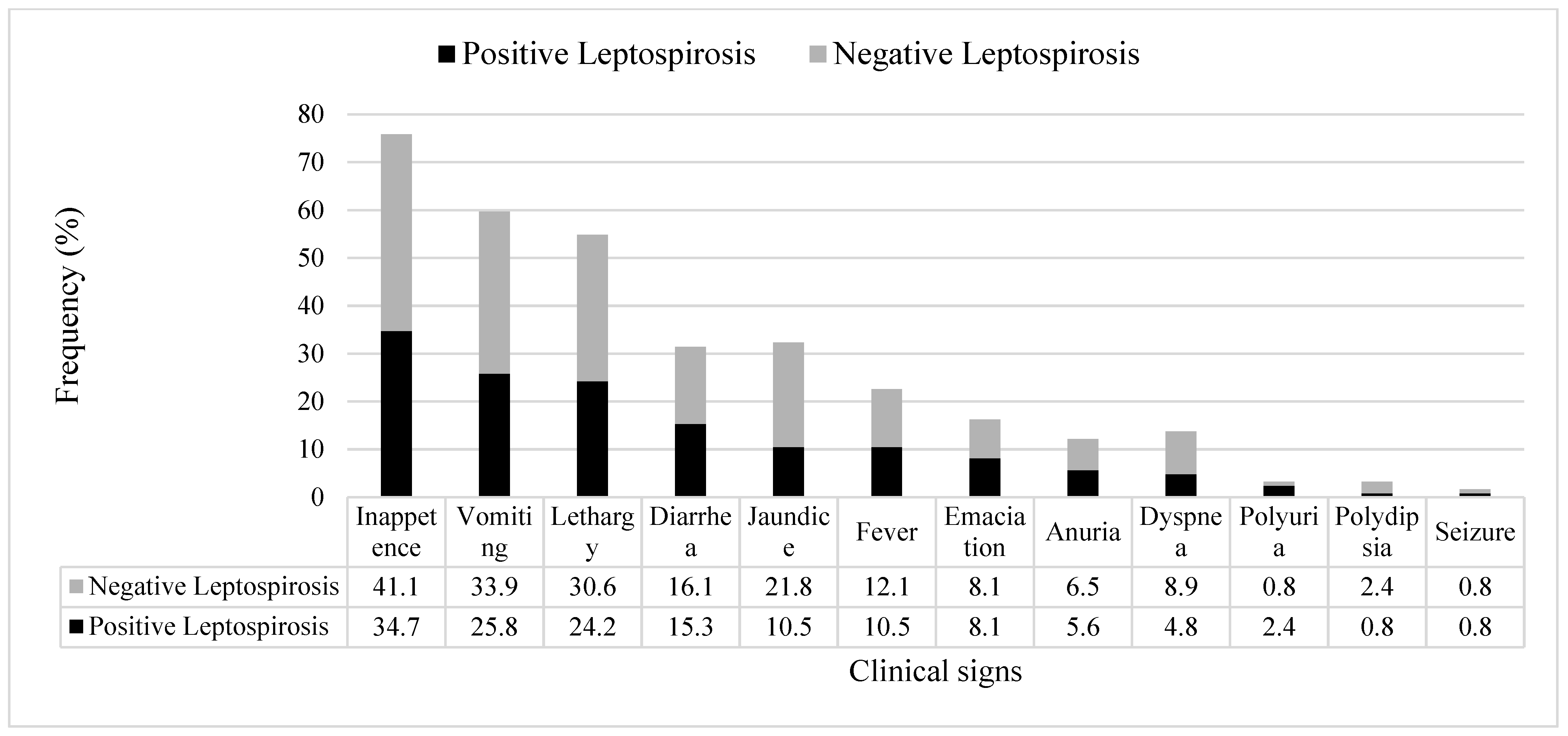
3. Results
3.1. Molecular Detection Using Polymerase Chain Reaction (PCR)
3.2. Isolation and Identification of Leptospira spp.
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [Green Version]
- Adler, B.; de la Pena Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Goarant, C. Leptospirosis: Risk factors and management challenges in developing countries. Res. Rep. Trop. Med. 2016, 7, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, J. Canine leptospirosis in Canada: A veterinarian’s perspective. Can. Med. Assoc. J. 2008, 178, 397–398. [Google Scholar] [CrossRef]
- Azocar-Aedo, L.; Monti, G. Meta-analyses of factors associated with leptospirosis in domestic dogs. Zoonoses Public Health 2016, 63, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef]
- Fiorello, C.V.; Straub, M.H.; Schwartz, L.M.; Liu, J.; Campbell, A.; Kownacki, A.K.; Foley, J.E. Multiple-host pathogens in domestic hunting dogs in Nicaragua’s Bosawás Biosphere Reserve. Acta Trop. 2017, 167, 183–190. [Google Scholar] [CrossRef]
- Suepaul, S.M.; Carrington, C.V.F.; Campbell, M.; Borde, G.; Adesiyun, A.A. Serovars of Leptospira isolated from dogs and rodents. Epidemiol. Infect. 2010, 138, 1059–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwijnenberg, R.J.G.; Smythe, L.D.; Symonds, M.L.; Dohnt, M.F.; Toribio, J.A. Cross-sectional study of canine leptospirosis in animal shelter populations in mainland Australia. Aust. Vet. J. 2008, 86, 317–323. [Google Scholar] [CrossRef]
- Hartskeerl, R.A.; Collares-Pereira, M.; Ellis, W.A. Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin. Microbiol. Infect. 2011, 17, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.H.; Ismail, R.; Lau, S.F.; Megat-Abdul-Rani, P.A.; Mohd-Mohidin, T.B.; Daud, F.; Bahaman, A.R.; Khairani-Bejo, S.; Radzi, R.; Khor, K.H. Risk factors and prediction of leptospiral seropositivity among dogs and dog handlers in Malaysia. Int. J. Environ. Res. Public Health 2019, 16, 1499. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Ramirez, C.V.; Gaxiola-Camacho, S.M.; Verdugo, I.E.; Ramírez, I.O.; Rivas-Llamas, J.R. Prevalence and risk factors associated with serovars of Leptospira in dogs, related human seropositive. J. Dairy Vet. Anim. Res. 2017, 6, 275–279. [Google Scholar]
- White, A.M.; Zambrana-Torrelio, C.; Allen, T.; Rostal, M.K.; Wright, A.K.; Ball, E.C. Hotspots of canine leptospirosis in the United States of America. Vet. J. 2017, 222, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Harkin, K.R.; Roshto, Y.M.; Sullivan, J.T.; Purvis, T.J.; Chengappa, M.M. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospirosis in dogs. J. Am. Vet. Med. Assoc. 2003, 222, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM small animal consensus statement on leptospirosis: Diagnosis, epidemiology, treatment, and prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, J.P.; Teixeira, M.G.; Costa, M.C.; Mendes, C.M.; Guimaraes, P.; Reis, M.G.; Ko, A.; Barreto, M.L. Factors associated with Leptospira sp. infection in a large urban center in Northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Monahan, A.M.; Miller, I.S.; Nally, J.E. Leptospirosis: Risks during recreational activities. J. Appl. Microbiol. 2009, 107, 707–716. [Google Scholar] [CrossRef] [PubMed]
- IRIS. Guideline Recommendations for Grading of AKI in Dogs and Cats. Available online: http://www.iris-kidney.com/guidelines/grading.html (accessed on 1 April 2021).
- Webster, C.R.L.; Cooper, J.C. Diagnostic approach to hepatobiliary disease. In Kirk’s Current Veterinary Therapy, 15th ed.; Bonagura, J., Twedt, D., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 569–575. [Google Scholar]
- Ahmed, S.A.; Sandai, D.A.; Musa, S.; Hoe, C.H.; Riadzi, M.; Lau, K.L.; Tang, T.H. Rapid diagnosis of leptospirosis by multiplex PCR. Malays. J. Med. Sci. 2012, 19, 9–16. [Google Scholar] [PubMed]
- Sabri, A.R.; Khairani-Bejo, S.; Zunita, Z.; Hassan, L. Molecular detection of Leptospira sp. in cattle and goats in Kelantan, Malaysia after a massive flood using multiplex polymerase chain reaction. Trop. Biomed. 2019, 36, 165–171. [Google Scholar]
- Tansuphasiri, U.; Thipsuk, C.; Phulsuksombati, D.; Chanyasanha, C. Duplex PCR-hybridization based detection of pathogenic Leptospira in environmental water samples obtained from endemic areas in northeast region of Thailand. Southeast Asian J. Trop. Med. Public Health 2006, 37, 729–741. [Google Scholar] [PubMed]
- World Organization for Animal Health (OIE). OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2004. [Google Scholar]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A single multilocus sequence typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dog Ages & Stages. DogTime. Available online: https://dogtime.com/dog-health/dog-ages-and-dog-stages/253-ages-stages (accessed on 23 September 2021).
- Benacer, D.; Thong, K.L.; Ooi, P.T.; Souris, M.; Lewis, J.W.; Ahmed, A.A.; Mohd-Zain, S.N. Serological and molecular identification of Leptospira spp. in swine and stray dogs from Malaysia. Trop. Biomed. 2017, 34, 89–97. [Google Scholar] [PubMed]
- Khor, K.H.; Tan, W.X.; Lau, S.F.; Mohd-Azri, R.; Rozanaliza, R.; Siti, K.B.; Abdul-Rani, B. Seroprevalence and molecular detection of leptospirosis from a dog shelter. Trop. Biomed. 2016, 33, 276–284. [Google Scholar] [PubMed]
- Lau, S.F.; Low, K.N.; Khor, K.H.; Roslan, M.A.; Bejo, S.K.; Radzi, R.; Bahaman, A.R. Prevalence of leptospirosis in healthy dogs and dogs with kidney disease in Klang valley, Malaysia. Trop. Biomed. 2016, 33, 469–475. [Google Scholar] [PubMed]
- Lau, S.F.; Wong, J.Y.; Khor, K.H.; Roslan, M.A.; Abdul-Rahman, M.S.; Bejo, S.K.; Radzi, R.; Bahaman, A.R. Seroprevalence of leptospirosis in working dogs. Top. Companion Anim. Med. 2017, 32, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Andre-Fontaine, G. Canine leptospirosis—Do we have a problem? Vet. Microbiol. 2006, 117, 19–24. [Google Scholar] [CrossRef]
- Van-de-Maele, I.; Claus, A.; Haesebrouck, F.; Daminet, S. Leptospirosis in dogs: A review with emphasis on clinical aspects. Vet. Rec. 2008, 163, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Canine Leptospirosis: A Perspective on Recent Trends. Available online: https://todaysveterinarypractice.com/canine-leptospirosis-a-perspective-on-recent-trends/ (accessed on 1 April 2021).
- Miotto, B.A.; Tozzi, B.F.; Penteado, M.S.; Guilloux, A.G.A.; Moreno, L.Z.; Heinemann, M.B.; Moreno, A.M.; Lilenbaum, W.; Hagiwara, M.K. Diagnosis of acute canine leptospirosis using multiple laboratory tests and characterization of the isolated strains. BMC Vet. Res. 2018, 14, 222. [Google Scholar] [CrossRef]
- Latosinski, G.S.; Fornazari, F.; Babboni, S.D.; Caffaro, K.; Paes, A.C.; Langoni, H. Serological and molecular detection of Leptospira spp. in dogs. Rev. Soc. Bras. Med. Trop. 2018, 51, 364–367. [Google Scholar] [CrossRef]
- Santanna, R.; Vieira, A.S.; Grapiglia, J.; Lilenbaum, W. High number of asymptomatic dogs as leptospiral carriers in an endemic area indicates a serious public health concern. Epidemiol. Infect. 2017, 145, 1852–1854. [Google Scholar] [CrossRef] [Green Version]
- Greene, C.E.; Sykes, J.E.; Brown, C.A.; Hartmann, K. Leptospirosis. In Infectious Diseases of the Dog and Cat, 3rd ed.; Greene, C.E., Ed.; Saunders Elsevier: St. Louis, MO, USA, 2006; pp. 402–417. [Google Scholar]
- Benacer, D.; Mohd-Zain, S.N.; Sim, S.Z.; Mohd-Khalid, M.K.; Galloway, R.L.; Souris, M.; Thong, K.L. Determination of Leptospira borgpetersenii serovar Javanica and Leptospira interrogans serovar Bataviae as the persistent Leptospira serovars circulating in the urban rat populations in Peninsular Malaysia. Parasit. Vectors 2016, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Ghada, A.K.; Bahaman, A.R.; Khairani-Bejo, S.; Zakaria, Z.; Garba, B. Serological and molecular prevalence of Leptospira infection in rat populations in Kuala Lumpur. Aust. J. Basic Appl. Sci. 2017, 11, 62–72. [Google Scholar]
- Gautam, R.; Wu, C.C.; Guptill, L.F.; Potter, A.; Moore, G.E. Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. J. Am. Vet. Med. Assoc. 2010, 237, 293–298. [Google Scholar]
- Ghneim, G.S.; Viers, J.H.; Chomel, B.B.; Kass, P.H.; Descollonges, D.A.; Johnson, M.L. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet. Res. 2007, 38, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, R.E. Canine leptospirosis. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.R.D.F.S.; Castro, V.; Mineiro, A.L.B.B.; Prianti, M.D.G.; Martins, G.H.C.; Santana, M.D.V.; Brito, L.M.; Silva, S.M.M.S. Sociodemographic and environmental analysis for the occurrence of anti-Leptospira antibodies in dogs of Teresina, Piauí, Brazil. Cienc. Saude Colet. 2018, 23, 1403–1414. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.P. Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Prev. Vet. Med. 2002, 56, 215–226. [Google Scholar] [CrossRef]
- Ward, M.P.; Glickman, L.T.; Guptill, L.F. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). J. Am. Vet. Med. Assoc. 2002, 220, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Guptill, L.F.; Prahl, A.; Ching, W.C. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002). J. Am. Vet. Med. Assoc. 2004, 224, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Meeyam, T.; Tablerk, P.; Petchanok, B.; Pichpol, D.; Padungtod, P. Seroprevalence and risk factors associated with leptospirosis in dogs. Southeast Asian J. Trop. Med. Public Health 2006, 37, 148–153. [Google Scholar]
- Ricardo, T.; Previtali, M.A.; Signorini, M. Meta-analysis of risk factors for canine leptospirosis. Prev. Vet. Med. 2020, 181, 105037. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.S.; Ismail, H.B.; Jaafar, M.H.B.; Rampal, S. The predictive factors for severe leptospirosis cases in Kedah. Trop. Med. Infect. Dis. 2020, 5, 79. [Google Scholar] [CrossRef]
- Garba, B.; Bahaman, A.R.; Khairani-Bejo, S.; Zakaria, Z.; Mutalib, A.R. Retrospective study of leptospirosis in Malaysia. EcoHealth 2017, 14, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Sembiring, E. Diagnostic approach in leptospirosis patients. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012089. [Google Scholar] [CrossRef]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A. WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef] [Green Version]
| Positive Leptospirosis (n = 53) | Negative Leptospirosis (n = 71) |
|---|---|
| Urea: 46.7 ± 51.3 mmol/L Creatinine: 326.2 ± 458.0 µmol/L | Urea: 34.0 ± 24.1 mmol/L Creatinine: 343.1 ± 339.6 µmol/L |
| ALT: 154.9 ± 138.6 U/L ALP: 370.5 ± 753.3 U/L | ALT: 179.6 ± 534.5 U/L ALP: 314.7 ± 400.4 U/L |
| Demographic | No. of Dogs (%) | Demographic | No. of Dogs (%) |
|---|---|---|---|
| Age (years old) * Young (≤1) Adult (<1–6) Senior (≥6) | 13 (10.5%) 48 (38.7%) 63 (50.8%) | Management Indoor Outdoor | 51 (41.1%) 73 (58.9%) |
| Breed * Large Medium Small | 31 (22.6%) 65 (52.4%) 28 (25.0%) | Type of household Single Multiple | 68 (54.8%) 56 (45.2%) |
| Sex Male Female | 76 (61.3%) 48 (38.7%) | Rat exposure Exposed Not exposed | 78 (62.9%) 46 (37.1%) |
| Vaccination status Vaccinated Non-vaccinated | 52 (41.9%) 72 (58.1%) | Clinical illness (days) Acute (≤7) Chronic (>7) | 102 (82.3%) 22 (17.7%) |
| Dog ID | Sample Obtained | Identification | |
|---|---|---|---|
| Serotyping | MLST | ||
| D2 | Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| D19 | Blood | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae | |
| D27 | Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| D41 | Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| D52 | Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| D63 * | Blood | Javanica | ST 143—L. borgpetersenii serogroup Javanica |
| D82 * | Urine | Australis | ST 51—L. interrogans serogroup Australis |
| Blood | Australis | ST 51—L. interrogans serogroup Australis | |
| D85 * | Urine | Bataviae | ST 50—L. interrogans serogroup Bataviae |
| Factors | Pearson Chi-Square | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Age | ||||
| Adult/Young | 0.013 | 1.000 | 1.07 | 0.31–3.67 |
| Senior/Young | 0.293 | 0.757 | 0.72 | 0.22–2.39 |
| Senior/Adult | 1.076 | 0.336 | 0.67 | 0.31–1.43 |
| Breed | ||||
| Medium/Small * | 7.342 | 0.010 | 4.19 | 1.42–12.38 |
| Large/Small * | 8.604 | 0.006 | 5.59 | 1.69–18.51 |
| Large/Medium | 0.429 | 0.663 | 1.33 | 0.56–3.14 |
| Sex | ||||
| Male/Female | 0.306 | 0.710 | 0.81 | 0.39–1.69 |
| Vaccination status | ||||
| Vaccinated/Not vaccinated | 0.203 | 0.715 | 0.85 | 0.41–1.75 |
| Management | ||||
| Outdoor/Indoor | 0.087 | 0.854 | 1.12 | 0.54–2.30 |
| Type of household | ||||
| Multiple/Single | 0.498 | 0.585 | 0.77 | 0.38–1.58 |
| Rat exposure | ||||
| Exposed/Not exposed * | 8.289 | 0.005 | 3.14 | 1.42–6.95 |
| Clinical illness | ||||
| Acute/Chronic | 2.921 | 0.087 | 0.45 | 0.18–1.14 |
| Factors | Pearson Chi-Square | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Type of management Outdoor/Indoor * | 4.742 | 0.045 | 4.31 | 1.10–16.93 |
| Factors | Simple Logistic Regression | Multiple Logistic Regression a | ||||
|---|---|---|---|---|---|---|
| b | Crude OR (95% CI) | p-Value | b | Adjusted OR (95% CI) | p-Value | |
| Sex (Male/Female) | −0.125 | 0.88 (0.40, 1.95) | 0.757 | |||
| Vaccination (Vaccinated/Not vaccinated) | −0.133 | 0.88 (0.40, 1.92) | 0.740 | |||
| Type of Management (Outdoor/Indoor) | −0.002 | 1.00 (0.45, 2.24) | 0.996 | |||
| Household (Multiple/Single) | −0.455 | 0.64 (0.29, 1.39) | 0.254 | |||
| Rat exposure (Exposed/Not exposed) | 1.347 | 3.85 (1.60, 9.23) | 0.003 | 1.258 | 3.52 (1.54, 8.03) | 0.003 |
| Clinical illness (Acute/Chronic) | 1.002 | 2.72 (0.99, 7.52) | 0.053 | 1.015 | 2.76 (1.01, 7.51) | 0.047 |
| Factors | Pearson Chi-Square | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Antibiotic | ||||
| Given/None given | 5.150 | 0.036 | 4.72 | 1.16–19.26 |
| Clinical illness | ||||
| Chronic/Acute * | 5.300 | 0.040 | 8.87 | 1.05–74.95 |
| Vaccination | ||||
| Vaccinated/Not vaccinated | 0.006 | 1.000 | 1.05 | 0.33–3.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahman, M.S.; Khor, K.H.; Khairani-Bejo, S.; Lau, S.F.; Mazlan, M.; Roslan, M.A. Risk and Predictive Factors of Leptospirosis in Dogs Diagnosed with Kidney and/or Liver Disease in Selangor, Malaysia. Animals 2021, 11, 3405. https://doi.org/10.3390/ani11123405
Abdul Rahman MS, Khor KH, Khairani-Bejo S, Lau SF, Mazlan M, Roslan MA. Risk and Predictive Factors of Leptospirosis in Dogs Diagnosed with Kidney and/or Liver Disease in Selangor, Malaysia. Animals. 2021; 11(12):3405. https://doi.org/10.3390/ani11123405
Chicago/Turabian StyleAbdul Rahman, Mohammad Sabri, Kuan Hua Khor, Siti Khairani-Bejo, Seng Fong Lau, Mazlina Mazlan, and Mohd Azri Roslan. 2021. "Risk and Predictive Factors of Leptospirosis in Dogs Diagnosed with Kidney and/or Liver Disease in Selangor, Malaysia" Animals 11, no. 12: 3405. https://doi.org/10.3390/ani11123405
APA StyleAbdul Rahman, M. S., Khor, K. H., Khairani-Bejo, S., Lau, S. F., Mazlan, M., & Roslan, M. A. (2021). Risk and Predictive Factors of Leptospirosis in Dogs Diagnosed with Kidney and/or Liver Disease in Selangor, Malaysia. Animals, 11(12), 3405. https://doi.org/10.3390/ani11123405






