1. Introduction
A remarkable increase in worldwide meat consumption has been recorded during the last few decades, with a noteworthy increase in developing countries. The global production of chicken meat has been raised from 100.46 million tonnes (Mt) in 2014 to 118.02 Mt in 2019 [
1]. Compared to other meat types, chicken meat is steadily and continuously raising worldwide owing to its low-price, health benefits, and sustainable production [
2]. Meat quality is considered a valuable assessment criteria associated with the prerequisites that must be met to accomplish the consumers’ demands and expectations. Consumers of the 21st Century are highly demanding ones with greater concern about meat quality, safety, and health benefits [
3], which in turn necessitates the development of new approaches to enhance the meat nutritive value [
4]. Currently, nutritional approaches are mainly depending on the application of natural constituents in poultry diets to enhance the nutritive value of the poultry products for better human health [
2,
3,
4].
There are numerous approaches for enrichment of broiler’s meat with valuable nutrients, for instance the enrichment with polyunsaturated fatty acids (PUFA). PUFA (ω3 and ω6 fatty acids, FA) are beneficial for the proper functions of the body, but these fatty acids cannot synthesize in the body and must be supplied by the diets. The addition of vegetable oils in the diets of broilers is an appropriate strategy to enrich the chicken meat with beneficial FA [
5], which is in concordance with the consumers’ interest and immunity perspectives [
5,
6,
7,
8]. However, enrichment of broiler’s meat with PUFA is accompanied by a great susceptibility to lipid peroxidation with a resultant decrease in the nutritional value, quality, and shelf-life of the meat [
5,
8], and for that reason, the dietary supplementation with antioxidant becomes necessary [
5]. Synthetic antioxidants are included in the diets of poultry to avoid or limit lipid peroxidation in the meat products [
9]. Due to the consumers’ awareness about consuming natural feed ingredients, nutritionists are seeking for natural antioxidants to replace synthetic ones [
5,
10]. These natural antioxidants have numerous advantages for instance lowering the incidence of metabolic diseases and improving the shelf life of meat, thus optimizing food safety and security [
5].
Rice bran oil (RBO) is receiving great interest among other conventionally vegetable oils owing to its good quality, extended shelf-life, and well-proportioned fatty acid composition as well as the presence of numerous antioxidant components [
11,
12]. RBO is rich in tocopherols, tocotrienols, and other bioactive phytochemicals, including phytosterols, γ-oryzanol, squalene, and triterpene alcohols [
13,
14]. These natural bioactive components have been reported to reveal antioxidant, anti-inflammatory, and hypocholesterolaemic properties as well as boost the immune response [
12]. RBO is one of the healthful edible oils because of its balanced fatty acids profile, with a ratio of 0.6:1.1:1 for saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and PUFA [
11]. Oleic acid (C18:1; 42%), linoleic acid (C18:2; 32%), and palmitic acid (C16:0; 20%) are the three main fatty acids in RBO and represent about 90% of the total fatty acids in RBO [
11,
12]. Even though RBO has small proportion of α-linolenic acid, it is sufficient for de novo synthesis of other ω3-PUFA such as eicosapentaenoic acid and docosahexaenoic acid in tissue phospholipids [
15].
What places RBO on top of other vegetable oils is its antioxidant components, which have been documented to have an outstanding nutritive significance and are recognized to induce substantial lipid-reducing effects and antioxidant properties based on research performed on mice and humans (see review, 12). Previous studies on the dietary supplementation of RBO in broilers showed enhanced growth performance, improved immune response, and reduced cholesterol concentration [
16,
17]. However, data concerning the impacts of dietary inclusion of RBO in the diets of broiler chickens on the antioxidant capacity, meat quality, meat fatty acid composition, hepatic lipid profile, and liver histomorphology are limited. Therefore, the present study aimed to investigate the effects of graded dietary inclusion levels of RBO on the growth performance, carcass traits, blood biochemical parameters, meat quality, abdominal fat color, lipid peroxidation, liver lipid content, and liver histological structure of broiler chickens. We hypothesized that the dietary inclusion of RBO might improve the meat quality, enhance the antioxidant capacity, and decrease meat and liver lipid content of broiler chickens owing to its unique FA profile and antioxidant constituents.
4. Discussion
The demands for healthy foods, such as nutrient-enriched animal products with PUFA and natural antioxidants, are rising. In addition, there is an increasing awareness among consumers about accessing the quality of animal products. RBO is considered one of the healthiest vegetable oils owing to its ideal FA composition and the presence of bioactive components such as γ-oryzanols, tocopherols, tocotrienols, polyphenols, sterols, and phenolic acids [
11,
12]. These bioactive components were reported to decrease the oxidative stress, blood cholesterol, and LDL levels in hyperlipidemic human subjects [
31] or animal models [
32,
33], and they suggested that RBO may decrease the risk factors of cardiovascular diseases. Therefore, RBO can be an appropriate candidate as a natural source of PUFA and antioxidant constituents in the broilers’ diets to enhance the production performance, health status, antioxidant capacity, and product quality.
In our study, there was an improvement of the final LW and total WG of the RBO groups compared to the control group. Our finding revealed that between the three RBO groups, a greater inclusion level (RBO
2%) resulted in higher total WG and greater average daily WG as well as better FCR. However, daily FI of broilers during the entire experimental period was decreased by the inclusion of RBO in diets. The beneficial impacts of RBO on final LW, WG, and FCR were reported previously by Anitha et al. [
34], which was enhanced when it included up to 3% in the diets of broilers. Similar findings concerning the FCR and LW enhancement of broilers were achieved by using 2% RBO [
17]. However, these earlier studies reported no significant difference in FI among the experimental groups [
17,
34]. The beneficial effects of RBO on the broiler’s performance may be due to its bioactive components, including γ-oryzanols, tocopherols, tocotrienols, polyphenols, sterols, and ferulic acid [
12,
17]. In previous studies, vegetable oil reduced the passage rate of feed in the gut, which permits more time for superior nutrient absorption and utilization [
35,
36], leading to a more effective utilization of nutrients from diet. The reduction in FI noticed herein of broiler chickens fed RBO may be attributed to PUFA and its high energy-yielding capacity [
8,
35,
37]. Attia et al. [
8] observed that the inclusion of vegetable oils rich in SFA, i.e., palmitic and stearic acids in the diets of broilers increased their FI when compared with the PUFA-enrich oils, i.e., linolenic and linoleic acids. Similarly, it was reported that SFA has low digestibility compared to UFA, especially during the initial stage of life [
8,
35,
37].
The carcass parameters, determined at day 35 of age, showed that broiler chickens who were fed the RBO-enriched diets had a greater dressing percentage and breast yield, but a lower abdominal fat per cent compared to the control ones. These findings suggested that RBO augments the availability of energy for muscle development, whereas the reduce in abdominal fat per cent in the RBO groups indicates a shifting in energy expenditure for meat growth instead of an accumulation in the body, in particular the abdominal cavity [
8]. It has been demonstrated that the sources and levels of PUFA considerably affected the carcass traits of broilers [
8,
35]. The dietary inclusion of oils rich in PUFA was reported to improve the oxidation and decrease FA synthesis, with a resultant reduction of abdominal fat accumulation in broiler chickens [
38,
39]. At a molecular level, Ahmed et al. [
40] reported that RBO treatment overwhelmed the elevated hepatic de novo lipogenesis of the insulin resistance rats via downregulation of lipogenic genes, i.e., superoxide dismutase and catalase. Accordingly, the suppressing effect of RBO may be owing to its un-saponifiable components, which was also found to downregulate sterol regulatory element binding protein-1 [
41]. All together, these results suggest that RBO may have a role in the lipid metabolism of broiler chickens; however, further investigations are required to confirm this mechanism.
Diet strategy is considered one of the documented approaches to augment the immune response in broiler chickens. The thymus gland, spleen, and bursa of Fabricius are the main lymphoid organs in poultry. The measurements of the lymphoid organs’ weights and immune response have been usually applied to evaluate the general immune status of birds [
42,
43]. In the present study, RBO linearly increased the relative weights of the immune organs, improved serum total protein, and globulin, enhanced HIND antibody titer, and augmented phagocytic activity of the RBO-broilers, within the normal reported values, compared to control. It has been documented that the relative weights of lymphoid organs reveal the growth and function of the immune system, the humoral and cellular immunity [
42,
43]. Accordingly, an increase in the weights of these organs can imply an immunocompetence [
42,
43,
44]. PUFA induces an immunomodulatory impact via influencing the intercellular signals which alter the leukocytes response due to antigenic stimulation [
44]. The FA composition rather than the particular dietary lipids is necessary to augment the cellular and humoral immunity in broilers [
8,
45]. Linoleic acid, n-6 FA, was reported to be associated with an increase in the lymphocyte proliferation, immune organs size, and antibody titer in mice [
46] and in broiler chickens [
8,
17]. RBO contain a considerable amount of linoleic acid (approximately 36%) and could be responsible for the observed immunomodulatory effect in the RBO groups. On the other hand, some plant components can augment the multiplication of advantageous microbiota, while diminishing the proliferation of harmful ones, consequently indirectly boosting host’s immunity [
47]. The flavonoids and phenolic compounds are known for their antioxidant, anti-inflammatory, and antimicrobial activities [
48]. Since RBO is rich in flavonoids and phenolic compounds, in the present study, possibly dietary supplementation with RBO notably enhanced the immune status of broiler chickens.
Analysis of serum enzyme activities is an imperious procedure in the assessment of poultry health problems. Enzymes, such as ALT and AST, are generally existing with high concentrations in the liver [
49] and are considered as particular indicators of hepatic cellular damage and inflammation [
50]. ALP is an enzyme produced in all tissue types and is mainly responsible for dephosphorylation of a substrate and activated in alkaline pH. Its raised blood values are commonly noted in hepatic damages [
49,
50]. In our study, RBO supplementation linearly reduced the serum AST, ALT, and ALP when compared with the control group. The current findings indicate that RBO supplementation did not induce any detrimental effects on the liver. Lower serum AST, ALT, and ALP levels, within the normal values, may probably indicate that RBO decreased tissue damage and enhanced liver functions in the RBO broilers, since higher activities of these markers were reported to be associated with liver damage and inflammation [
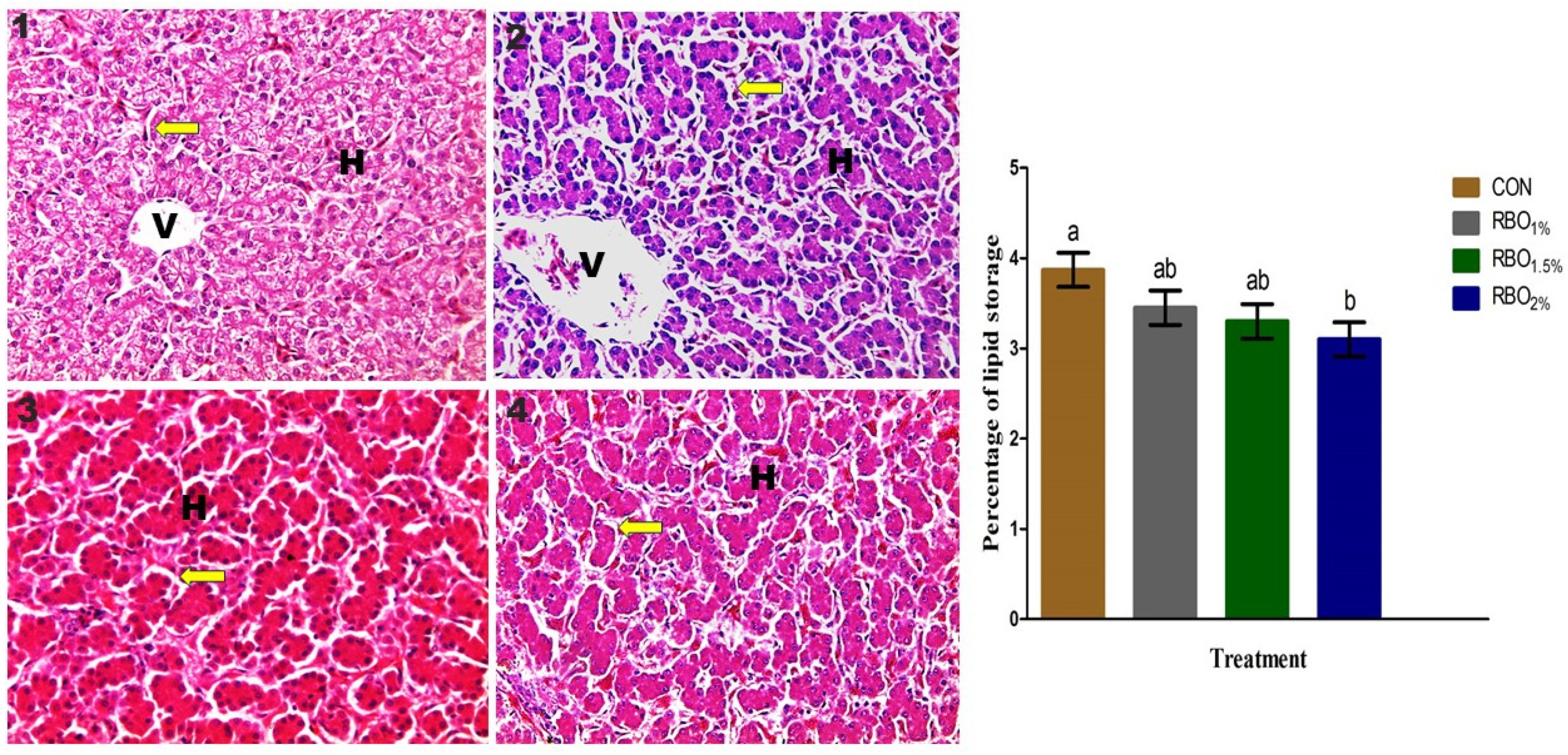
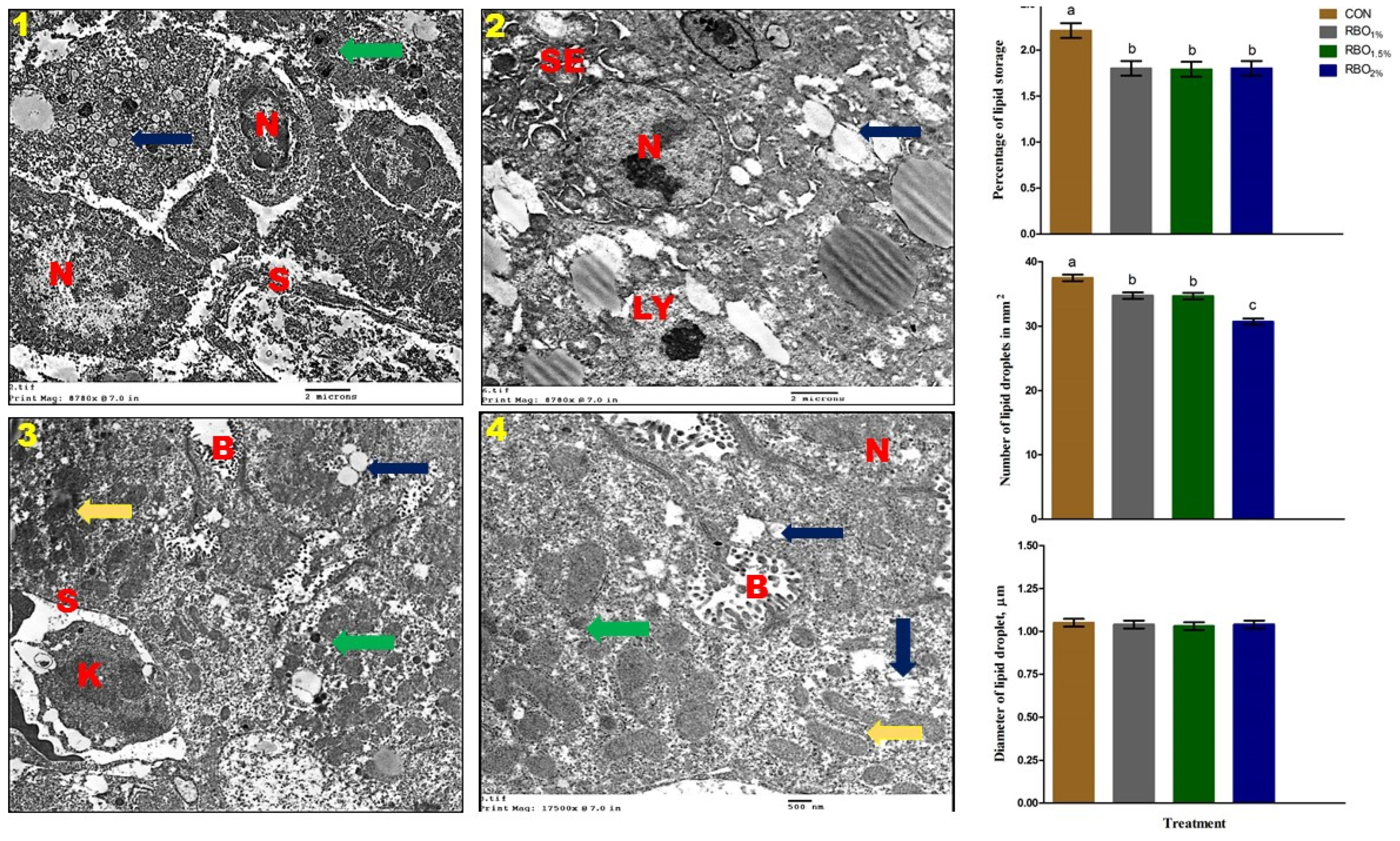
51]. Along with serum biochemical constituents, histological structure can be used to determine liver abnormalities and help in assessing disease diagnosis in poultry. In support of our findings, the general histological structure and ultrastructural of the liver of the experimental groups are normal and comparable to previous observations of the birds’ liver [
43,
52]. No pathological lesions were observed in the broiler chickens’ livers of the current experiment. The lipid content of the experimental groups whether assessed using images from light or electron microscope revealed that RBO feeding linearly decreased the percentage of hepatic lipid storage and number of lipid droplets compared to control. Regarding the hepatic chemical assessments, the broiler chickens consumed the RBO diets had significantly lower liver cholesterol, triglycerides, and MDA contents when compared with those consumed the control diet. The liver plays a central role in lipogenesis in birds, and contrary to mammals, the fat synthesis occurs mainly in the liver and is restricted in the adipose tissue [
53]. Based on the obtained findings, we suggested that RBO can improve liver lipid profile and induce liver protective effects through its phenolic and antioxidant compounds [
32,
33]. The presence of phenolic compounds and antioxidants allows RBO to protect hepatic tissue from lipid peroxidation and thus decreased serum levels of ALT, AST, and ALP. Previous studies by Wang et al. [
32] and Zhang et al. [
33] showed that rice bran phenolic extract exerts its hypolipidemic effect through activation of AMP-activated protein kinase, wherein phenolic compounds found in rice bran such as p-coumaric acid, ferulic acid, and rutin play a fundamental role.
It is worth noting that RBO supplementation resulted in a reduction in serum levels of total lipids, total cholesterol, LDL, and triglycerides, while it increased serum HDL level. The favorable actions of RBO on total lipids, total cholesterol, and LDL are possibly due to its richness in non-saponifiable components, including sterols (b-sitosterol, campesterol and stigmasterol), c-oryzanol, and tocotrienols. Lichtenstien et al. [
54] showed a higher content of b-sitosterol, campesterol and stigmasterol in RBO than other vegetable oils. Plant sterols are observed to be accountable for a 30–40% reduction in the cholesterol absorption [
55]. Furthermore, oryzanol and tocotrienols found in RBO lessen the rate of endogenous cholesterol synthesis through diminishing the HMG-CoA reductase enzyme [
32,
56], and increasing the expression of cholesterol 7-alpha-hydroxylase, which is the rate-limiting enzyme in the synthesis of bile acids from cholesterol. Another suggested mechanism accountable for the reduction of total cholesterol due to feeding RBO is its omega-3 FA content which can inhibit the apolipoprotein-B100 and LDL synthesis and accordingly result in a decrease in total cholesterol [
54,
57]; however, the exact mechanism in broiler chickens is still unclear and necessitates further investigations.
Meat color is one of the critical indicators of freshness and wholesomeness of any meat product, relying on it, customers accomplish an initial impression of the product [
58]. Numerous factors were reported to influence the color of broiler’s meat including genetics, sex, age, diet composition, the heme pigments, and pre-slaughter condition [
58]. In the current study, dietary RBO had no influence on L* (lightness), a* (redness), and b* (yellowness) values of breast or thigh meat at 24 h post-mortem among the experimental groups. The present findings are in line with the reports of Khatun et al. [
59] and Jankowski et al. [
60], who found that meat color was not influenced by the dietary oils supplementation. However, these results contradict the observations of Turcu et al. [
5] and Qi et al. [
61] and who found that dietary supplementation with various oils influenced the meat color of broiler chickens. The inconsistency between these studies may be due to various types of the studied plant oils. Measurement of the meat pH is vital because there is a relationship between pH and physicochemical properties of the meat, such as color and hardness [
5]. In this study, the pH values of the breast and thigh muscles are within the ranges of normal pH limits [
5]. Our results are in agreement with those of Khatun et al. [
59] and Jankowski et al. [
60] who observed no differences in the meat pH at 24 h post-mortem in broiler chickens fed various dietary oils. In goose, the inclusion of full-fat rice bran at 6–18% did not influence the meat quality traits, involving color parameters, and pH [
62]. The lack of differences observed in meat color in the current trial might be owing to the similarity in the pH values for all groups. Furthermore, in this trial, the lack of variances between the experimental groups for the pH of the meat could be owing to the fact that RBO had no effects on the glycogen content of the meat. Glycogen plays a key role in the pH value of meat [
63]; nevertheless, the meat glycogen content was not measured in the present trial, which necessitates further research. On the other hand, there was non-significant difference in broiler abdominal fat coloration in the present study, which is probably due to the low contents of beta-carotene, lutein, and zeaxanthin found in RBO [
64]. To our knowledge, there have been no previous reports on the effects of dietary RBO on broiler meat or abdominal fat colorations.
It is well acknowledged that lipid peroxidation is one of the major processes that affect the quality of meat and its products [
65]. The inclusion of RBO at different levels in the broiler diets has been performed to decrease the lipid content while increase the antioxidant contents of the meat in order to avoid its quality deterioration. In the present trial, the antioxidant effect of RBO on meat lipid peroxidation was noticed both in thigh and breast meat, compared to control samples. Dietary inclusion of RBO linearly and quadratically improved the meat GPx content, while reduced its EE %, triglycerides, cholesterol, and MDA contents. On the other hand, with the increasing RBO level there was no variation in CP content in the broiler meat, which may indicate that RBO had no influence on protein synthesis in the meat. In broiler chickens, in ovo injection of RBO increased the GPx content but decreased the MDA content in the breast and heart muscles [
66]. Full fat rice bran inclusion linearly and quadratically reduced crude fat content in goose meat but did not affect the meat CP content [
62]. The γ-oryzanol, tocopherols, and tocotrienols components of RBO are thought as the major ingredients responsible for the hypolipidemic and antioxidant activities of RBO [
67]. Although the mechanism underlying this effect is not clear at the moment, oryzanol, and tocopherols of RBO are supposed to be the main cause for this beneficial outcome. Furthermore, phenolic compounds (e.g., polyphenols) induced the antioxidant potential which may diminish the formation of free radicals and subsequently counteract the peroxidation of PUFA [
68].
Chicken meat is considered as good sources of protein and essential FA for humans. Diet manipulation has a considerable influence on the broiler meat composition. Meat FA composition is considered as a precious indicator of meat quality from the human health standpoint. Vegetable oils are valuable dietary constituents as a source of FA that can possibly be redirected in the poultry products [
65]. Interestingly, our findings revealed that the inclusion of RBO in the broiler diets increased the meat MUFA, PUFA, n-6, and n-3 FA contents, but decreased the SFA level. The improvement of PUFA in the meat of RBO groups chiefly caused by the diminution of SFA and the elevation of C18:2n-6 and C18:3n-3 FA in the meat. The most effective of RBO supplementation was the enrichment of the breast meat with PUFA, n-6 FA, n-3 FA, and PUFA:SFA, and lowering the SFA content. PUFA show an important role in lessening the occurrence of cardiovascular and inflammatory diseases in humans [
69]. To our knowledge, there is inadequate data in the literature regarding the dietary inclusion of RBO and the modification of the meat FA composition in poultry. According to Turcu, et al. [
5] and Attia et al. [
8], the dietary supplementation with PUFA-rich oils in broiler diets resulted in a modification in the meat FA profile, namely enrichment the meat with PUFA and a decrease in SFA level. Sun et al. [
62] showed that full-fat rice bran inclusion decreased MUFA and increased PUFA level in the goose meat. In pigs, Jayaraman et al. [
70] reported that the inclusion full-fat rice bran as a replacement of corn in their diets linearly decreased SFA (palmitic and stearic acids), but increased n-6 FA and n-3 FA in meat. The hypolipidemic action of the phenolic acids presented in RBO may be contributed to the enhancement of the FA profile of broiler meat through lipid homeostasis. Previous researchers have proposed that the FA content of poultry meat could be modified through dietary manipulation by adding plant oils and antioxidants [
5,
8,
17,
62,
68], which in turn could extend the shelf-life and improve the quality of meat products.