The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocols
2.3. Evaluation of Sedation
2.4. Surgery
2.5. Recovery
2.6. Statistical Analysis
3. Results
3.1. Heart Rate (HR) and Respiratory Rate (RR)
3.2. Sedation (SDX) and Ataxia (ATX)
3.3. Schemes Modified from Singh
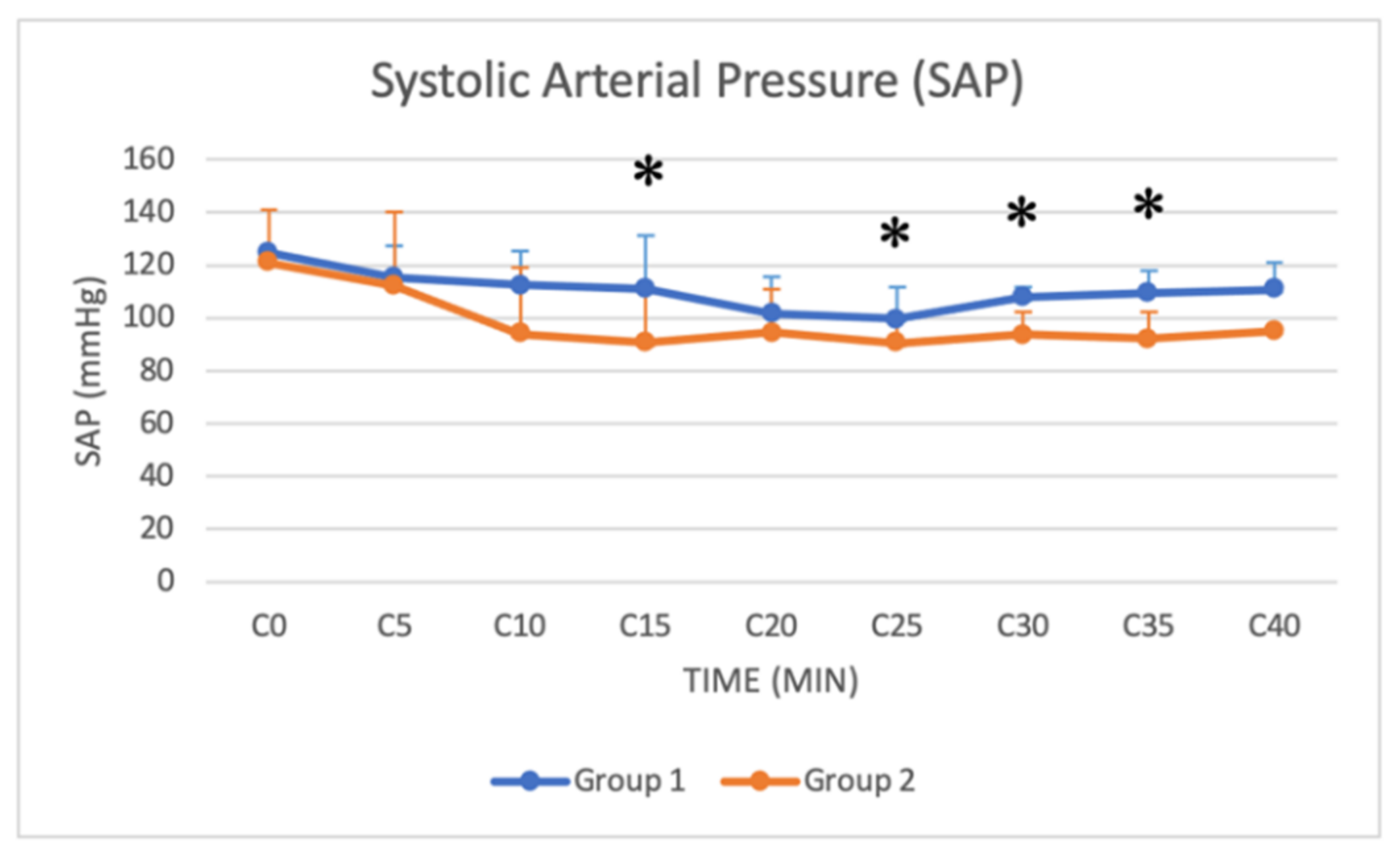
3.4. Arterial Blood Pressure
3.5. Pain Scale
4. Discussion
4.1. Aims
4.2. Interpretation of Clinical Observations
4.3. Physiological and Hemodynamic Considerations
4.4. Interpretation of Pain Scale Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- De la Cruz-Cruz, L.A.; Guerrero-Legarreta, I.; Ramirez-Necoechea, R.; Roldan-Santiago, P.; Mora-Medina, P.; Hernandez-Gonzalez, R.; Mota-Rojas, D. The behaviour and productivity of water buffalo in different breeding systems: A review. Vet. Med. 2014, 59, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, F.; Pacelli, C.; Grasso, F.; Braghieri, A.; De Rosa, G. The behaviour and welfare of buffaloes (Bubalus bubalis) in modern dairy enterprises. Animal 2013, 7, 1704–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzoni, L.; Chincarini, M.; Giammarco, M.; Fusaro, I.; Gloria, A.; Contri, A.; Ferri, N.; Vignola, G. Maternal and Neonatal Behaviour in Italian Mediterranean Buffaloes. Animals 2021, 11, 1584. [Google Scholar] [CrossRef]
- Martins, L.T.; Gonçalves, M.C.; Tavares, K.C.S.; Gaudêncio, S.; Santos Neto, P.C.; Dias, A.L.G.; Gava, A.; Saito, M.E.; Oliveira, C.A.; Mezzalira, A.; et al. Castration methods do not affect weight gain and have diverse impacts on the welfare of water buffalo males. Livest. Sci. 2011, 140, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Van der Saag, D.; White, P.; Ingram, L.; Manning, J.; Windsor, P.; Thomson, P.; Lomax, S. Effects of Topical Anaesthetic and Buccal Meloxicam Treatments on Concurrent Castration and Dehorning of Beef Calves. Animals 2018, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, J.F.; Nutsch, A.L.; Barbur, L.A.; Bradburn, R.M. A survey of castration methods and associated livestock management practices performed by bovine veterinarians in the United States. BMC Vet. Res. 2010, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Meléndez, D.M.; Marti, S.; Pajor, E.A.; Sidhu, P.K.; Gellatly, D.; Moya, D.; Janzen, E.D.; Coetzee, J.F.; Schwartzkopf-Genswein, K.S. Effect of meloxicam and lidocaine administered alone or in combination on indicators of pain and distress during and after knife castration in weaned beef calves. PLoS ONE 2018, 13, e0207289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyke, R.; Connor, M.; Miele, A. An investigation into the perceptions of veterinarians towards perioperative pain management in calves. Animals 2021, 11, 1882. [Google Scholar] [CrossRef]
- Straticò, P.; Varasano, V.; Suriano, R.; Mariscoli, M.; Robbe, D.; Giammarco, M.; Vignola, G.; Petrizzi, L. Analgesic effects of intravenous flunixin and intrafunicular lidocaine or their combination for castration of lambs. Veter Rec. Open 2018, 5, e000266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straticò, P.; Carluccio, A.; Varasano, V.; Guerri, G.; Suriano, R.; Robbe, D.; Cerasoli, I.; Petrizzi, L. Analgesic Effect of Butorphanol during Castration in Donkeys under Total Intravenous Anaesthesia. Animals 2021, 11, 2346. [Google Scholar] [CrossRef]
- EU Commission Regulation (EU). N° 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union L 2010, 15, 1–72. [Google Scholar]
- Tiwari, S.K.; Kumar, A.; Vainio, O. Reversal of sedative and clinicophysiological effects of epidural xylazine and detomidine with atipamezole and yohimbine in buffaloes (Bubalus bubalis). Vet. Rec. 1998, 143, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Shekidef, M.H.; Al-Akraa, A.M.; Ghanem, M.M. Studies on the effect of medetomidine versus romifidine in buffalo calves. Assiut. Vet. Med. J. 2007, 53, 292–309. [Google Scholar]
- Riebold, T.W. Ruminants. In Veterinary Anaesthesia and Analgesia, 5th ed.; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley Blackwell: Ames, IA, USA, 2015; pp. 912–927. [Google Scholar]
- Purohit, G.N.; Gaur, M.; Kumar, A.; Shekher, C.; Ruhil, S. Perspectives of cesarean section in buffaloes. Asian Pac. J. Reprod. 2013, 2, 229–237. [Google Scholar] [CrossRef]
- Alshara, M.A.; Somroo, H.; Memon, M.A.; Kalhoro, A.B. Effects of xylazine on blood glucose levels in young male buffaloes. Pakistan Vet. J. 2000, 20, 200–202. [Google Scholar]
- Valverde, A.; Doherty, T.J.; Dyson, D.; Valliant, A.E. Evaluation of pentobarbital as a drug for standing sedation in cattle. Vet. Surg. 1989, 18, 235–238. [Google Scholar] [CrossRef]
- Singh, G.D.; Kinjavdekar, P.; Aithal, H.P.; Pawde, A.M.; Zama, M.; Singh, J.; Tiwary, R. Clinicophysiological and haemodynamic effects of fentanyl with xylazine, medetomidine and dexmedetomidine in isoflurane-anaesthetised water buffaloes (Bubalus bubalis). J. S. Afr. Vet. Assoc. 2013, 84, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suriano, R.; Varasano, V.; Robbe, D.; Carluccio, A.; Straticò, P.; Contri, A.; Petrizzi, L. Intraoperative analgesic effect of intrafunicolar lidocaine injection during orchiectomy in isoflurane anaesthetized Martina Franca donkeys. J. Equine Vet. Sci. 2014, 34, 793–798. [Google Scholar] [CrossRef]
- Capucille, D.J.; Poore, M.H.; Rogers, G.M. Castration in cattle: Techniques and animal welfare issues. Compend. Cont. Educ. Pract. Vet. 2002, 24, 66–73. [Google Scholar]
- Hussain, S.A.; Uppal, S.K. Comparison of blood acid base gas parameters in venous and arterial blood of healthy buffaloes. Buffalo Bull. 2014, 33, 328–331. [Google Scholar]
- Alonso, B.B.; La Rosa, L.; Carregaro, A.B.; Gasthuys, F.; Schauvliege, S. Recovery Quality after Romifidine Versus Detomidine Infusion During Isoflurane Anesthesia in Horses. J. Equine Vet. Sci. 2020, 94, 103243. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.; Luna, S.P.L.; do Amaral, J.B.; Rodrigues, K.A.; Sant’Anna, A.C.; Daolio, M.; Brondani, J.T. Validation of the UNESP-Botucatu unidimensional composite pain scale for assessing postoperative pain in cattle. BMC Vet. Res. 2014, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodh, D.; Singh, K.; Gopinathan, A.; Mohindroo, J.; Singh Saini, N. Comparative evaluation of halothane and isoflurane maintenance anesthesia in water buffaloes (Bubalus bubalis). J. Appl. Anim. Res. 2014, 42, 269–277. [Google Scholar] [CrossRef]
- Pawde, A.M.; Kinjavdekar, A.P.; Aithal, H.P.; Pratap, K.; Bisht, G.S. Detomidine–Diazepam–Ketamine Anaesthesia in Buffalo (Bubalus bubalis) Calves. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2000, 47, 175–179. [Google Scholar] [CrossRef]
- Malik, V.; Kinjavdekar, P.A.; Aithal, H.P.; Pawde, A.M.; Surbhi. Sedative, analgesic, cardiopulmonary and haemodynamic effects of medetomidine-butorphanol and midazolam-butorphanol on thiopental-propofol anaesthesia in water buffaloes (Bubalus bubalis). J. Appl. Anim. Res. 2011, 39, 284–287. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, N.; Dimri, U.; Hoque, M.; Maiti, S.K.; Gupta, O.P.; Shahi, A. Romifidine–Ketamine Anaesthesia in Atropine and Triflupromazine Pre-medicated Buffalo Calves. J. Vet. Med. A Physiol. Clin. Med. 2004, 51, 420–424. [Google Scholar] [CrossRef]
- Haga, H.A.; Lykkjen, S.; Revold, T.; Ranheim, B. Effect of intratesticular injection of lidocaine on cardiovascular responses to castration in isoflurane-anesthetized stallions. Am. J. Vet. Res. 2006, 67, 403–408. [Google Scholar] [CrossRef]
- Bodh, D.; Kiranjeet, S.; Mohindroo, J.; Gopinathan, A.; Mahajan, S.K.; Saini, N.S. Evaluation of midazolam and midazolam-butorphanol premedications for general anaesthesia in buffaloes (Bubalus bubalis). Indian J. Vet. Surg. 2015, 36, 77–81. [Google Scholar]
- Peshin, P.K.; Nigam, J.M. Effects of detomidine in young water buffalo. J. Vet. Anaesth. 1994, 21, 35–38. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, J.; Peshin, P.K.; Gahlawat, J.S.; Singh, P.; Nigam, J.M. Evaluation of Xylazine-Ketamine anaesthesia in Buffaloes (Bubalus bubalis). Zentralbl. Veterinarmed. A 1985, 32, 54–58. [Google Scholar] [CrossRef]











| Parameters | Score | Interpretation |
|---|---|---|
| Ataxia | 0 | Absent (no ataxia) |
| 1 | Mild (knuckling of the fetlocks) | |
| 2 | Moderate (crossing of the hindlimbs) | |
| 3 | Strong (attempting or assuming recumbency) | |
| Sedation | 0 | No sedation (alert, eyes open) |
| 1 | Mild (drooping of eyelids) | |
| 2 | Moderate (lowering the head, minimum to moderate reaction to noise nociception and handling) | |
| 3 | Deep (no reaction to noise nociception and handling) | |
| Depth of anaesthesia | 0 | No sedation (alert, eyes open) |
| 1 | Mild (drooping of eyelids, mild sensory and motor deficit) | |
| 2 | Moderate (drooping of eyelids, moderate sensory and motor deficit) | |
| 3 | Strong (drooping of eyelids, severe sensory and motor deficit) | |
| Muscle relaxation | 1 | Absent (tightly closed jaws and stiff limbs, no flaccidity of the abdomen) |
| 2 | Mild (moderate resistance to the opening of the jaws and bending of the limbs, no flaccidity of the abdomen) | |
| 3 | Moderate (mild resistance to the opening of the jaws and bending of the limbs, no flaccidity of the abdomen) | |
| 4 | Excellent (no resistance to the opening of the jaws, bending of the limbs and flaccid abdomen) | |
| Response to acoustic stimulus | 1 | Strong reaction |
| 2 | Weak response | |
| 3 | Occasional response | |
| 4 | No response | |
| Response to tactile stimulus | 1 | No analgesia (strong reaction) |
| 2 | Mild analgesia (weak response) | |
| 3 | Moderate analgesia (occasional response) | |
| 4 | Excellent analgesia (no response) | |
| Eyelid reflex | 0 | - Completely abolished reflexes |
| 1 | + Mildly abolished reflexes | |
| 2 | ++ Moderately abolished reflexes | |
| 3 | +++ Slightly intact reflexes | |
| 4 | ++++ Intact reflexes | |
| Salivation | 0 | - Absent |
| 1 | + Mild | |
| 2 | ++ Moderate | |
| 3 | +++ Extensive | |
| 4 | ++++ Profuse | |
| Regurgitation/tympanism/ | No | Absent |
| reflux | Yes | Present |
| Group 1 | Group 2 | |
|---|---|---|
| Age (months) | 3.15 ± 1.31 | 2.3 ± 0.25 |
| Body weight (kg) | 176.50 ± 43.64 | 102.20 ± 20.31 |
| Catheterisation time (minutes) | 13.00 ± 11.17 | 5.01 ± 4.50 |
| Catheterisation attempts (number) | 3.14 ± 1.17 | 1.5 ± 1.26 |
| Intubation (minutes) | 7.40 ± 11.36 | 2.50 ± 2.01 |
| #ET tube | 16.20 ± 2.39 | 12.80 ± 1.03 |
| Top-ups of xylazine | 0.80 ± 0.63 | 0.90 ± 0.99 |
| Top-ups of ketamine | 1.20 ± 2.57 | 0.30 ± 0.48 |
| Group | Anaesthesia Time (min) | Surgical Time (min) | Time to Extubation (min) | Time to Sternal Recumbency (min) | Time to Quadrupedal Station (min) | Recovery Score |
|---|---|---|---|---|---|---|
| Group 1 | 39.2 ± 13.56 | 23.60 ± 11.68 | 7.20 ± 5.95 | 8.40 ± 6.07 | 18.70 ± 20.87 | 1.7 ± 1.05 |
| Group 2 | 30.9 ± 5.52 | 15.50 ± 3.77 | 10.80 ± 4.31 | 13.00 ± 4.64 | 20.90 ± 15.63 | 2.30 ± 1.25 |
| Group | T0 | T2 | T5 | T10 | T15 | T20 | T25 | T30 | T35 | T40 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 1 | 68.40 ± 19.17 | 47.60 ± 5.39 | 42.80 ± 5.67 | 40.00 ± 5.65 | 36.60 ± 6.39 | 36.00 ± 5.65 | 36.20 ± 6.89 | 34.00 ± 4.32 a | 33.2 ± 3.42 a | 38.5 ± 7.09 ab |
| 2 | 57.40 ± 7.77 | 44.80 ± 5.20 | 42.80 ± 4.23 | 40.00 ± 4.71 | 36.00 ± 9.61 | 38.40 ± 8.26 | 42.20 ± 8.56 | 41.70 ± 5.88 a | 41.00 ± 5.45 a | 48.00 ± 15.52 ab | |
| RR | 1 | 22.2 ± 6.49 | 23 ± 8.80 | 19.7 ± 5.61 | 17.1 ± 3.95 | 14.3 ± 6.21 | 16.6 ± 9.66 | 14.5 ± 12.60 | 13.1 ± 5.80 | 15.3 ± 11.05 | 9.00 ± 4.29 |
| 2 | 19.80 ± 4.26 | 19.40 ± 5.66 | 19.60 ± 5.56 | 17.60 ± 6.58 | 15.80 ± 5.99 | 16.60 ± 4.00 | 14.70 ± 5.85 | 16.00 ± 12.50 | 18.30 ± 15.76 | 15.00 ± 10.98 |
| Group | T0 | T2 | T5 | T10 | T15 | T20 | T25 | T30 | T35 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Depth of anaesthesia | 1 | 0 c | 0.40 ± 0.51 | 0.90 ± 0.56 ab | 1.60 ± 0.69 a | 2.50 ± 0.52 | 2.60 ± 0.51 | 2.70 ± 0.48 | 2.90 ± 0.31 | 2.80 ± 0.42 |
| 2 | 0.40 ± 0.51 c | 0.70 ± 0.67 | 1.50 ± 0.70 ab | 2.00 ± 0.94 a | 2.30 ± 0.94 | 2.50 ± 0.97 | 2.50 ± 0.97 | 2.60 ± 0.96 | 2.60 ± 0.96 | |
| Salivation | 1 | 0.30 ± 0.48 | 0.30 ± 0.48 | 0.60 ± 0.5 bc | 0.80 ± 0.63 ab | 0.90 ± 0.56 b | 0.80 ± 0.42 | 0.70 ± 0.48 | 0.70 ± 0.48 | 0.70 ± 0.48 |
| 2 | 0.3 ± 0.48 | 0.70 ± 0.48 | 1.1 ± 0.73 bc | 1.50 ± 0.70 ab | 1.50 ± 0.70 b | 1.20 ± 0.78 | 1.10 ± 0.87 | 1.00 ± 0.81 | 1.00 ± 0.81 | |
| Sedation acoustic stimulus | 1 | 1 | 1.10 ± 0.31 | 1.40 ± 0.69 ab | 2.80 ± 1.13 a | 3.60 ± 0.84 | 3.30 ± 0.94 | 3.50 ± 1.08 | 3.50 ± 0.97 | 3.70 ± 0.67 |
| 2 | 1 | 1.60 ± 0.69 | 2.30 ± 0.82 ab | 3.50 ± 0.70 a | 3.70 ± 0.67 | 3.60 ± 0.69 | 3.70 ± 0.67 | 3.70 ± 0.67 | 3.70 ± 0.67 | |
| Sedation tactile stimulus | 1 | 1 | 1.20 ± 0.42 | 1.60 ± 0.84 bc | 2.90 ± 0.99 | 3.50 ± 0.84 | 3.30 ± 0.94 | 3.60 ± 0.84 | 3.80 ± 0.63 | 3.80 ± 0.42 |
| 2 | 1 | 1.50 ± 0.52 | 2.20 ± 0.78 bc | 3.20 ± 0.91 | 3.60 ± 0.69 | 3.60 ± 0.69 | 3.60 ± 0.69 | 3.60 ± 0.69 | 3.60 ± 0.69 | |
| Muscle relaxation | 1 | 1 | 1 ab | 1.50 ± 0.52 bc | 2.10 ± 0.87 a | 2.60 ± 0.96 | 2.80 ± 1.03 | 3.40 ± 0.69 | 3.50 ± 0.52 | 3.40 ± 0.69 |
| 2 | 1 | 1.50 ± 0.52 ab | 2.00 ± 0.81 bc | 2.90 ± 0.99 a | 3.20 ± 0.91 | 3.20 ± 0.91 | 3.30 ± 0.94 | 3.50 ± 0.97 | 3.50 ± 0.97 | |
| Eyelid reflex | 1 | 4 | 4 | 3.50 ± 0.52 | 1.70 ± 1.15 | 1.60 ± 1.17 | 1.40 ± 0.84 | 1.30 ± 0.82 | 1.40 ± 0.84 | 1.50 ± 0.97 |
| 2 | 4 | 3.7 ± 0.48 | 3.30 ± 0.82 | 2.00 ± 1.33 | 1.70 ± 1.56 | 1.90 ± 1.44 | 1.80 ± 1.54 | 1.40 ± 1.42 | 1.50 ± 1.43 | |
| Reflux/regurgitation/ tympanism | 1 | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| 2 | NO | NO | NO | NO | NO | NO | NO | 1 buffalo | NO |
| Groups | 30” | 60” | 90” | P6H | 12H | 24H | |
|---|---|---|---|---|---|---|---|
| Group 1 | Score | 0.90 ± 1.59 | 0.90 ± 1.66 | 0.40 ± 0.69 | 0.40 ± 1.26 | 0 | 0 |
| Attitude | A (1/10) G (1/10) | F (2/10) G (1/10) | F (2/10) | H (1/10) | - | - | |
| Group 2 | Score | 1.4 ± 2.06 | 1.5 ± 2.27 | 0.6 ± 1.07 | 0.1 ± 0.31 | 0.6 ± 1.89 | 0.2 ± 0.63 |
| Attitude | F (2/10) H (1/10) | F (2/10) H (1/10) | F (1/10) | - | E (1/10) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerri, G.; Cerasoli, I.; Straticò, P.; De Amicis, I.; Giangaspero, B.; Varasano, V.; Paolini, A.; Carluccio, A.; Petrizzi, L. The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia. Animals 2021, 11, 3433. https://doi.org/10.3390/ani11123433
Guerri G, Cerasoli I, Straticò P, De Amicis I, Giangaspero B, Varasano V, Paolini A, Carluccio A, Petrizzi L. The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia. Animals. 2021; 11(12):3433. https://doi.org/10.3390/ani11123433
Chicago/Turabian StyleGuerri, Giulia, Ilaria Cerasoli, Paola Straticò, Ippolito De Amicis, Brunella Giangaspero, Vincenzo Varasano, Andrea Paolini, Augusto Carluccio, and Lucio Petrizzi. 2021. "The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia" Animals 11, no. 12: 3433. https://doi.org/10.3390/ani11123433
APA StyleGuerri, G., Cerasoli, I., Straticò, P., De Amicis, I., Giangaspero, B., Varasano, V., Paolini, A., Carluccio, A., & Petrizzi, L. (2021). The Clinical Effect of Xylazine Premedication in Water Buffalo Calves (Bubalus bubalis) Undergoing Castration under General Anaesthesia. Animals, 11(12), 3433. https://doi.org/10.3390/ani11123433









