Aristolochic Acid I-Induced Hepatotoxicity in Tianfu Broilers Is Associated with Oxidative-Stress-Mediated Apoptosis and Mitochondrial Damage
Abstract
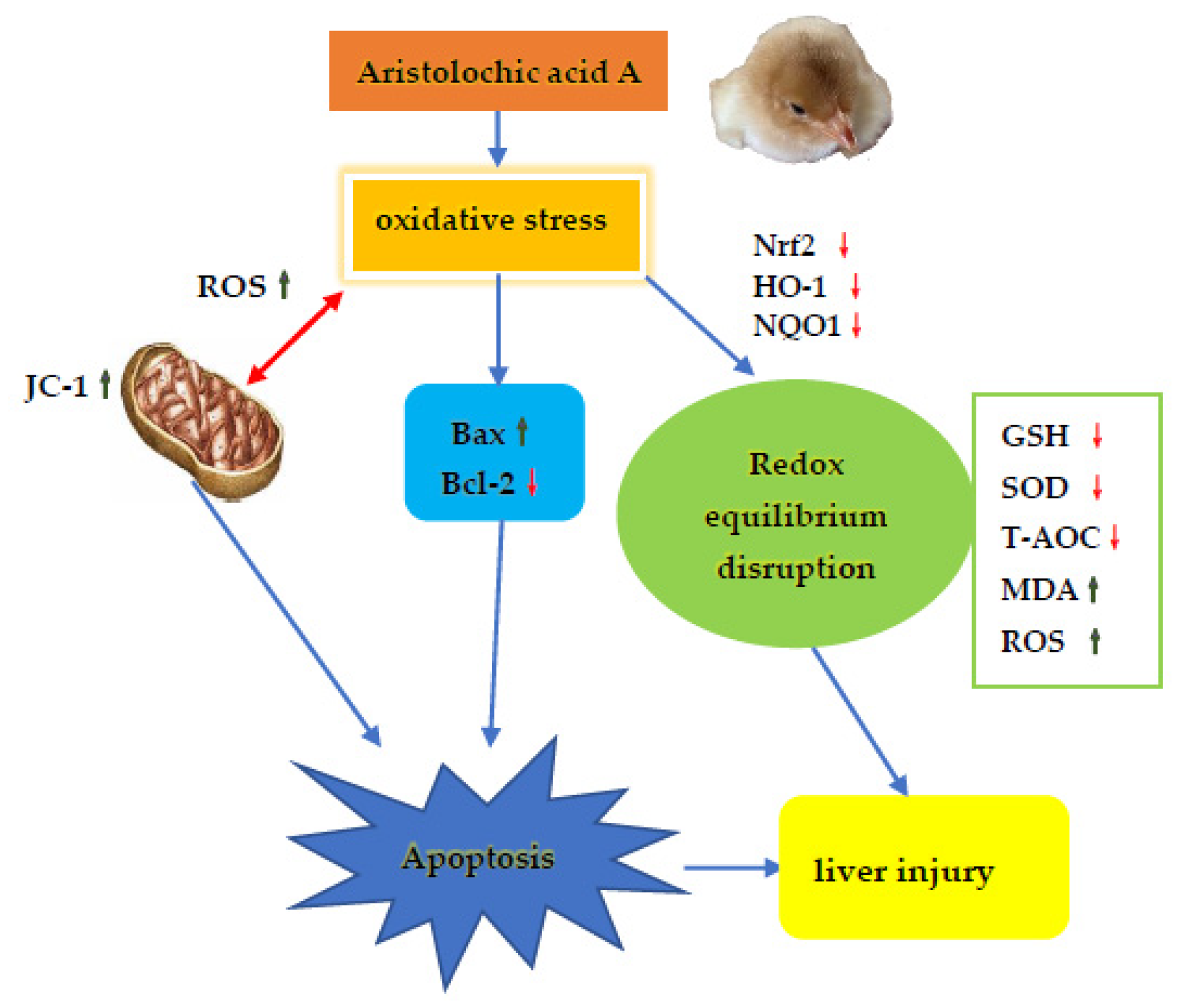
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
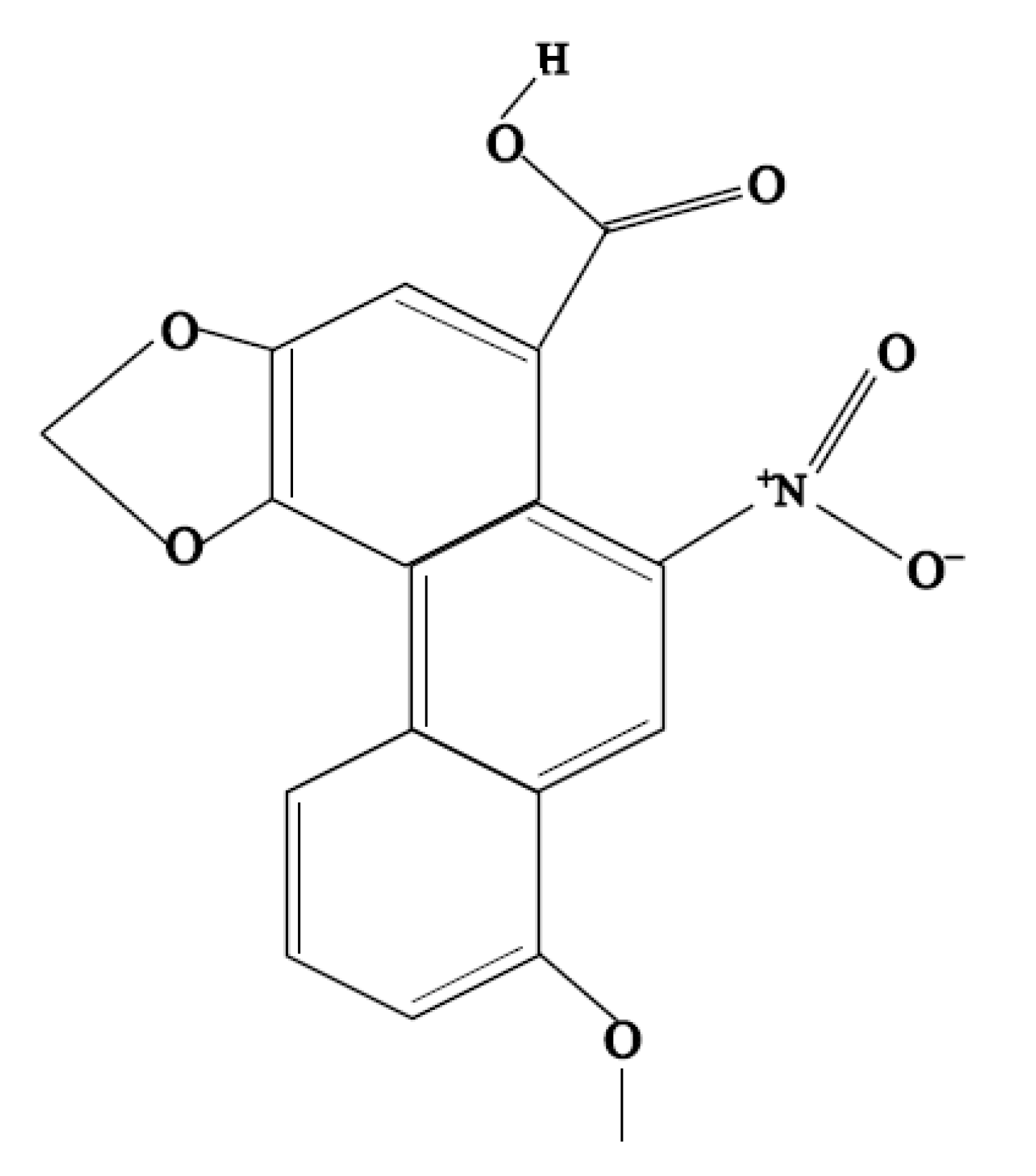
2.1. Experimental Materials
2.2. Experimental Animals
2.3. Subchronic Poisoning with Aristolochic Acid A in Tianfu Broilers
2.3.1. Sample Collection and Liver Cell Separation
2.3.2. Biochemical Analysis
2.3.3. Histopathology
2.3.4. Reactive Oxygen Species (ROS), Mitochondrial Membrane Potential (MMP) and Apoptosis by Flow Cytometry
2.3.5. Ultrastructure Observations
2.3.6. Fluorescence Real-Time Quantification PCR (QRT-PCR)
2.4. Statistical Analyses
3. Results
3.1. AA-I Elevated Liver Index in Broilers
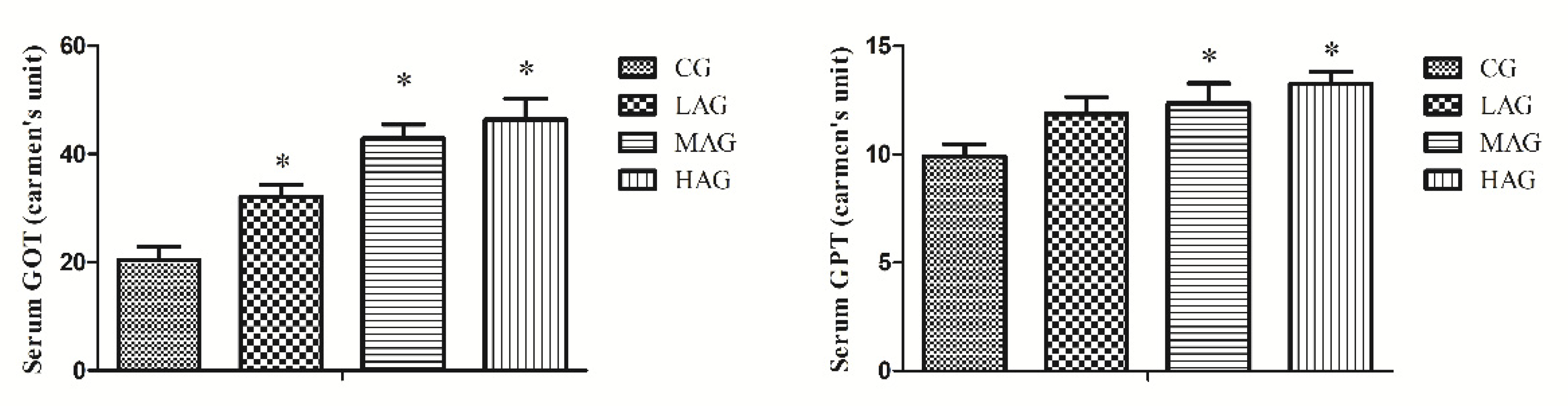
3.2. AA-I Elevated Hepatic Enzymes in Broilers
3.3. AA-I Deformed Hepatocyte Structures
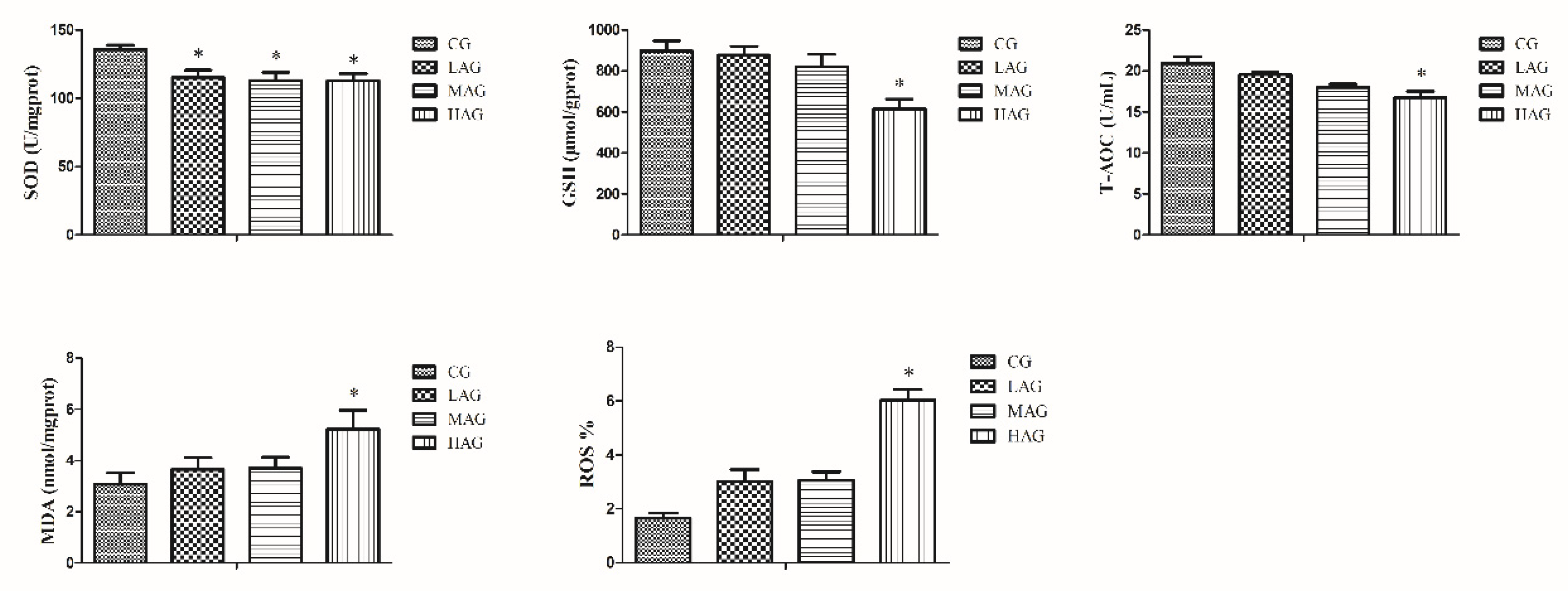
3.4. AA-I Impacted Antioxidant System and Aroused Oxidative Stress
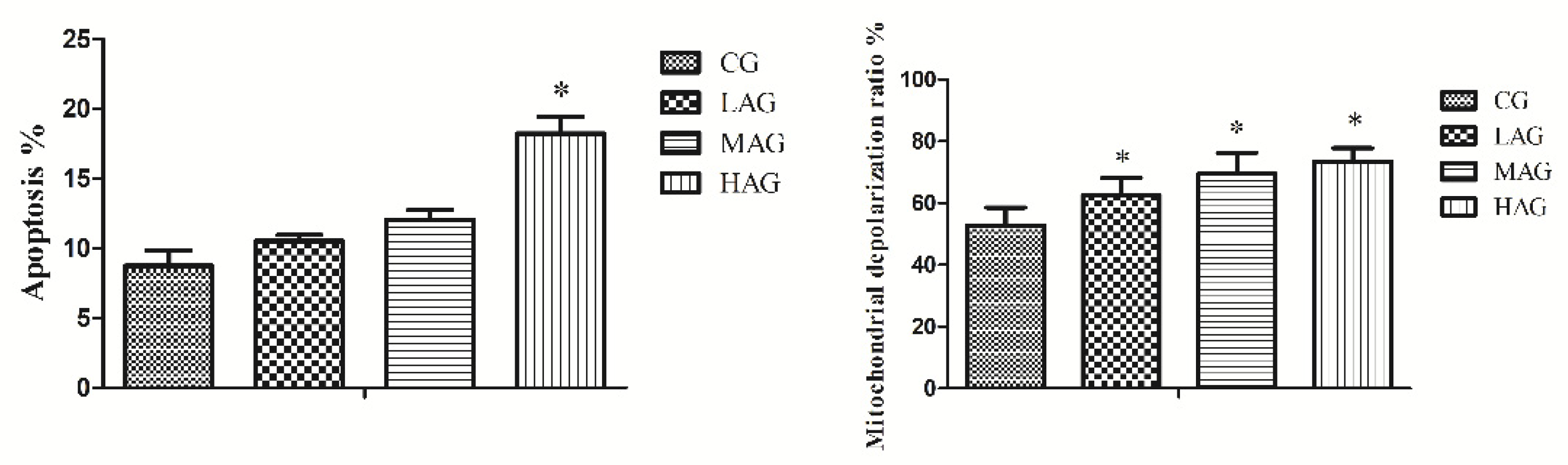
3.5. AA-I Induced Mitochondrial Damage and Apoptosis in Hepatocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.Y.; Chen, P.C.; Wang, J.D. Chinese Herbs Containing Aristolochic Acid Associated with Renal Failure and Urothelial Carcinoma: A Review from Epidemiologic Observations to Causal Inference. BioMed. Res. Int. 2014, 2014, 569325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandagoda, N.; Lord, G.M. Preventing Aristolochic Acid Nephropathy. Clin. J. Am. Soc. Nephro. 2015, 10, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Vanherweghem, J.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Vanhaelen-Fastre, R. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Abdullah, R.; Diaz, L.N.; Wesseling, S.; Rietjens, I. Risk assessment of plant food supplements and other herbal products containing aristolochic acids using the margin of exposure (MOE) approach. Food Addit. Contam. 2017, 34, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibutani, S.; Dong, H.; Suzuki, N.; Ueda, S.; Miller, F.; Grollman, A.P. Selective Toxicity of Aristolochic Acids I and II. Drug Metab. Dispos. 2007, 35, 1217–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.Y.; Weber, E.J.; Sidorenko, V.S.; Chapron, A.; Yeung, C.K.; Gao, C.Y.; Mao, Q.C.; Shen, D.; Wang, J.; Rosenquist, T.A. Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2017, 2, e95978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; He, B.S.; Liu, Y.Q.; Huo, M.L.; Fu, W.Q.; Yang, C.Y.; Wei, J.F.; Abliz, Z. In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta. Pharm. Sin. B 2020, 10, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Geng, X.D.; Chi, K.; Liu, C.; Liu, R.; Chen, X.M.; Hong, Q.; Cai, G.Y. Ultrasound enhances the therapeutic potential of mesenchymal stem cells wrapped in greater omentum for aristolochic acid nephropathy. Stem. Cell Res. Ther. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Prabu, S.M.; Muthumani, M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol. Biol. Rep. 2012, 39, 11201–11216. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Pistol, G.C.; Gras, M.; Palade, M.; Taranu, I. A comparison between the effects of ochratoxin A and aristolochic acid on the inflammation and oxidative stress in the liver and kidney of weanling piglets. N-S Arch. Pharmacol. 2018, 391, 1147–1156. [Google Scholar] [CrossRef]
- Poon, S.L.; Pang, S.T.; McPherson, J.R.; Yu, W.; Huang, K.K.; Guan, P.; Weng, W.H.; Siew, E.Y.; Liu, Y.; Heng, H.L. Genome-Wide Mutational Signatures of Aristolochic Acid and Its Application as a Screening Tool. Sci. Transl. Med. 2013, 5, 197ra101. [Google Scholar] [CrossRef]
- Ang, L.P.; Ng, P.W.; Lean, Y.L.; Kotra, V.; Kifli, N.; Goh, H.P.; Lee, K.S.; Ming, L.C. Herbal products containing aristolochic acids: A call to revisit the context of safety. J. Herb. Med. 2021, 28, 100447. [Google Scholar] [CrossRef]
- van Montfoort, J.E.; Hagenbuch, B.; Groothuis, G.M.M.; Koepsell, H.; Meier, P.J.; Meijer, D.K.F. Drug uptake systems in liver and kidney. Curr. Drug Metab. 2003, 4, 185–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.M.; Zhao, X.H.; Sun, Z.H.; Li, G.C.; Liu, G.C.; Sun, L.R.; Hou, J.Q.; Zhou, W. Recognition of the toxicity of aristolochic acid. J. Clin. Pharm. Ther. 2019, 44, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Ran, C.L.; Yin, L.Z.; Lin, J.C.; Shu, G. Acute and Subchronic Toxicity Studies of Aristolochic Acid A in Tianfu Broilers. Animals 2021, 11, 1556. [Google Scholar] [CrossRef]
- Draghia, L.P.; Lukinich-Gruia, A.T.; Oprean, C.; Pavlovic, N.M.; Tatu, C.A. Aristolochic acid I: An investigation into the role of food crops contamination, as a potential natural exposure pathway. Environ. Geochem. Health 2021, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Xu, D.; Du, X.X.; Ran, C.L.; Shu, G. Protective Effects of Salidroside against Carbon Tetrachloride (CCl4)-Induced Liver Injury by Initiating Mitochondria to Resist Oxidative Stress in Mice. Int. J. Mol. Sci. 2019, 20, 3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Na, R.; Yu, P.; Shi, B.; Yan, S. Effects of dietary supplementation of chitosan on immune and antioxidative function in beef cattle. Czech. J. Anim. Sci. 2015, 60, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Marin, D.E.; Braicu, C.; Gras, M.A.; Pistol, G.C.; Taranu, L. Low level of ochratoxin A affects genome-wide expression in kidney of pig. Toxicon 2017, 136, 67. [Google Scholar] [CrossRef]
- Bunel, V.; Antoine, M.H.; Nortier, J.; Duez, P.; Stevigny, C. In Vitro Effects of Panax ginseng in Aristolochic Acid-Mediated Renal Tubulotoxicity: Apoptosis versus Regeneration. Planta. Med. 2015, 81, 363–372. [Google Scholar] [CrossRef]
- Wu, T.K.; Pan, Y.R.; Wang, H.F.; Yu, Y.L. Vitamin E (αtocopherol) ameliorates aristolochic acidinduced renal tubular epithelial cell death by attenuating oxidative stress and caspase3 activation. Mol. Med. Rep. 2018, 17, 31–36. [Google Scholar]
- Pesti, G.M. Nutrient requirements of poultry. Anim. Feed. Sci. Tech. 1995, 56, 177–178. [Google Scholar] [CrossRef]
- Wang, Y.W.; Guo, Y.M.; Ning, D. Changes of hepatic biochemical parameters and proteomics in broilers with cold-induced ascites. J. Anim. Sci. Biotechnol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uni, Z.; Gal-Garber, O.; Geyra, A.; Sklan, D.; Yahav, S. Changes in Growth and Function of Chick Small Intestine Epithelium Due to Early Thermal Conditioning. Poult. Sci. 2001, 80, 438–445. [Google Scholar] [CrossRef]
- Thoolen, B.; Maronpot, R.R.; Takanori, H.; Abraham, N.; Colin, R.; Thomas, N.; Wolfgang, K.; Karin, K.; Ulrich, D.; Dai, N.; et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Hepatobiliary System. Toxicol. Pathol. 2010, 38, 5S–81S. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zuo, Z.C.; Chen, K.J.; Gao, C.X.; Yang, Z.Z.; Zhao, S.; Li, J.Z.; Song, H.T.; Peng, X.; Fang, J. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins 2018, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.; Luxia, Z.; Wenke, W.; Haiyan, W. Association Between Aristolochic Acid and CKD: A Cross-sectional Survey in China—ScienceDirect. Am. J. Kidney Dis. 2013, 61, 918–922. [Google Scholar]
- Cosyns, J.P.; Dehoux, J.P.; Guiot, Y.; Goebbels, R.M.; Cvyd, S. Chronic aristolochic acid toxicity in rabbits: A model of Chinese herbs nephropathy. Kidney Int. 2001, 59, 2164–2173. [Google Scholar] [CrossRef] [Green Version]
- Mengs, U. Tumour induction in mice following exposure to aristolochic acid. Arch. Toxicol. 1988, 61, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Bellamri, M.; Brandt, K.; Brown, C.V.; Wu, M.T.; Turesky, R.J. Cytotoxicity and genotoxicity of the carcinogen aristolochic acid I (AA-I) in human bladder RT4 cells. Arch. Toxicol. 2021, 95, 1–11. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Hou, D.Z.; Laraib, Y.; Xue, Y.; Shen, Q. Comparison of the effects of raw and cooked adzuki bean on glucose/lipid metabolism and liver function in diabetic mice. Cereal Chem. 2021, 98, 10456. [Google Scholar] [CrossRef]
- Wang, X.; Xia, H.J.; Liu, Y.P.; Qiu, F.; Di, X. Simultaneous determination of three glucuronide conjugates of scutellarein in rat plasma by LC–MS/MS for pharmacokinetic study of breviscapine. J. Chromatogr. B 2014, 965, 79–84. [Google Scholar] [CrossRef]
- Traesel, G.K.; Menegati, S.E.L.T.; Santos, A.C.D.; Boas, G.R.V.; Justi, P.N.; Kassuya, C.A.L.; Argandoa, E.J.S.; Oesterreich, S.A. Oral acute and subchronic toxicity studies of the oil extracted from pequi (Caryocar brasiliense, Camb.) pulp in rats. Food Chem. Toxicol. 2016, 97, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World. J. Gastroenterol. 2015, 21, 711–725. [Google Scholar] [CrossRef]
- Khattab, H.; Fouad, A.; Hamza, M.A.; Mohey, M.; El-Akel, W.; Ghoneim, H.; Abul-Fotouh, A.; Esmat, G. Relation of ALT and AST levels to the histopathological changes in liver biopsies of patients with chronic hepatitis C genotype 4. Arab. J. Gastroenterol. 2015, 16, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Lee, Y.T.; Hsieh, H.S.; Hwang, D.F. Short-term toxicity of aristolochic acid, aristolochic acid-I and aristolochic acid-II in rats. Food Chem. Toxicol. 2008, 46, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Wang, H.; Bian, B.; Zhao, H. The Disturbance of Hepatic and Serous Lipids in Aristolochic Acid Ι Induced Rats for Hepatotoxicity Using Lipidomics Approach. Molecules 2019, 24, 3745. [Google Scholar] [CrossRef] [Green Version]
- Schmeiser, H.H.; Frei, E.; Wiessler, M.; Stiborova, M. Comparison of DNA adduct formation by aristolochic acids in various in vitro activation systems by 32P-post-labelling: Evidence for reductive activation by peroxidases. Carcinogenesis 1997, 18, 1055–1062. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar]
- Youl, E.; Husson, C.; El Khattabi, C.; El Mere, S.; Antoine, M.H. Characterization of cytotoxic effects of aristolochic acids on the vascular endothelium. Toxicol. In Vitro 2020, 65, 104811. [Google Scholar] [CrossRef]
- Zhang, Y.; Shiyang, X.Y.; Li, Y.; Shi, X.; Xiong, B. Exposure to aristolochic acid I compromises the maturational competency of porcine oocytes via oxidative stress-induced DNA damage. Aging-Us 2019, 11, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Li, L.R.; Dong, H.; Song, E.Q.; Xu, X.Y.; Liu, L.C.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem.-Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Ran, C.L.; Yin, L.Z.; Amevor, F.K. Protective effect of dietary supplementation of Bupleurum falcatum L saikosaponins on ammonia exposure induced ileum injury in broilers. Poult. Sci. 2020, 100, 100803. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; Whyard, T.C.; Waltzer, W.C.; Grollman, A.P.; Rosenquist, T. Aristolochic acid-induced apoptosis and G2 cell cycle arrest depends on ROS generation and MAP kinases activation. Arch. Toxicol. 2015, 89, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.R.; Chen, L.; Cui, H.M.; Peng, X.; Wu, B.Y. Research Advances on Pathways of Nickel-Induced Apoptosis. Int. J. Mol. Sci. 2015, 17, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.R.; Ouyang, Y.J.; Wang, J.Q.; Cui, H.M.; Tang, H.Q. Cu-induced spermatogenesis disease is related to oxidative stress-mediated germ cell apoptosis and DNA damage. J. Hazard Mater. 2021, 416, 125903. [Google Scholar] [CrossRef]
- Zorov, B.D. Reactive Oxygen Species (ROS)-induced ROS Release: A New Phenomenon Accompanying Induction of the Mitochondrial Permeability Transition in Cardiac Myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Giordano, S.; Zhang, J.H. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, J.; Wang, J.; Feng, X.; Yang, X. Mitochondrial dysfunction is involved in aristolochic acid I-induced apoptosis in renal proximal tubular epithelial cells. Hum. Exp. Toxicol. 2019, 39, 096032711989709. [Google Scholar] [CrossRef] [PubMed]




| Numerical Score | Description | Definition |
|---|---|---|
| 0 | Within normal limits | Tissue considered to be normal, under the conditions of the study and considering the age, sex, and strain of the animal concerned. Alterations may be present, which, under other circumstances, would be considered deviations from normal. |
| 1 | Minimal | The amount of change present barely exceeds that which is considered to be within normal limits. |
| 2 | Slight | In general, the lesion is easily identified but of limited severity. |
| 3 | Moderete | The lesion is prominent, but there is significant potential for increased severity. |
| 4 | Severe | The degree of change is as complete as possible (occupies the majority of the organ). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yin, L.; Lin, J.; Fu, H.; Peng, X.; Chang, L.; Zheng, Y.; Zhao, X.; Shu, G. Aristolochic Acid I-Induced Hepatotoxicity in Tianfu Broilers Is Associated with Oxidative-Stress-Mediated Apoptosis and Mitochondrial Damage. Animals 2021, 11, 3437. https://doi.org/10.3390/ani11123437
Xu D, Yin L, Lin J, Fu H, Peng X, Chang L, Zheng Y, Zhao X, Shu G. Aristolochic Acid I-Induced Hepatotoxicity in Tianfu Broilers Is Associated with Oxidative-Stress-Mediated Apoptosis and Mitochondrial Damage. Animals. 2021; 11(12):3437. https://doi.org/10.3390/ani11123437
Chicago/Turabian StyleXu, Dan, Lizi Yin, Juchun Lin, Hualin Fu, Xi Peng, Lijen Chang, Yilei Zheng, Xiaoling Zhao, and Gang Shu. 2021. "Aristolochic Acid I-Induced Hepatotoxicity in Tianfu Broilers Is Associated with Oxidative-Stress-Mediated Apoptosis and Mitochondrial Damage" Animals 11, no. 12: 3437. https://doi.org/10.3390/ani11123437





