Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Importance of Thermoregulation in Laboratory Animals
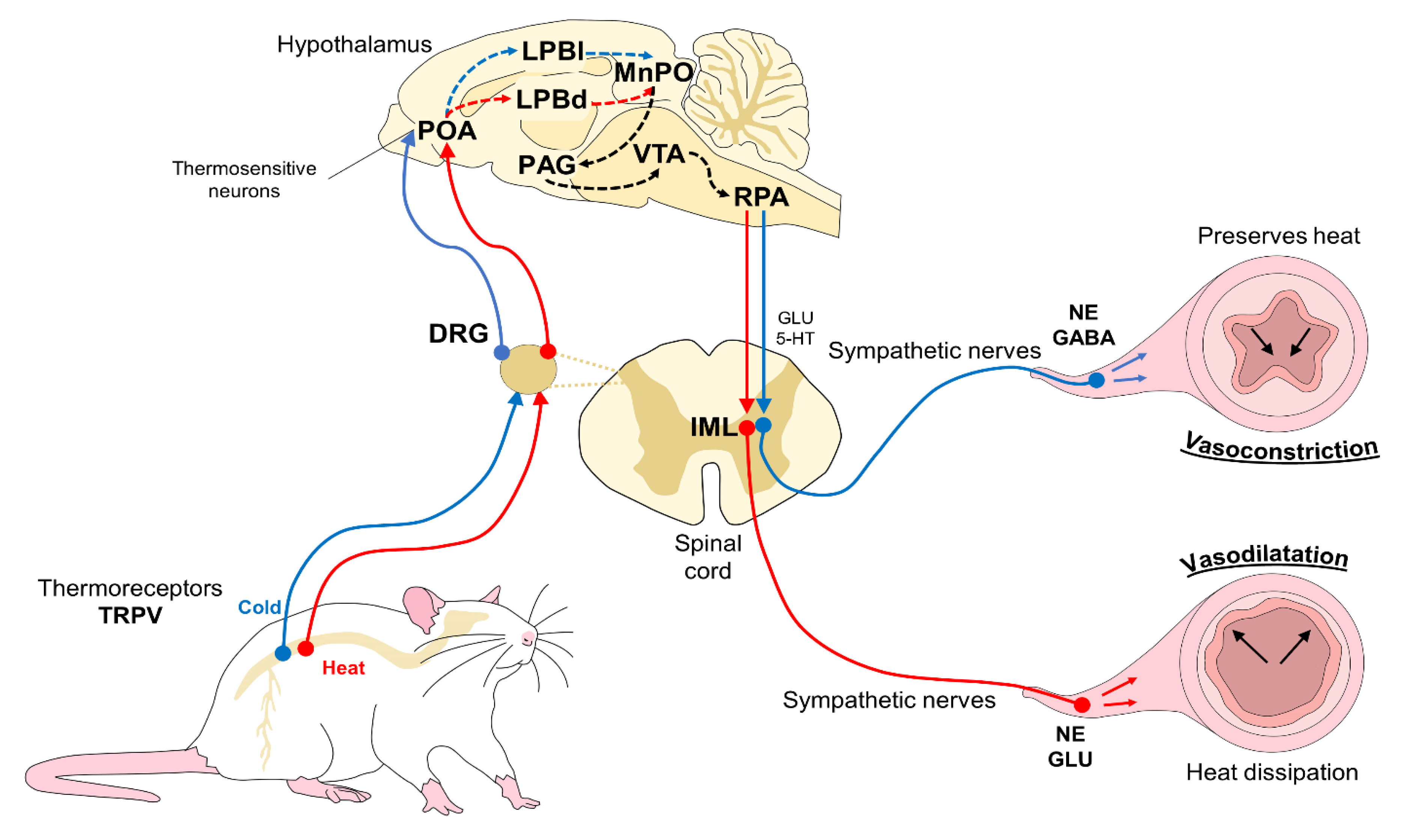
2.1. Peripheral Physiological Vascular Responses [Vasomotion of Cutaneous Blood Vessels]
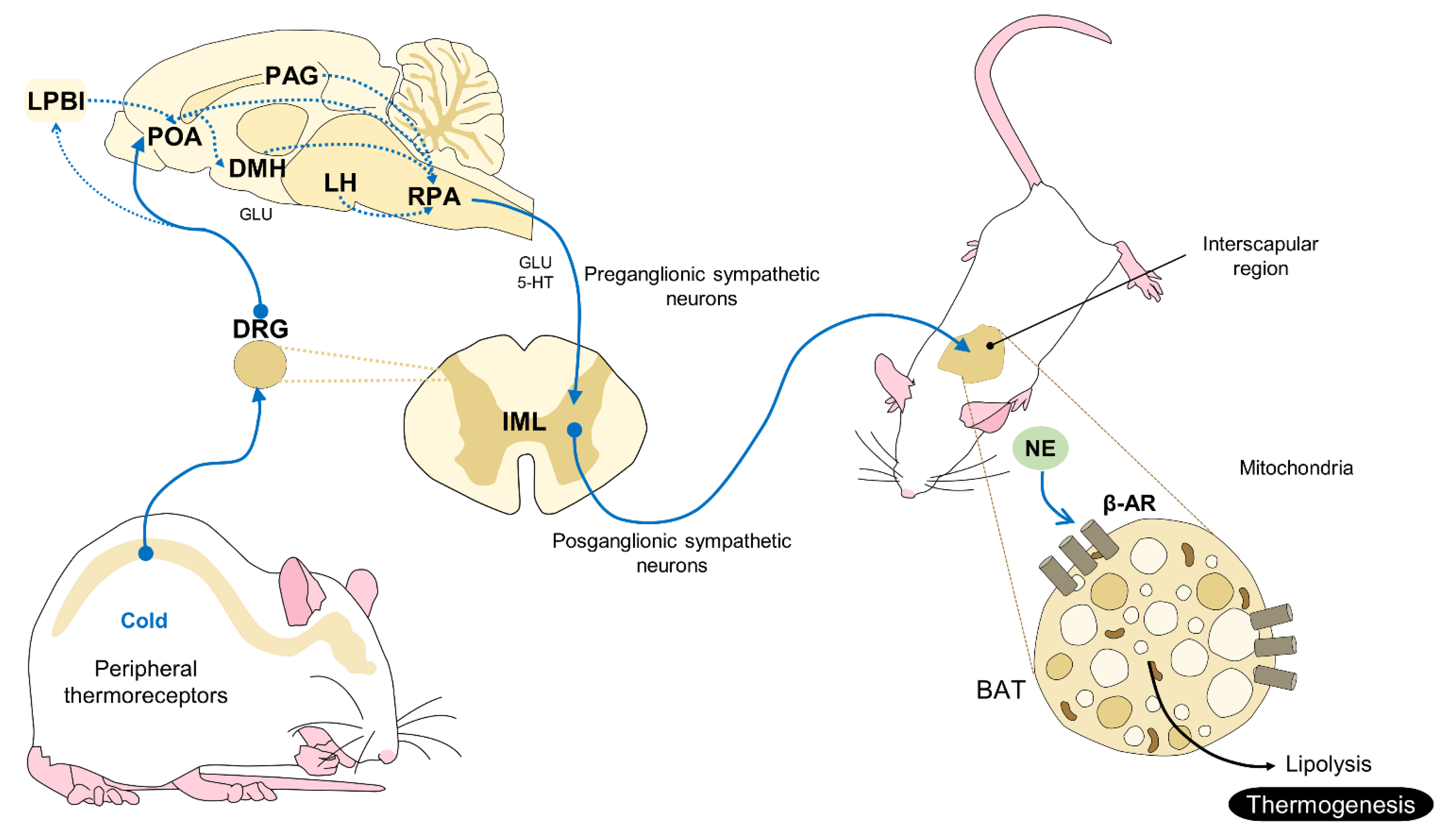
2.2. A Non-Shivering Mechanism of Thermogenesis: BAT
3. Thermal Windows Used with Laboratory Animals
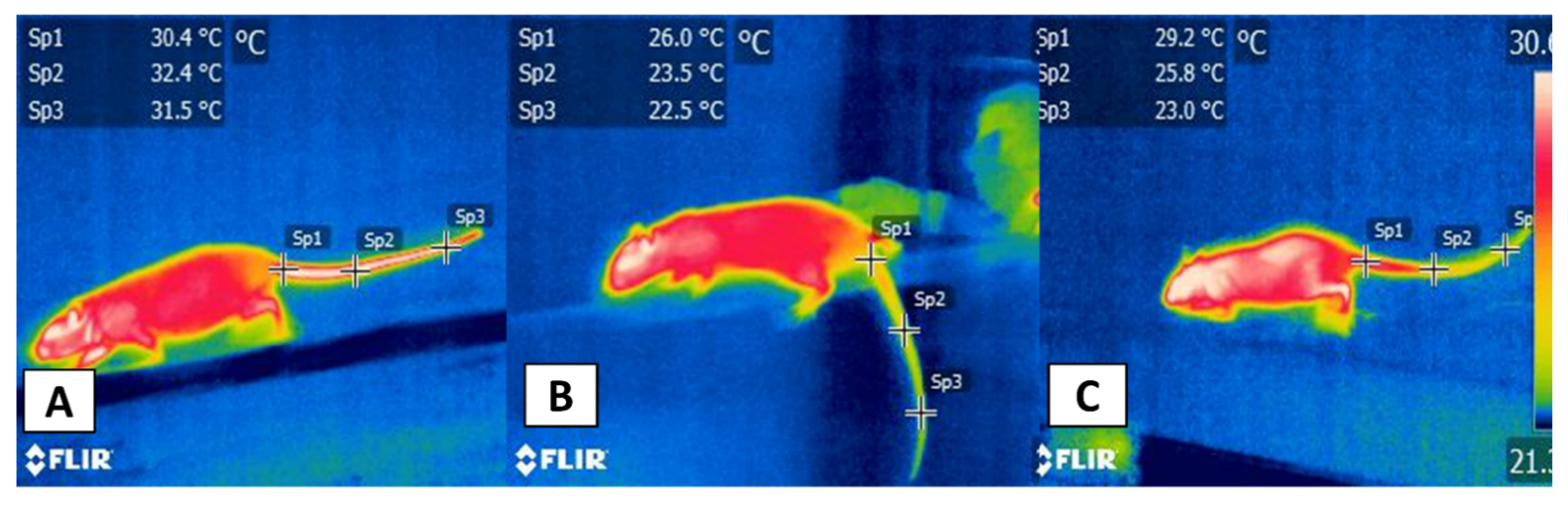
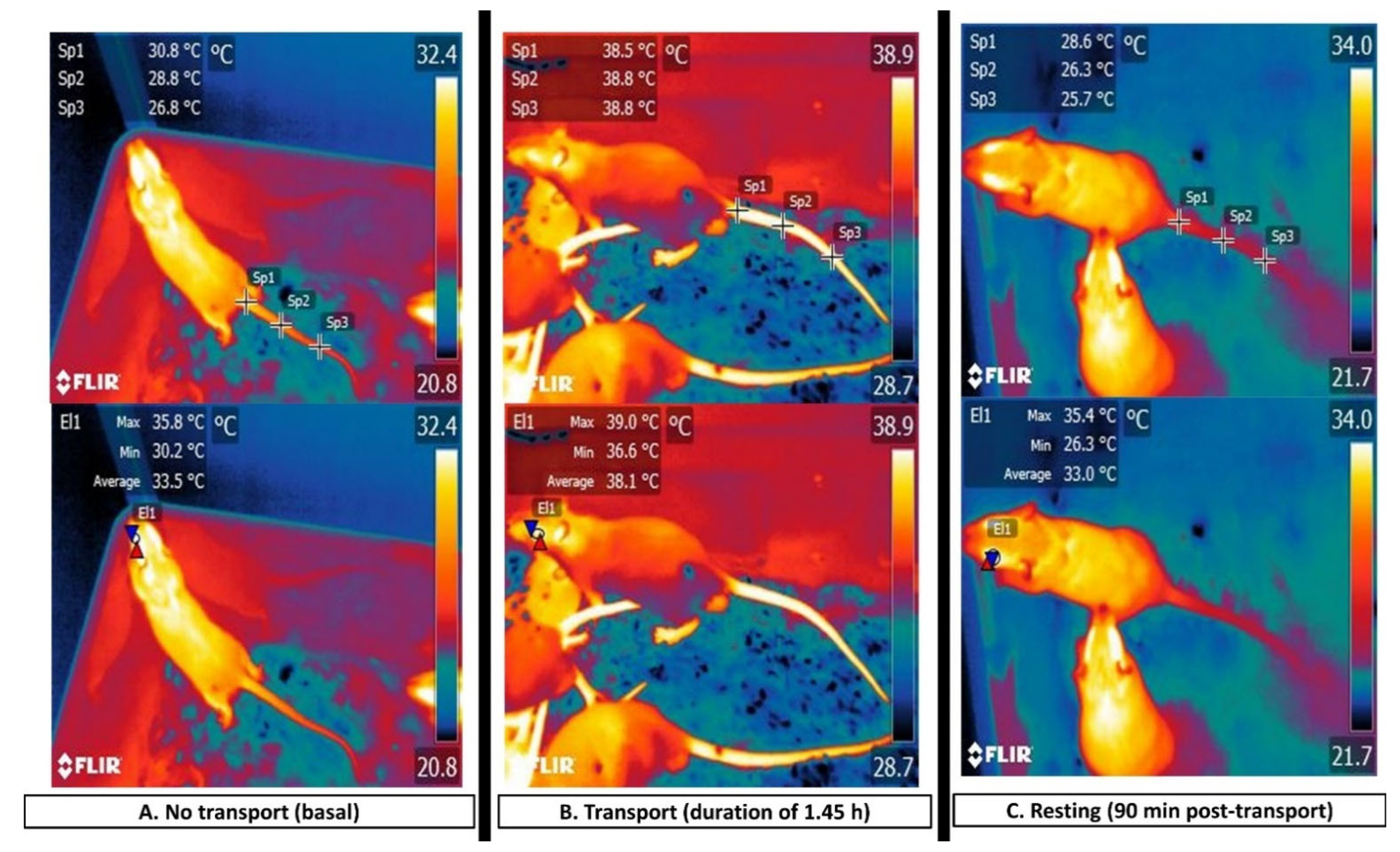
3.1. Thermal Windows: The Tail
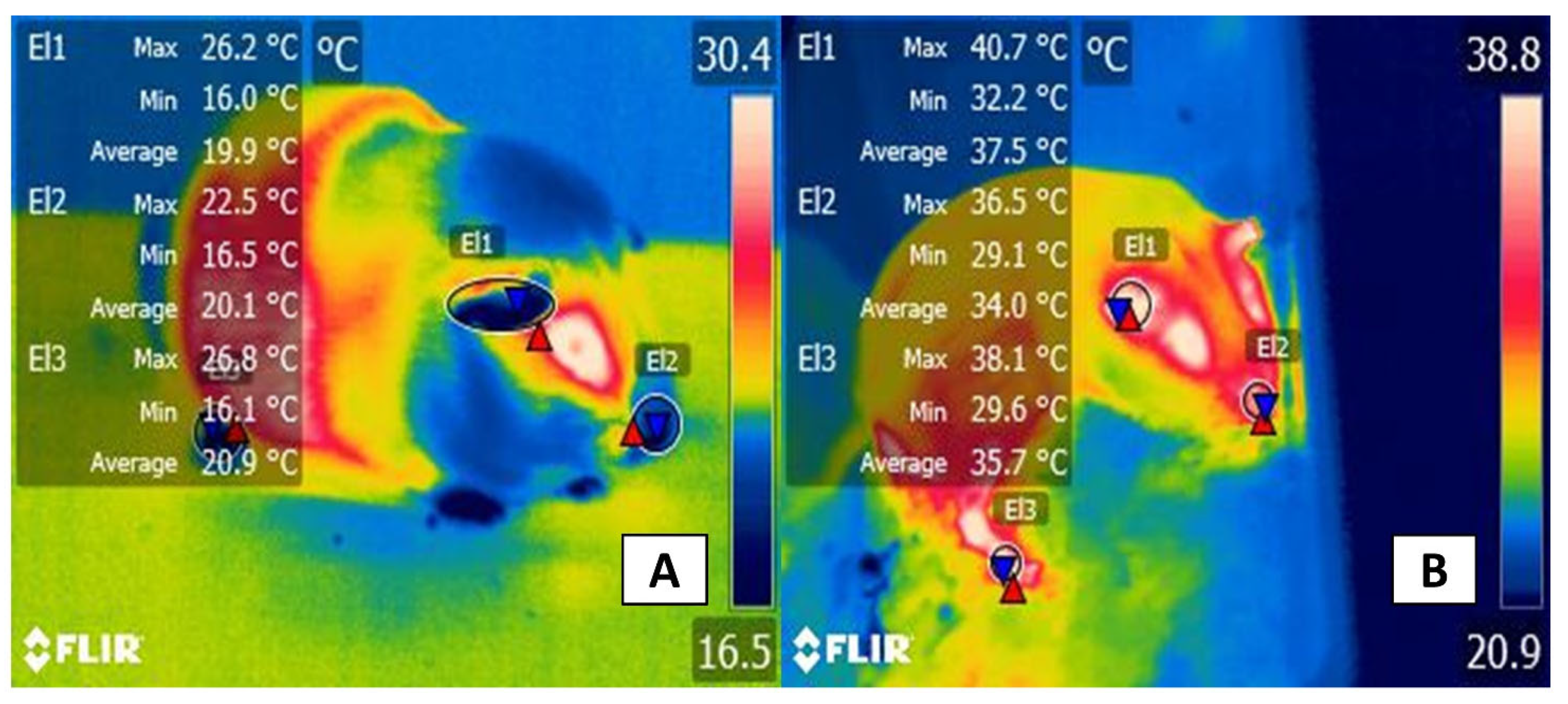
3.2. The Ocular Window
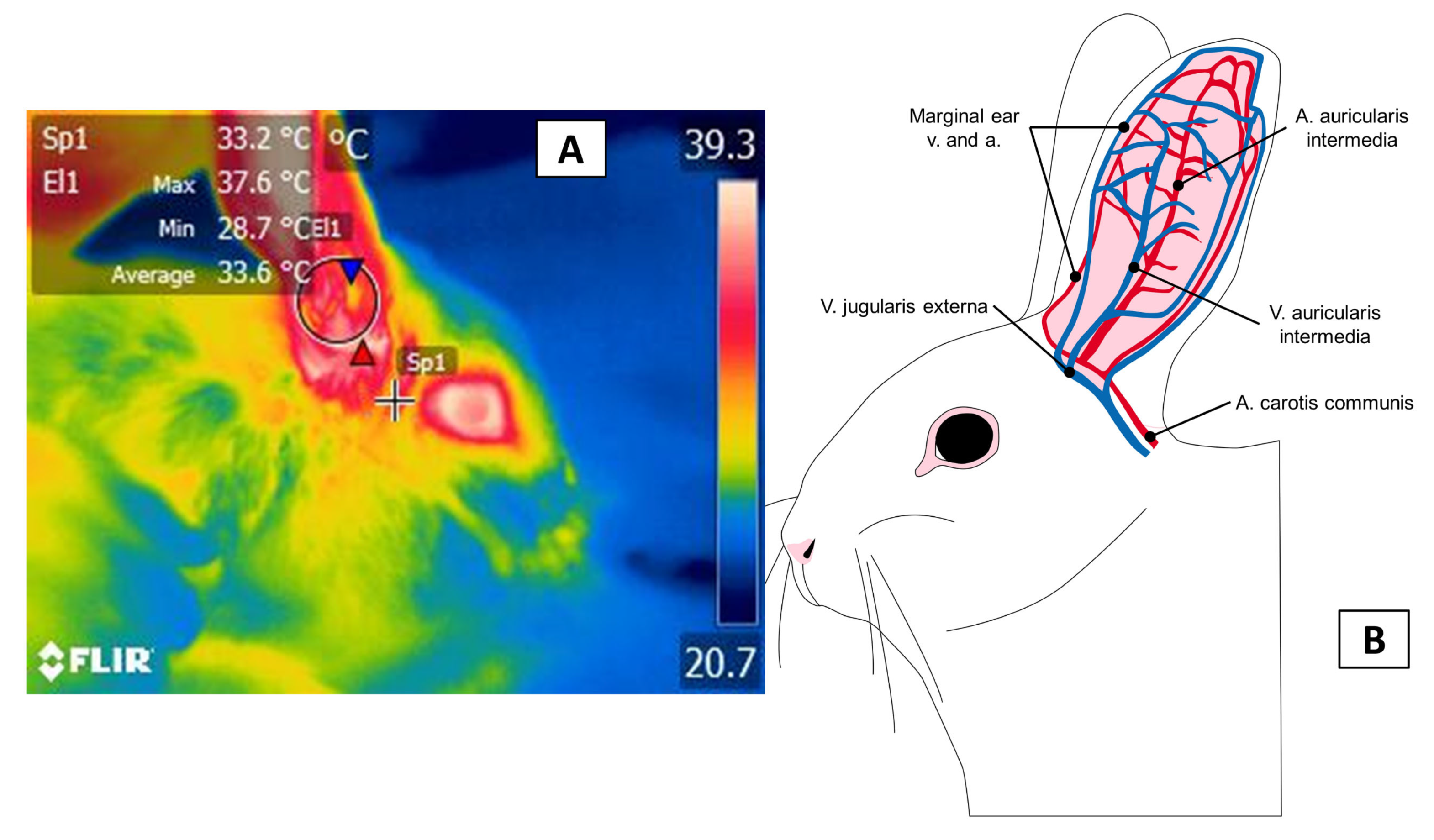
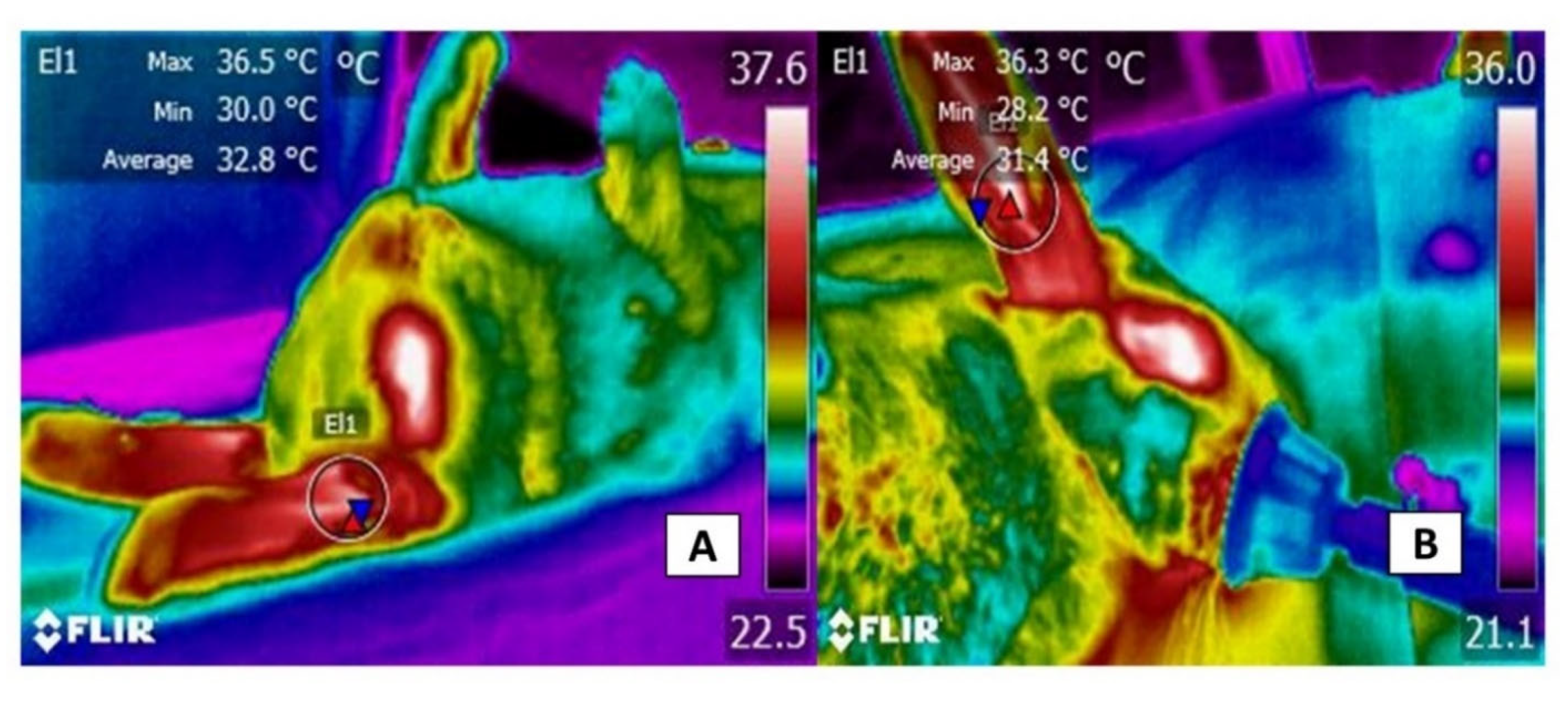
3.3. The Auricular Pavilion
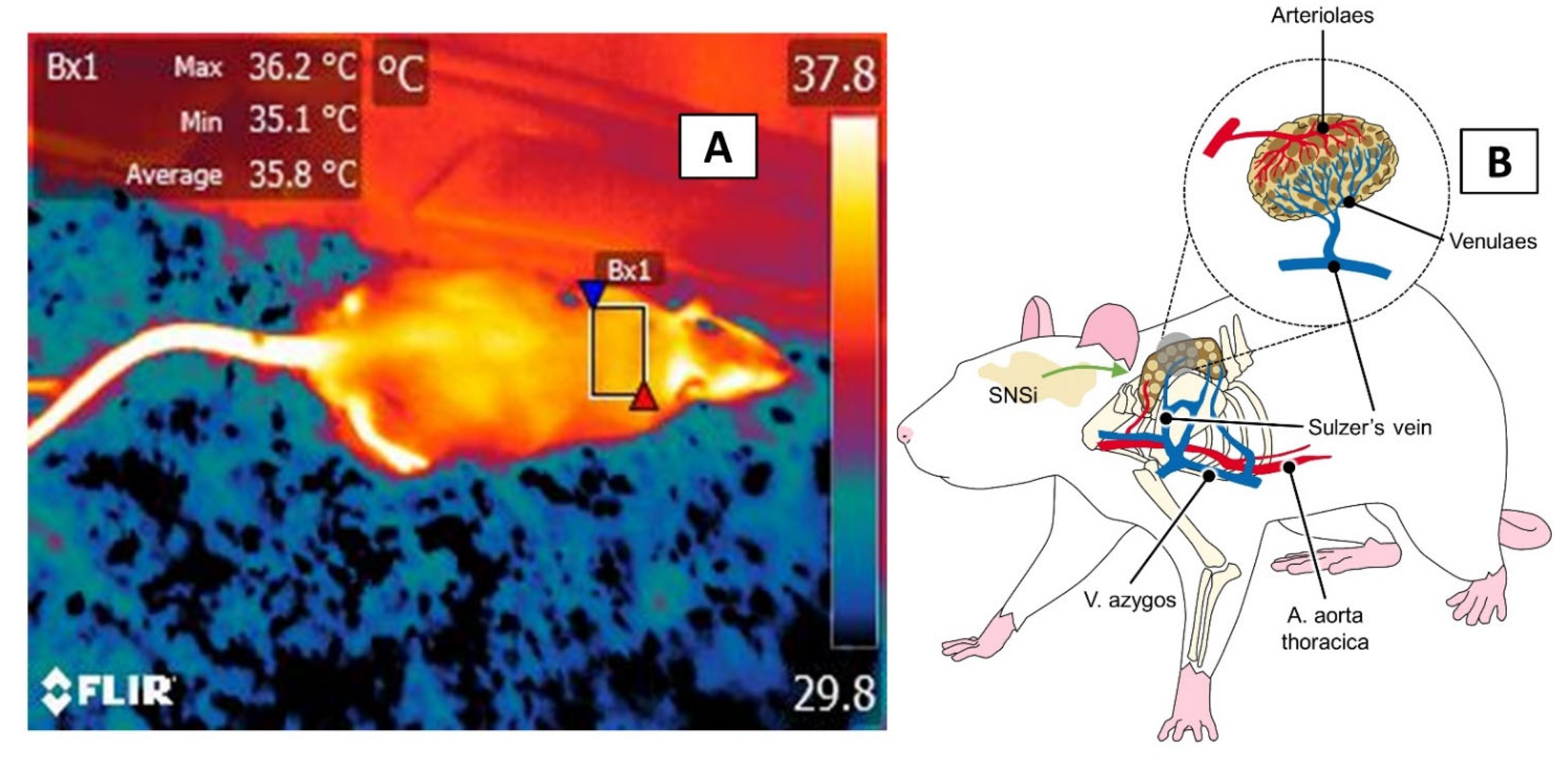
3.4. The Interscapular, or BAT, Window
4. Areas of Opportunity and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- National Research Council. Use of Laboratory Animals in Biomedical and Behavioral Research; National Academies Press: Washington, DC, USA, 1988; pp. 1–112. [Google Scholar] [CrossRef]
- Van Zutphen, L.F.M.B. Use of animals in research: A science—Society controversy? The European perspective. ALTEX 2002, 19, 140–144. [Google Scholar]
- Badyal, D.; Desai, C. Animal use in pharmacology education and research: The changing scenario. Indian J. Pharmacol. 2014, 46, 257. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Pratap, K.; Sinha, J.; Desiraju, K.; Bahal, D.; Kukreti, R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016, 29, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Thermal. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Rezende, E.L.; Bacigalupe, L.D. Thermoregulation in endotherms: Physiological principles and ecological consequences. J. Comp. Physiol. B 2015, 185, 709–727. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of body temperature by the nervous system. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.; Vianna, D.M.L.; Carrive, P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1239–R1247. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Liedtke, W.B. Deconstructing mammalian thermoregulation. Proc. Natl. Acad. Sci. USA 2017, 114, 1765–1767. [Google Scholar] [CrossRef] [Green Version]
- Škop, V.; Guo, J.; Liu, N.; Xiao, C.; Hall, K.D.; Gavrilova, O.; Reitman, M.L. Mouse thermoregulation: Introducing the concept of the thermoneutral point. Cell Rep. 2020, 31, 107501. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of rodent thermoregulation on animal models in the research environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, B.N.; Rohr, S.A.; Pajor, E.A.; Lucas, J.R.; Garner, J.P. Some like it hot: Mouse temperature preferences in laboratory housing. Appl. Anim. Behav. Sci. 2009, 116, 279–285. [Google Scholar] [CrossRef]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CAB International: Wallingford, UK, 2000; pp. 1–22. [Google Scholar]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Gaskill, B.N.; Garner, J.P. Stressed out: Providing laboratory animals with behavioral control to reduce the physiological effects of stress. Lab Anim. 2017, 46, 142–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Nakai, S.; Tanaka, M.; Kanosue, K. Neuronal circuitries involved in thermoregulation. Auton. Neurosci. 2000, 85, 18–25. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K.; Madden, C.J. Central control of thermogenesis in mammals. Exp. Physiol. 2008, 93, 773–797. [Google Scholar] [CrossRef]
- Malheiros-Lima, M.R.; Pires, W.; Fonseca, I.A.T.; Joviano-Santos, J.V.; Ferreira, A.J.; Coimbra, C.C.; Lima, N.R.V.; Wanner, S.P. Physical exercise-induced cardiovascular and thermoregulatory adjustments are impaired in rats subjected to cutaneous artery denervation. Front. Physiol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angilletta, M.J.; Youngblood, J.P.; Neel, L.K.; VandenBrooks, J.M. The neuroscience of adaptive thermoregulation. Neurosci. Lett. 2019, 692, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Da Scarpellini, C.S.; Cristina-Silva, C.; Biancardi, V.; Gargaglioni, L.H.; Almeida, M.C.; Bícego, K.C. Hypothalamic TRPV4 channels participate in the medial preoptic activation of warmth-defence responses in Wistar male rats. Pflüg. Arch. Eur. J. Physiol. 2019, 471, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 2017, 7, 5031. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S.B.G.; Saper, C.B. Role of the median preoptic nucleus in the autonomic response to heat-exposure. Temperature 2018, 5, 4–6. [Google Scholar] [CrossRef] [Green Version]
- McAllen, R.M.; McKinley, M.J. Efferent thermoregulatory pathways regulating cutaneous blood flow and sweating. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 305–316. [Google Scholar]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef]
- Kamijo, Y.-I.; Lee, K.; Mack, G.W. Active cutaneous vasodilation in resting humans during mild heat stress. J. Appl. Physiol. 2005, 98, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, N.; Kenny, G.P.; McGarr, G.W.; Amano, T.; Honda, Y.; Kondo, N.; Nishiyasu, T. TRPV4 channel blockade does not modulate skin vasodilation and sweating during hyperthermia or cutaneous postocclusive reactive and thermal hyperemia. Am. J. Physiol. Integr. Comp. Physiol. 2021, 320, R563–R573. [Google Scholar] [CrossRef]
- Ootsuka, Y.; Blessing, W.W. Inhibition of medullary raphé/parapyramidal neurons prevents cutaneous vasoconstriction elicited by alerting stimuli and by cold exposure in conscious rabbits. Brain Res. 2005, 1051, 189–193. [Google Scholar] [CrossRef]
- El Bitar, N.; Pollin, B.; Karroum, E.; Pincedé, I.; Mouraux, A.; Le Bars, D. Thermoregulatory vasomotor tone of the rat tail and paws in thermoneutral conditions and its impact on a behavioral model of acute pain. J. Neurophysiol. 2014, 112, 2185–2198. [Google Scholar] [CrossRef] [Green Version]
- Oladimeji, A.M.; Johnson, T.G.; Metwally, K.; Farghly, M.; Mahrose, K.M. Environmental heat stress in rabbits: Implications and ameliorations. Int. J. Biometeorol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Johnson, J.M.; Kellogg, D.L. Skin vasoconstriction as a heat conservation thermoeffector. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 175–192. [Google Scholar]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Bovell, D.L. The evolution of eccrine sweat gland research towards developing a model for human sweat gland function. Exp. Dermatol. 2018, 27, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.M.; Landis, S.C. Development and properties of the secretory response in rat sweat glands: Relationship to the induction of cholinergic function in sweat gland innervation. Dev. Biol. 1987, 123, 179–190. [Google Scholar] [CrossRef]
- Xu, M.; Wang, D. Distribution and density of sweat glands in Mongolian gerbils (Meriones unguiculatus) and Brandt´s voles (Lisiopodomys brandtii). Acta Theriol. Sin. 2015, 35, 80–86. [Google Scholar]
- Labbé, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Carpentier, A.C.; Lecomte, R.; Richard, D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef] [Green Version]
- Labbé, S.M.; Caron, A.; Lanfray, D.; Monge-Rofarello, B.; Bartness, T.J.; Richard, D. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 2015, 9, 150. [Google Scholar] [CrossRef]
- Tupone, D.; Madden, C.J.; Morrison, S.F. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: Potential clinical applications for altering BAT thermogenesis. Front. Neurosci. 2014, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Vialard, F.; Olivier, M. Thermoneutrality and Immunity: How does cold stress affect disease? Front. Immunol. 2020, 11, 588387. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- Terrien, J. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaskill, B.N.; Gordon, C.J.; Pajor, E.A.; Lucas, J.R.; Davis, J.K.; Garner, J.P. Impact of nesting material on mouse body temperature and physiology. Physiol. Behav. 2013, 110–111, 87–95. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Gordon, C.J.; Pajor, E.A.; Lucas, J.R.; Davis, J.K.; Garner, J.P. Heat or insulation: Behavioral titration of mouse preference for warmth or access to a nest. PLoS ONE 2012, 7, e32799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nääs, I.A.; Garcia, R.G.; Caldara, F.R. Infrared thermal image for assessing animal health and welfare. J. Anim. Behav. Biometeorol. 2014, 2, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, Z.; Bergamo, M.; Rodríguez-Rodríguez, C.; Saatchi, K.; Häfeli, U.O. Refinement and validation of infrared thermal imaging (IRT): A non-invasive technique to measure disease activity in a mouse model of rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Van der Vinne, V.; Pothecary, C.A.; Wilcox, S.L.; McKillop, L.E.; Benson, L.A.; Kolpakova, J.; Tam, S.K.E.; Krone, L.B.; Fisk, A.S.; Wilson, T.S.; et al. Continuous and non-invasive thermography of mouse skin accurately describes core body temperature patterns, but not absolute core temperature. Sci. Rep. 2020, 10, 20680. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows in large rumiants to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Vogel, B.; Wagner, H.; Gmoser, J.; Wörner, A.; Löschberger, A.; Peters, L.; Frey, A.; Hofmann, U.; Frantz, S. Touch-free measurement of body temperature using close-up thermography of the ocular surface. MethodsX 2016, 3, 407–416. [Google Scholar] [CrossRef]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria, 6th ed.; World Association of Veterinary Anatomist: Oslo, Norway, 2017; pp. 1–178. [Google Scholar]
- Gordon, C.J.; Puckett, E.; Padnos, B. Rat tail skin temperature monitored noninvasively by radiotelemetry: Characterization by examination of vasomotor responses to thermomodulatory agents. J. Pharmacol. Toxicol. Methods 2002, 47, 107–114. [Google Scholar] [CrossRef]
- Gordon, C.J. Thermal biology of the laboratory rat. Physiol. Behav. 1990, 47, 963–991. [Google Scholar] [CrossRef]
- Fiebig, K.; Jourdan, T.; Kock, M.H.; Merle, R.; Thöne-Reineke, C. Evaluation of infrared thermography for temperature measurement in adult male NMRI nude mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 715–724. [Google Scholar] [CrossRef]
- Conley, K.E.; Porter, W.P. Heat loss regulation: Role of appendages and torso in the deer mouse and the white rabbit. J. Comp. Physiol. B 1985, 155, 423–431. [Google Scholar] [CrossRef]
- Farrar, K.L.; Field, A.E.; Norris, S.L.; Jacobsen, K.O. Comparison of rectal and infrared thermometry temperatures in anesthetized swine (Sus scrofa). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Brunell, M.K. Comparison of noncontact infrared thermometry and 3 commercial subcutaneous temperature transponding microchips with rectal thermometry in rhesus macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 479–484. [Google Scholar] [PubMed]
- Vianna, D.M.L.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, J.; Bockhop, F.; Mitkovski, M.; Martin, S.; Ronnenberg, A.; Krueger-Burg, D.; Schneider, K.; Röhse, H.; Wüstefeld, L.; Cosi, F.; et al. Vascular response to social cognitive performance measured by infrared thermography: A translational study from mouse to man. FASEB BioAdv. 2020, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Kunczik, J.; Zieglowski, L.; Tolba, R.; Abdelrahman, A.; Zechner, D.; Vollmar, B.; Janssen, H.; Thum, T.; Czaplik, M. Remote welfare monitoring of rodents using thermal imaging. Sensors 2018, 18, 3653. [Google Scholar] [CrossRef] [Green Version]
- Weitkamp, J. Effect of Tickling and Gentling on Eye and Tail Temperature of Laboratory Rats during Manual Restraint, Using Infrared Thermography. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, March 2020. [Google Scholar]
- Luong, L.N.L.; Carrive, P. Orexin microinjection in the medullary raphe increases heart rate and arterial pressure but does not reduce tail skin blood flow in the awake rat. Neuroscience 2012, 202, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Lecorps, B.; Rödel, H.G.; Féron, C. Short-term thermal responses after exposure to predator odor (TMT) in the house mouse. Mamm. Biol. 2019, 94, 25–29. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Carvajal-de la Fuente, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of Fever and Application of Infrared Thermography (IRT) in the Detection of Sick Domestic Animals: Recent Advances. Animals 2021, 11, 2316. [Google Scholar] [CrossRef]
- Bell, C.; Rogers, S.; Taylor, J.; Busby, D. Improving the recognition of equine affective states. Animals 2019, 9, 1124. [Google Scholar] [CrossRef] [Green Version]
- Gjendal, K.; Franco, N.H.; Ottesen, J.L.; Sørensen, D.B.; Olsson, I.A.S. Eye, body or tail? Thermography as a measure of stress in mice. Physiol. Behav. 2018, 196, 135–143. [Google Scholar] [CrossRef]
- Wirth, S.; Gebhardt-Henrich, S.; Riemer, S.; Hattendorf, J.; Zinsstag, J.; Hediger, K. The influence of human interaction on guinea pigs: Behavioral and thermographic changes during animal-assisted therapy. Physiol. Behav. 2020, 225, 113076. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stookey, J.M.; Stafford, K.J.; Tucker, C.B.; Rogers, A.R.; Dowling, S.K.; Verkerk, G.A.; Schaefer, A.L.; Webster, J.R. Effects of local anesthetic and a nonsteroidal antiinflammatory drug on pain responses of dairy calves to hot-iron dehorning. J. Dairy Sci. 2009, 92, 1512–1519. [Google Scholar] [CrossRef]
- Nijland, N. The Influence of Different Types of Behaviour on the Eye Temperature of Mice Using Infrared Thermograohy. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, January 2021. [Google Scholar]
- Ding, J.E.; Kim, Y.H.; Yi, S.M.; Graham, A.D.; Li, W.; Lin, M.C. Ocular surface cooling rate associated with tear film characteristics and the maximum interblink period. Sci. Rep. 2021, 11, 15030. [Google Scholar] [CrossRef] [PubMed]
- Lecorps, B.; Rödel, H.G.; Féron, C. Assessment of anxiety in open field and elevated plus maze using infrared thermography. Physiol. Behav. 2016, 157, 209–216. [Google Scholar] [CrossRef]
- Maynard, R.L.; Downes, N. Cardiovascular System. In Anatomy and Histology of the Labor; Maynard, R., Downes, N., Eds.; Academic Press: Oxford, UK, 2019; pp. 77–90. [Google Scholar]
- Moore, D.M.; Zimmerman, K.; Smith, S.A. Hematological assessment in pet rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2015, 18, 9–19. [Google Scholar] [CrossRef]
- Ludwig, N.; Gargano, M.; Luzi, F.; Carenzi, C.; Verga, M. Technical note: Applicability of infrared thermography as a non invasive measurements of stress in rabbit. World Rabbit Sci. 2007, 15, 199–206. [Google Scholar] [CrossRef] [Green Version]
- McCafferty, D.J. The value of infrared thermography for research on mammals: Previous applications and future directions. Mamm. Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Hutu, I.; Patras, I.; Gherghel, D.; Lungu, B. Application of infrared thermograohy in rabbit orthopaedic models. In Proceedings of the Life Sciences, a Challenge for the Future, Iasi, Romania, 17–18 October 2018; University of Agricultural Sciences and Veterinary Medicine: Iasi, Romania, 2018; pp. 9–14. [Google Scholar]
- Redaelli, V.; Luzi, F.; Verga, M. Infrared hermography (IRT) in nude mice: An alternative method for body temperature measurement. In Proceedings of the Annual Meeting, Snad Las, Helsinki, 17 June 2010; pp. 14–17. [Google Scholar]
- De Lima, V.; Piles, M.; Rafel, O.; López-Béjar, M.; Ramón, J.; Velarde, A.; Dalmau, A. Use of infrared thermography to assess the influence of high environmental temperature on rabbits. Res. Vet. Sci. 2013, 95, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Ludwig, N.; Gargano, M.; Milazzo, M.; Carenzi, C.; Verga, M. Evaluation of skin temperature change as stress indicator in rabbit through infrared thermography. Ital. J. Anim. Sci. 2007, 6, 769. [Google Scholar] [CrossRef]
- Gilbert, C.; McCafferty, D.J.; Giroud, S.; Ancel, A.; Blanc, S. Private heat for public warmth: How huddling shapes individual thermogenic responses of rabbit pups. PLoS ONE 2012, 7, e33553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wokke, E.S. Refinement: Evaluating Stress and Accuracy of Different Intraperitoneal Techniques in Mice. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2017. [Google Scholar]
- Xu, Z.; Agbigbe, O.; Nigro, N.; Yakobi, G.; Shapiro, J.; Ginosar, Y. Use of high-resolution thermography as a validation measure to confirm epidural anesthesia in mice: A cross-over study. Int. J. Obstet. Anesth. 2021, 46, 102981. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Pastorelli, G.; Faustini, M.; Luzi, F.; Redaelli, V.; Turin, L. Passiflora Incarnata powder extract in postweaning piglets feeding slightly improves wellbeing and immune parameters. Livest. Sci. 2020, 235, 104000. [Google Scholar] [CrossRef]
- Mrzilkova, J.; Michenka, P.; Seremeta, M.; Kremen, J.; Dudak, J.; Zemlicka, J.; Musil, V.; Minnich, B.; Zach, P. Morphology of the vasculature and blood supply of the brown adipose tissue examined in an animal model by micro-CT. Biomed Res. Int. 2020, 2020, 7502578. [Google Scholar] [CrossRef]
- Warner, A.; Kjellstedt, A.; Carreras, A.; Böttcher, G.; Peng, X.-R.; Seale, P.; Oakes, N.; Lindén, D. Activation of β 3 -adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am. J. Physiol. Metab. 2016, 311, E901–E910. [Google Scholar] [CrossRef] [Green Version]
- Farrell, W.J.; Alberts, J.R. Ultrasonic vocalizations by rat pups after adrenergic manipulations of brown fat metabolism. Behav. Neurosci. 2000, 114, 805–813. [Google Scholar] [CrossRef]
- Boileau, A.; Farish, M.; Turner, S.P.; Camerlink, I. Infrared thermography of agonistic behaviour in pigs. Physiol. Behav. 2019, 210, 112637. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Papa, S.; Marsella, G.; Grignaschi, G.; Bosi, A.; Ludwig, N.; Luzi, F.; Vismara, I.; Rimondo, S.; Veglianese, P.; et al. A refinement approach in a mouse model of rehabilitation research. Analgesia strategy, reduction approach and infrared thermography in spinal cord injury. PLoS ONE 2019, 14, e0224337. [Google Scholar] [CrossRef] [PubMed]
- Jaén-Téllez, J.A.; Sánchez-Guerrero, M.J.; López-Campos, J.I.; Valera, M.; González-Redondo, P. Acute stress assessment using infrared thermography in fattening rabbits reacting to handling under winter and summer conditions. Span. J. Agric. Res. 2020, 18, e0502. [Google Scholar] [CrossRef]
- Brzezinski, R.Y.; Ovadia-Blechman, Z.; Lewis, N.; Rabin, N.; Zimmer, Y.; Levin-Kotler, L.; Tepper-Shaihov, O.; Naftali-Shani, N.; Tsoref, O.; Grossman, E.; et al. Non-invasive thermal imaging of cardiac remodeling in mice. Biomed. Opt. Express 2019, 10, 6189. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body temperature measurement in mice during acute illness: Implantable temperature transponder versus surface infrared thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef]
- Song, C.; Appleyard, V.; Murray, K.; Frank, T.; Sibbett, W.; Cuschieri, A.; Thompson, A. Thermographic assessment of tumor growth in mouse xenografts. Int. J. Cancer 2007, 121, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Vainer, B.G. A Novel High-Resolution Method for the Respiration Rate and Breathing Waveforms Remote Monitoring. Ann. Biomed. Eng. 2018, 46, 960–971. [Google Scholar] [CrossRef]
- Franco, N.H.; Gerós, A.; Oliveira, L.; Olsson, I.A.S.; Aguiar, P. ThermoLabAnimal—A high-throughput analysis software for non-invasive thermal assessment of laboratory mice. Physiol. Behav. 2019, 207, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Geraldo, A.M.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11. in press. [Google Scholar]
- Mazur-Milecka, M.; Kocejko, T.; Ruminski, J. Deep instance segmentation of laboratory animals in thermal images. Appl. Sci. 2020, 10, 5979. [Google Scholar] [CrossRef]
- Unger, S.D.; Rollins, M.A.; Thompson, C.M. Hot- or Cold-Blooded? A Laboratory Activity That Uses Accessible Technology to Investigate Thermoregulation in Animals. Am. Biol. Teach. 2020, 82, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Całkosiński, I.; Dobrzyński, M.; Rosińczuk, J.; Dudek, K.; Chrószcz, A.; Fita, K.; Dymarek, R. The use of infrared thermography as a rapid, quantitative, and noninvasive method for evaluation of inflammation response in different anatomical regions of rats. Biomed. Res. Int. 2015, 2015, 972535. [Google Scholar] [CrossRef]


| Reference | Research Objective | Species | Thermal Window | Basal Temperature (°C) | Experimental Phase Temperature (°C) | Ambiental Conditions |
|---|---|---|---|---|---|---|
| Fiebig et al. [57] | Compare rectal, subcutaneous, intraperitoneal temperature with infrared | Male NMRI nude mice (10 months old) | Dorsal region | 37.36 ± 0.63 | Compared to rectal temperature Mean difference of 0.56 | 22 °C ± 1 °C Relative humidity 53–56% |
| Vianna and Carrive [61] | Thermal response to conditioned fear | Male Wistar rats (350–500 g) | Regions of interest: Tail Paws Head Dorso Eyes | Basal 31.5 34.8 32.8 31.9 35.2 | Fear 32.9 26.3 27.3 36.4 34.3 | Ambient temperature 26–27 °C |
| Vogel et al. [53] | Thermal response to anesthesia with isoflurane | Mice C57BL/6J or CD-1 | Ocular region | (1) 37.0 ± 0.3 (2) 37.3 ± 0.2 (3) 37.5 ± 0.2 | 35.6 ± 0.2 36.4 ± 0.2 36.9 ± 0.1 | |
| Vogel et al. [53] | Comparison with rectal temperature and ocular surface | Mice C57BL/6J CD-1, Wistar rats and New Zealand White rab-bits | Ocular region | Rat T° Rectal 35.7 ± 0.1 Rabbit T° Rectal 38.2 ± 0.2 Mice T° rectal 37.5 ± 0.2 | 36.5 ± 0.2 39.1 ± 0.2 37.2 ± 0.2 °C | |
| Gjendal et al. [69] | Thermal response to three stressors (1) Anesthesia (2) Scruff (3) Peritoneal injection | Male C57BL/6 mice (n = 80) | Maximum temperature of Eye Tail Body Surface | Before isoflurane Eye: 37.32 Tail: 31.16 Body: 32.88 Scruff Eye: 37.04 Body: 32.4 Intraperitoneal Eye: 36.99 Body: 32.46 | After isoflurane 34.70 28.13 30.29 37.25 32.13 37.19 32.25 | Ambient temperature between 20–24 °C |
| Marks et al. [10] | Cold exposure | Wistar rats, weight 450– 550 g | Interscapular region Dorsal Tail | Average temperature Interscapular: 34.9 Dorsal: 34.4 Tail: 28.6 | After exposure to cold (4 °C) 34.14 ± 0.17 30.96 ± 0.31 8.69 ± 0.49 | Room temperature of 22–24 °C |
| Lecorps et al. [66] | Thermal response to predator odor 2,5-dihydro-2,4,5-trimethylthiazole (TMT), and water | Adult male house mice (4-month-old) | Tail Body surface | 5 min before TMT 22.10 ± 0.9 °C 5 min before TMT 35.76 ± 0.08 °C | 4 and 5 min after TMT, respectively 23.32 ± 1.3 °C 23.23 ± 1.65 °C 2, 3 and 4 min after TMT, respectively 35.90 ± 0.08 °C 35.98 ± 0.11 °C 36.03 ± 0.11 °C | A separated experimental room Reverse light-dark cycle |
| Franco et al. [98] | Hypothermia induced by lipopolysaccharides (E. coli) | Mice C57BL/6 | Body surface Interscapular region | 31.58 32.43 | Ambient temperature at 20–24 °C | |
| Całkosiński et al. [102] | Induced inflammatory response with carrageenan 1% | Female rats, 10-weeks-old, weight 150–160 g | Pleura Lower lip Right hindlimb Left hindlimb | Basal 34.00 ± 0.59 31.86 ± 0.57 24.66 ± 0.76 24.93 ± 0.85 | 72 h after the administration 35.91 ± 0.65 33.73 ± 0.66 26.26 ± 0.98 26.36 ± 0.82 | Room temperature at 20 ± 1 °C |
| El Bitar et al. [33] | Thermoregulation assessment in an acute pain model Comparison between Tcore, Tambient and Ttail | Adult male Sprague-Dawley rats, 320–370 g | Tail | 32.0 °C | Thermographic pictures every minute from a 20-min sequence 27.8 | Experiments conducted between 9 am and 5 pm Ambient temperature 24.1 °C Mean core temperature of 38.2 °C |
| Nosrati et al. [49] | Measure disease activity in a collagen-induced rheumatoid arthritis model | Female 7-week-old DBA1/J mice 18–20 g | Wrist joints Front paws Ankle joints Hind paws Upper back | Quantified as temperature index TItotal (± SD) Baseline arthritis 3.37 ± 0.12 Baseline control 3.31 ± 0.06 | Progressive increase at day 28 3.53–3.85 Same | Fluorescent light to prevent radiation Ambient temperature 24 ± 1°C |
| Brunell [60] | Compare IRT to rectal temperature | 20 female and 30 male rhesus macaques, 3.5 to 11 years, weight of 3.4 to 11.7 kg | Chest Abdomen | Correlation coefficient with rectal temperature (first week) −0.079 −0.079 | After approximately 3 weeks −0.012 0.060 | Ambient temperature of 68 to 72 °F Relative humidity of 30 to 70% 12:12-h light:dark cycle |
| Farrar et al. [59] | Compare rectal an infrared thermometry | Female Yorkshire-cross swine, 12–15-week-old, 30–45 pounds | Area surrounding the eyes Neck Base of the ear | Mean baseline rectal 38 Eye basal ~37.1 °F Neck basal ~36.9 °F | ~38.7 ~38.6 ~38.7 | Distance between 24 and 32 inches |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals 2021, 11, 3448. https://doi.org/10.3390/ani11123448
Verduzco-Mendoza A, Bueno-Nava A, Wang D, Martínez-Burnes J, Olmos-Hernández A, Casas A, Domínguez A, Mota-Rojas D. Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals. 2021; 11(12):3448. https://doi.org/10.3390/ani11123448
Chicago/Turabian StyleVerduzco-Mendoza, Antonio, Antonio Bueno-Nava, Dehua Wang, Julio Martínez-Burnes, Adriana Olmos-Hernández, Alejandro Casas, Adriana Domínguez, and Daniel Mota-Rojas. 2021. "Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals" Animals 11, no. 12: 3448. https://doi.org/10.3390/ani11123448
APA StyleVerduzco-Mendoza, A., Bueno-Nava, A., Wang, D., Martínez-Burnes, J., Olmos-Hernández, A., Casas, A., Domínguez, A., & Mota-Rojas, D. (2021). Experimental Applications and Factors Involved in Validating Thermal Windows Using Infrared Thermography to Assess the Health and Thermostability of Laboratory Animals. Animals, 11(12), 3448. https://doi.org/10.3390/ani11123448











