Effect of Actin Alpha Cardiac Muscle 1 on the Proliferation and Differentiation of Bovine Myoblasts and Preadipocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source
2.2. Isolation, Culture, and Differentiation of Primary Bovine Myoblasts and Preadipocytes
2.3. Interference and Overexpression of Adenovirus Packaging
2.4. Optimal Adenovirus MOI Determination
2.5. Immunofluorescence Staining
2.6. Oil Red O Staining
2.7. Flow Cytometry
2.8. Real-Time Quantitative PCR
2.9. Western Blot
2.10. Statistical Analysis
3. Results
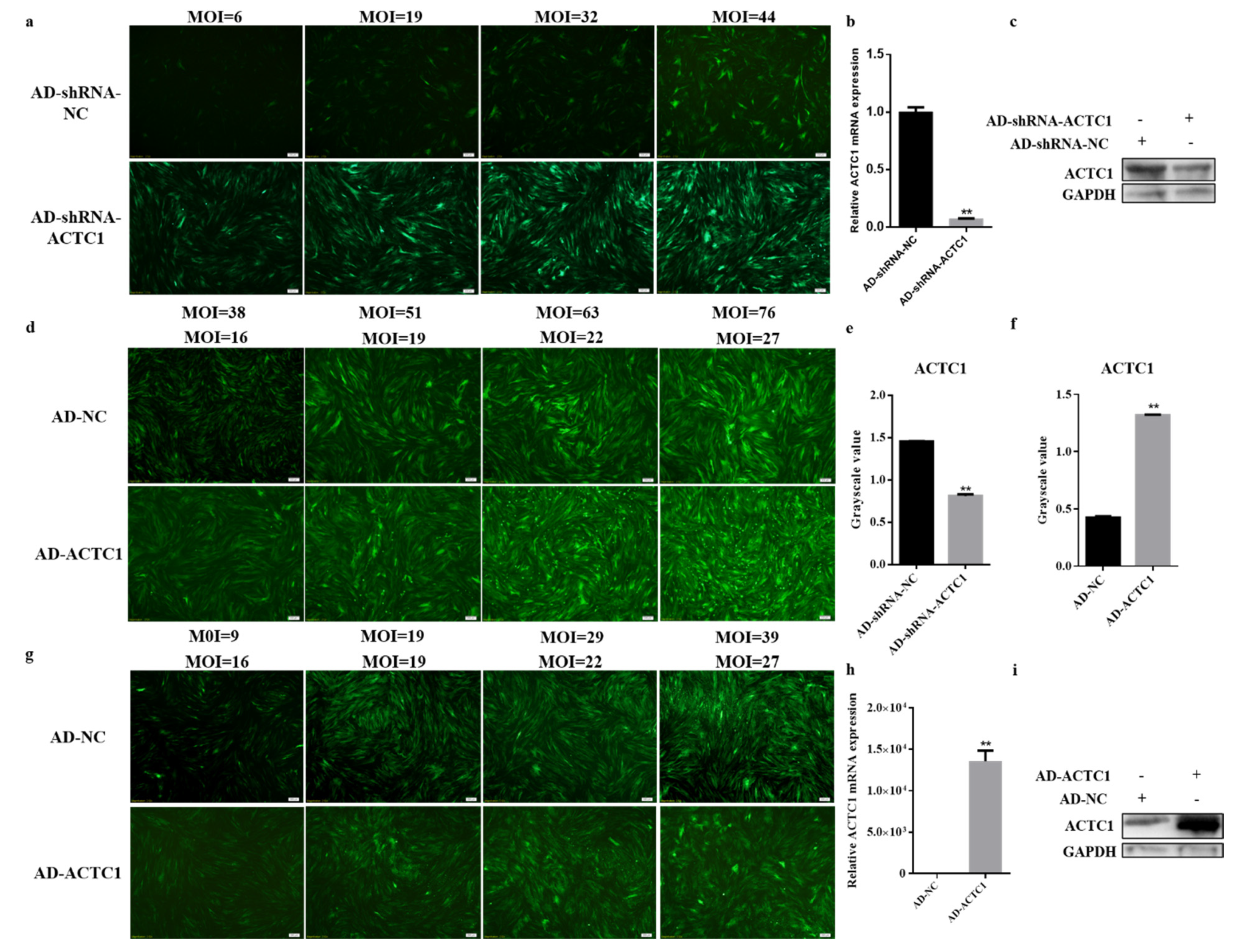
3.1. Screening of Adenovirus MOI
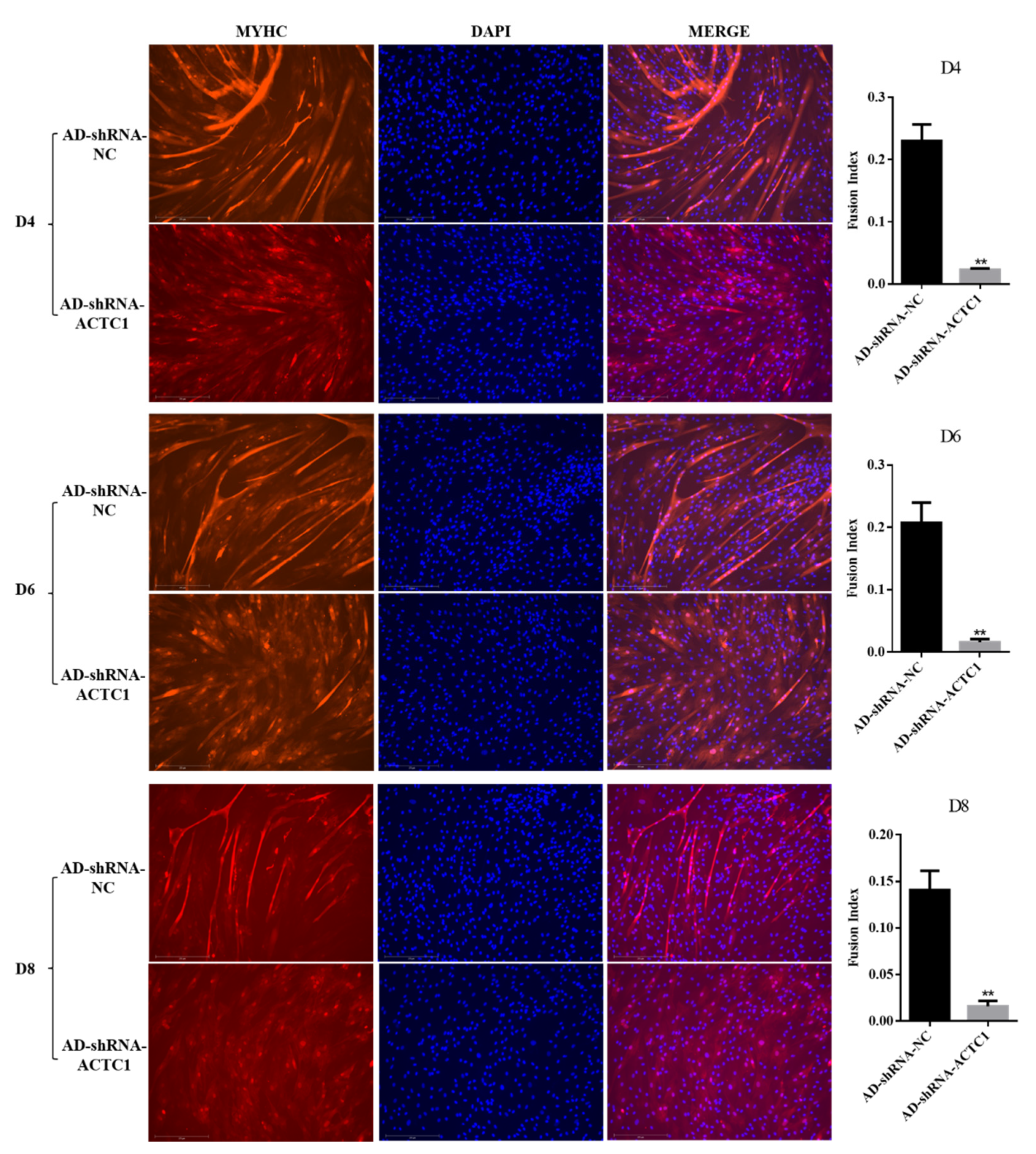
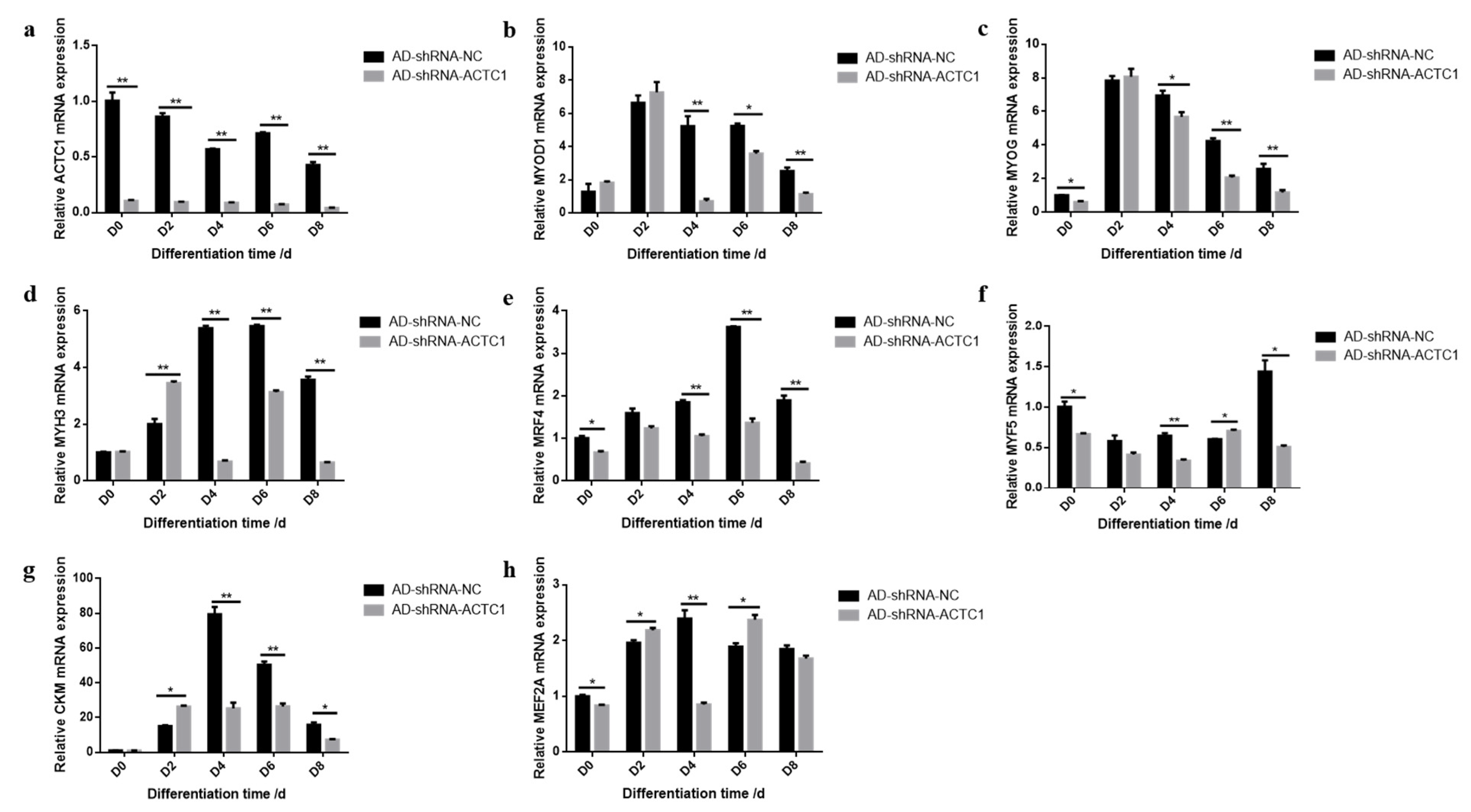
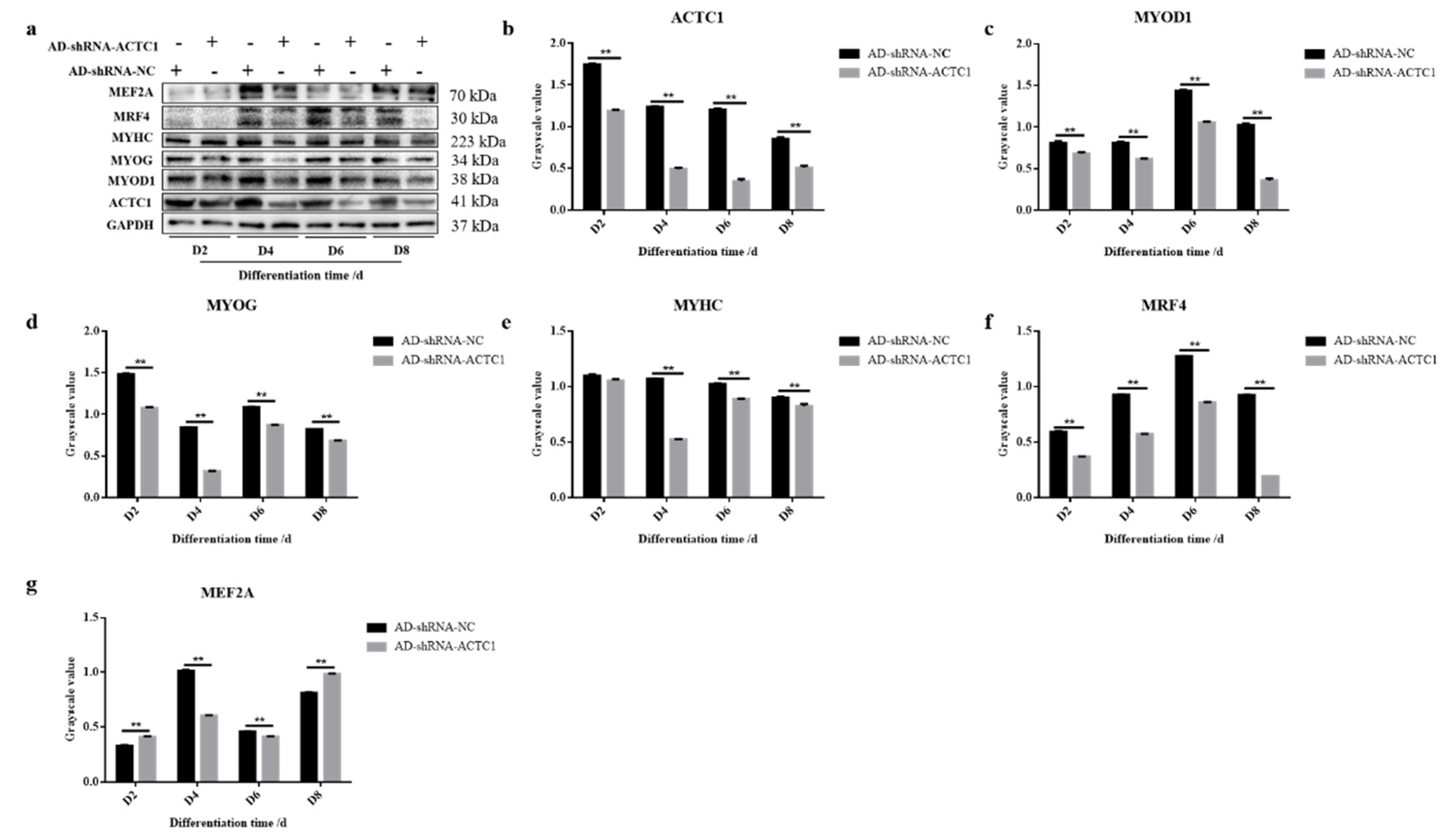
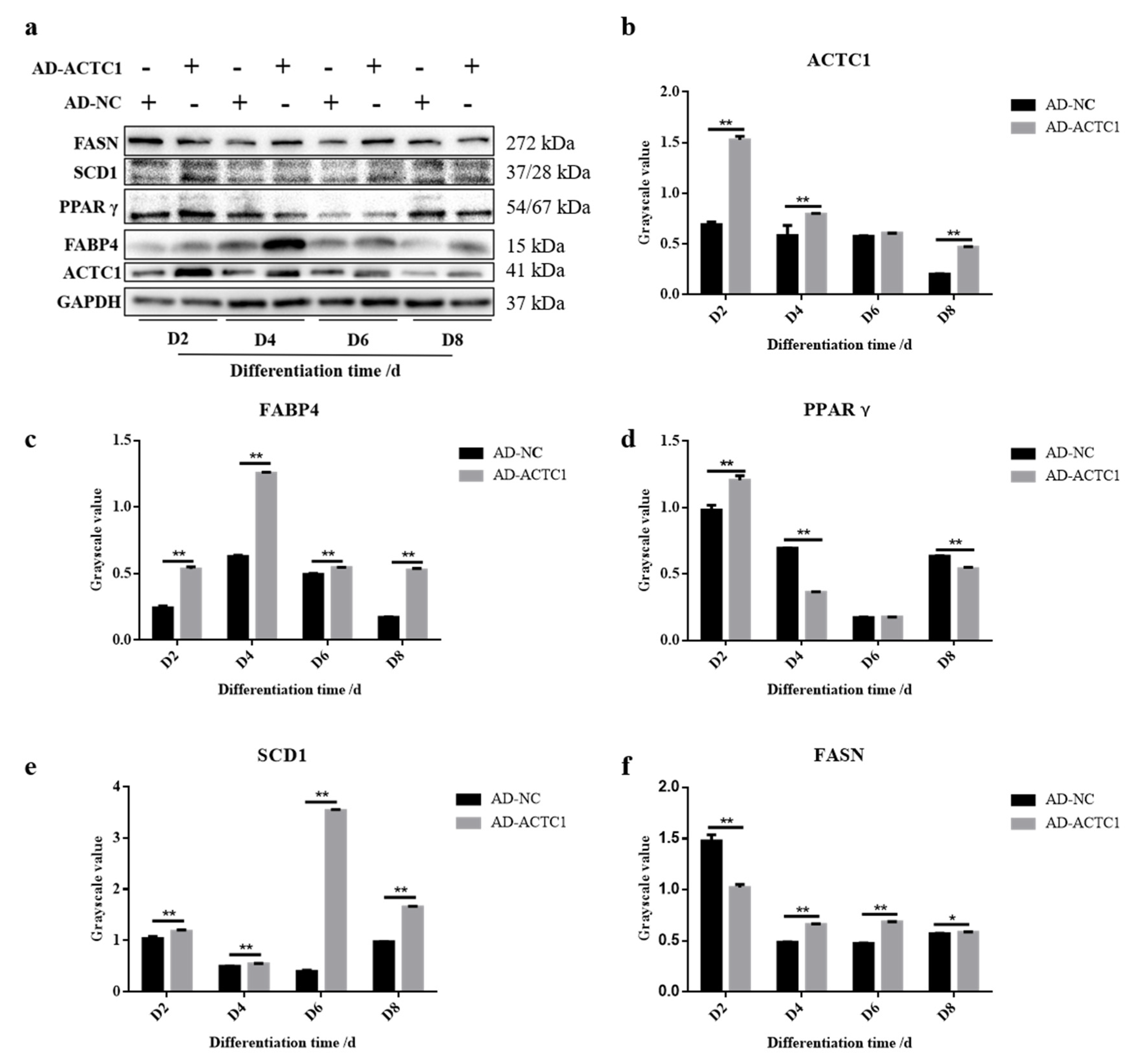
3.2. Effect of ACTC1 on Differentiation of Bovine Myoblasts
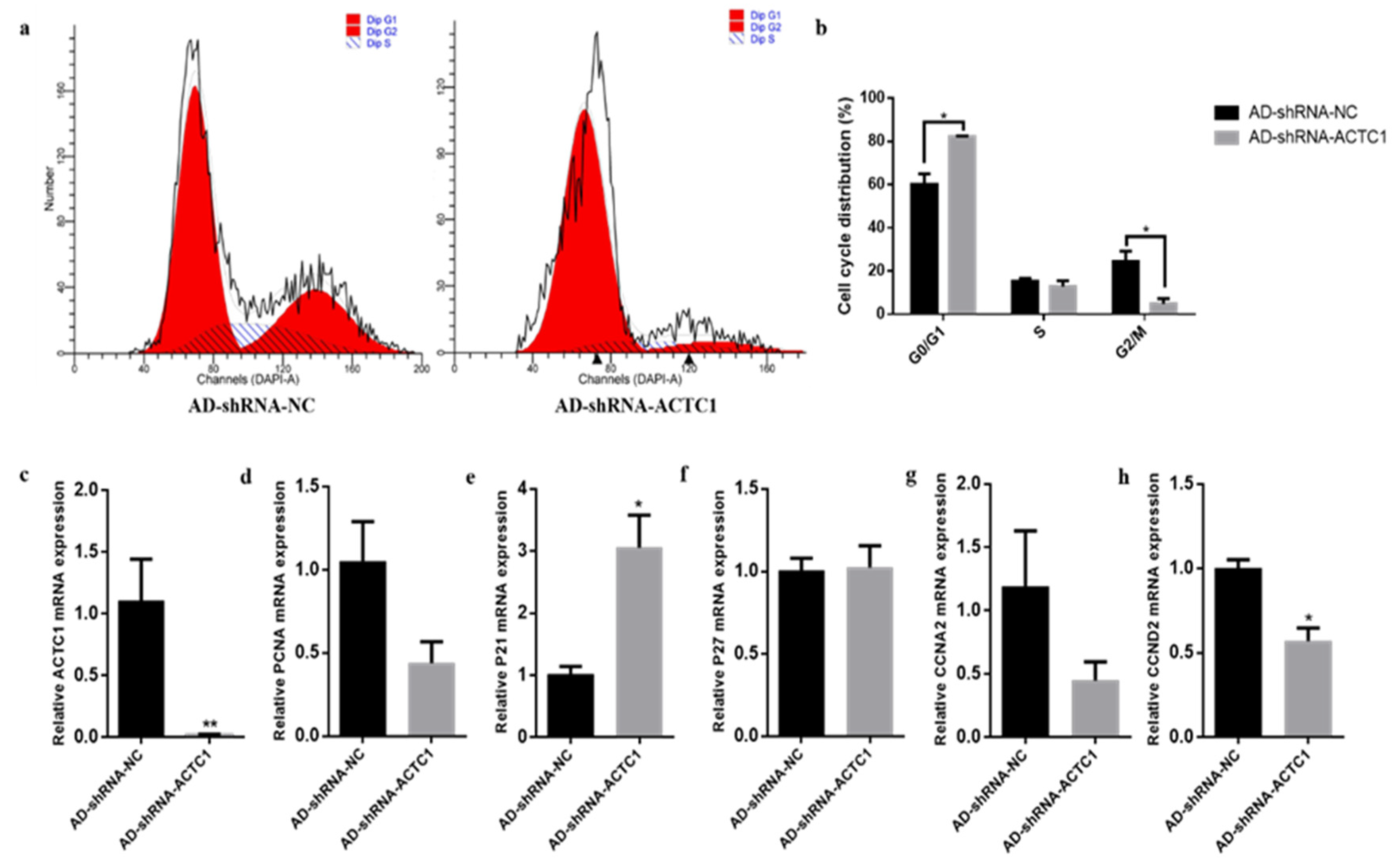
3.3. Effect of ACTC1 on the Proliferation of Bovine Myoblasts
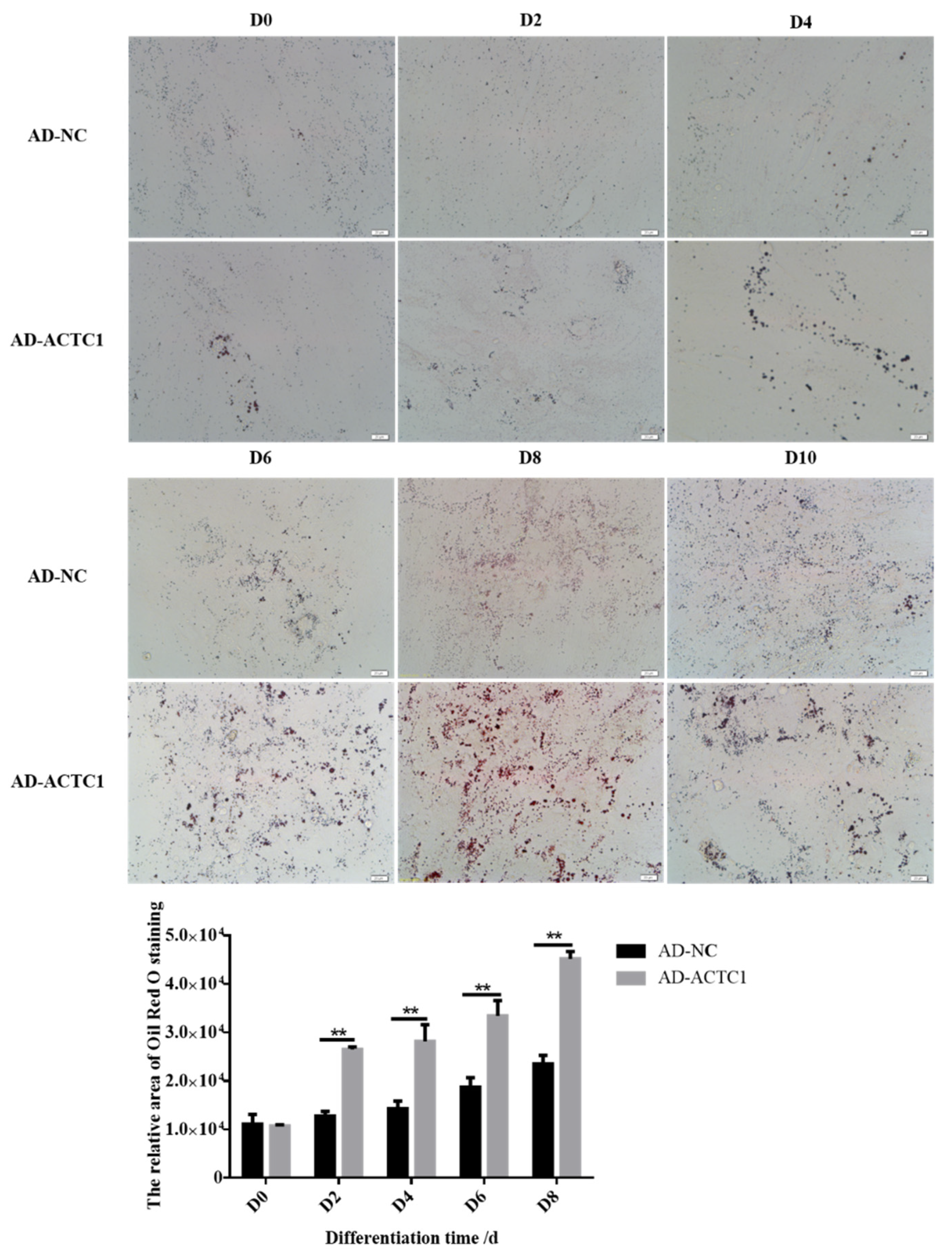
3.4. Impact of ACTC1 on Bovine Preadipocyte Differentiation
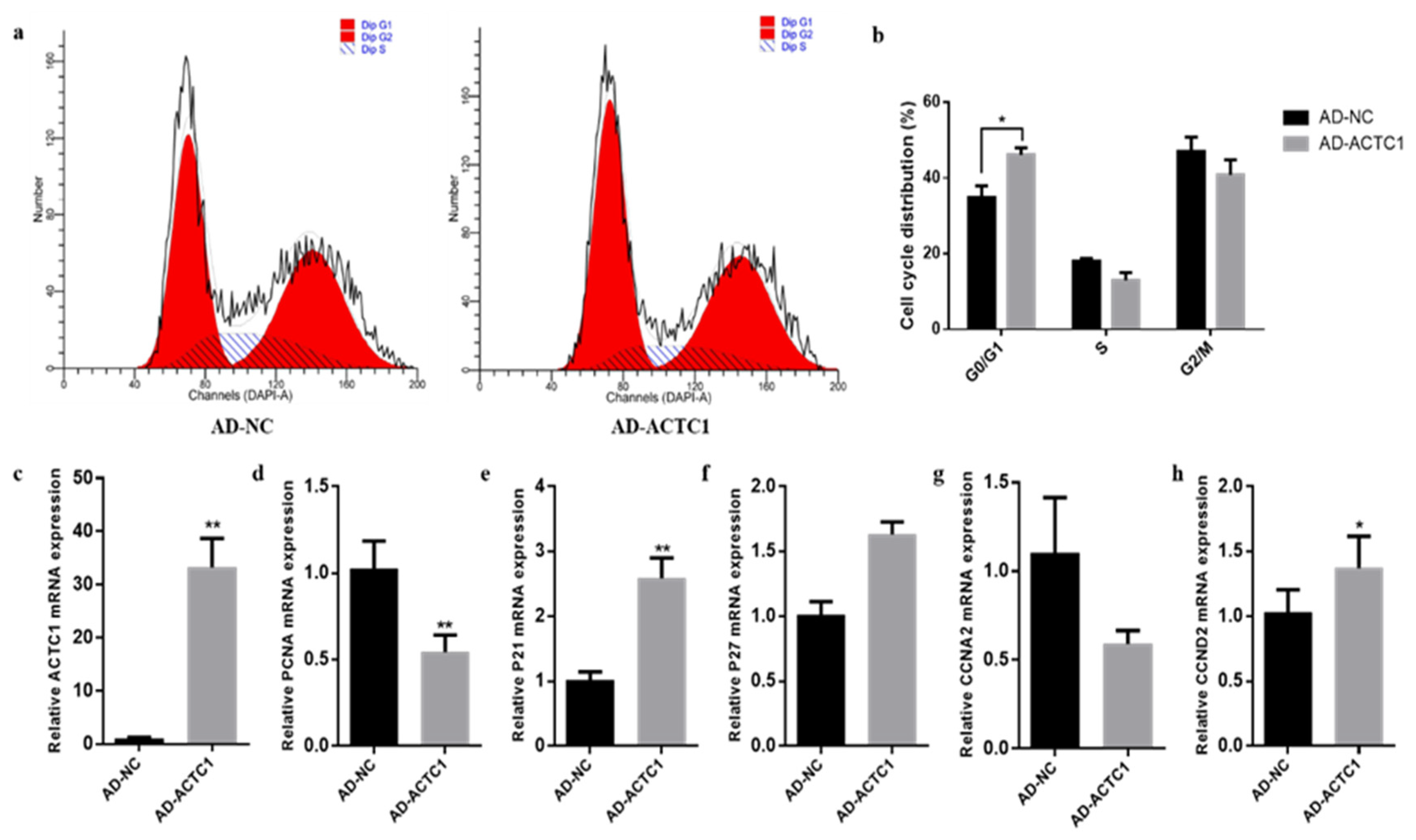
3.5. Effect of ACTC1 on Proliferation of Bovine Preadipocytes
3.6. Model Diagram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poleti, M.D.; Regitano, L.; Souza, G.; Cesar, A.; Simas, R.C.; Silva-Vignato, B.; Oliveira, G.B.; Andrade, S.; Cameron, L.C.; Coutinho, L.L. Longissimus dorsi muscle label-Free quantitative proteomic reveals biological mechanisms associated with intramuscular fat deposition. J. Proteom. 2018, 179, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating meat quality and composition. Proc. Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef]
- McNally, E.; Dellefave, L. Sarcomere mutations in cardiogenesis and ventricular noncompaction. Trends Cardiovasc. Med. 2009, 19, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Matsson, H.; Eason, J.; Bookwalter, C.S.; Klar, J.; Gustavsson, P.; Sunnegårdh, J.; Enell, H.; Jonzon, A.; Vikkula, M.; Gutierrez, I.; et al. Alpha-Cardiac actin mutations produce atrial septal defects. Hum. Mol. Genet. 2008, 17, 256–265. [Google Scholar] [CrossRef]
- Suurmeijer, A.J.; Clément, S.; Francesconi, A.; Bocchi, L.; Angelini, A.; Van Veldhuisen, D.J.; Spagnoli, L.G.; Gabbiani, G.; Orlandi, A. Alpha-actin isoform distribution in normal and failing human heart: A morphological, morphometric, and biochemical study. J. Pathol. 2003, 199, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Buron, F.; Arib, G.; Le Fourn, V.; Regamey, A.; Bodenmann, I.; Girod, P.A.; Mermod, N. Influence of cytoskeleton organization on recombinant protein expression by CHO cells. Biotechnol. Bioeng. 2020, 117, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Augière, C.; Mégy, S.; El Malti, R.; Boland, A.; El Zein, L.; Verrier, B.; Mégarbané, A.; Deleuze, J.F.; Bouvagnet, P. A Novel Alpha Cardiac Actin (ACTC1) Mutation Mapping to a Domain in Close Contact with Myosin Heavy Chain Leads to a Variety of Congenital Heart Defects, Arrhythmia and Possibly Midline Defects. PLoS ONE 2015, 10, e0127903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonne, G.; Carrier, L.; Richard, P.; Hainque, B.; Schwartz, K. Familial hypertrophic cardiomyopathy: From mutations to functional defects. Circ. Res. 1998, 83, 580–593. [Google Scholar] [CrossRef]
- Fokstuen, S.; Lyle, R.; Munoz, A.; Gehrig, C.; Lerch, R.; Perrot, A.; Osterziel, K.J.; Geier, C.; Beghetti, M.; Mach, F.; et al. A DNA resequencing array for pathogenic mutation detection in hypertrophic cardiomyopathy. Hum. Mutat. 2008, 29, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, J.; Klausen, I.C.; Pedersen, A.K.; Egeblad, H.; Bross, P.; Kruse, T.A.; Gregersen, N.; Hansen, P.S.; Baandrup, U.; Borglum, A.D. Alpha-Cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Investig. 1999, 103, R39–R43. [Google Scholar] [CrossRef] [Green Version]
- Marian, A.J.; Roberts, R. Familial hypertrophic cardiomyopathy: A paradigm of the cardiac hypertrophic response to injury. Ann. Med. 1998, 30 (Suppl. 1), 24–32. [Google Scholar] [PubMed]
- Olson, T.M.; Michels, V.V.; Thibodeau, S.N.; Tai, Y.S.; Keating, M.T. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science 1998, 280, 750–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangrez, A.Y.; Kilian, L.; Stiebeling, K.; Dittmann, S.; Yadav, P.; Schulze-Bahr, E.; Frey, N.; Frank, D. Data on the role of cardiac α-actin (ACTC1) gene mutations on SRF-Signaling. Data Brief. 2020, 28, 105071. [Google Scholar] [CrossRef]
- Yang, Q.L.; Bian, Y.Y.; Wang, B.; Zuo, L.; Zhou, M.Y.; Shao, H.; Zhang, Y.M.; Liu, L.W. Novel phenotype-Genotype correlations of hypertrophic cardiomyopathy caused by mutation in α-Actin and myosin-Binding protein genes in three unrelated Chinese families. J. Cardiol. 2019, 73, 438–444. [Google Scholar] [CrossRef]
- Boutilier, J.K.; Taylor, R.L.; Ram, R.; McNamara, E.; Nguyen, Q.; Goullée, H.; Chandler, D.; Mehta, M.; Balmer, L.; Laing, N.G.; et al. Variable cardiac α-Actin (Actc1) expression in early adult skeletal muscle correlates with promoter methylation. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, J.; Bugaisky, G.; Buckingham, M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J. Biol. Chem. 1986, 261, 1838–1843. [Google Scholar] [CrossRef]
- Dennis, R.A.; Przybyla, B.; Gurley, C.; Kortebein, P.M.; Simpson, P.; Sullivan, D.H.; Peterson, C.A. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol. Genom. 2008, 32, 393–400. [Google Scholar] [CrossRef]
- Moll, R.; Holzhausen, H.J.; Mennel, H.D.; Kuhn, C.; Baumann, R.; Taege, C.; Franke, W.W. The cardiac isoform of alpha-Actin in regenerating and atrophic skeletal muscle, myopathies and rhabdomyomatous tumors: An immunohistochemical study using monoclonal antibodies. Virchows Arch. 2006, 449, 175–191. [Google Scholar] [CrossRef]
- Ilkovski, B.; Clement, S.; Sewry, C.; North, K.N.; Cooper, S.T. Defining alpha-Skeletal and alpha-Cardiac actin expression in human heart and skeletal muscle explains the absence of cardiac involvement in ACTA1 nemaline myopathy. Neuromuscul. Disord. 2005, 15, 829–835. [Google Scholar] [CrossRef]
- Moss, J.B.; Olson, E.N.; Schwartz, R.J. The myogenic regulatory factor MRF4 represses the cardiac alpha-Actin promoter through a negative-Acting N-Terminal protein domain. J. Biol. Chem. 1996, 271, 31688–31694. [Google Scholar] [CrossRef] [Green Version]
- Ohtaki, S.; Wanibuchi, M.; Kataoka-Sasaki, Y.; Sasaki, M.; Oka, S.; Noshiro, S.; Akiyama, Y.; Mikami, T.; Mikuni, N.; Kocsis, J.D.; et al. ACTC1 as an invasion and prognosis marker in glioma. J. Neurosurg. 2017, 126, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Song, M.J.; Jung, Y.; Lee, W.S.; Jang, H.H. Proteomic Analysis of Primary Colon Cancer and Synchronous Solitary Liver Metastasis. Cancer Genom. Proteom. 2019, 16, 583–592. [Google Scholar] [CrossRef]
- Mei, C.; Junjvlieke, Z.; Raza, S.; Wang, H.; Cheng, G.; Zhao, C.; Zhu, W.; Zan, L. Copy number variation detection in Chinese indigenous cattle by whole genome sequencing. Genomics 2020, 112, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Keel, B.N.; Zarek, C.M.; Keele, J.W.; Kuehn, L.A.; Snelling, W.M.; Oliver, W.T.; Freetly, H.C.; Lindholm-Perry, A.K. RNA-Seq Meta-Analysis identifies genes in skeletal muscle associated with gain and intake across a multi-Season study of crossbred beef steers. BMC Genom. 2018, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Liu, S.; Campanile, G.; Salzano, A.; Gasparrini, B.; Plastow, G.; Zhang, C.; Wang, Z.; Liang, A.; et al. Genome-Wide association study for buffalo mammary gland morphology. J. Dairy Res. 2020, 87, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Yang, W.C.; Li, P.W.; Wang, H.B.; Zhang, Y.Y.; Zan, L. Myocyte enhancer factor 2A promotes proliferation and its inhibition attenuates myogenic differentiation via myozenin 2 in bovine skeletal muscle myoblast. PLoS ONE 2018, 13, e0196255. [Google Scholar] [CrossRef]
- Meissburger, B.; Perdikari, A.; Moest, H.; Müller, S.; Geiger, M.; Wolfrum, C. Regulation of adipogenesis by paracrine factors from adipose stromal-Vascular fraction—A link to fat depot-Specific differences. Biochim. Biophys. Acta 2016, 1861, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wang, Y.; Zhang, L.; Ning, Y.; Zan, L. The Expression Pattern of PLIN2 in Differentiated Adipocytes from Qinchuan Cattle Analysis of Its Protein Structure and Interaction with CGI-58. Int. J. Mol. Sci. 2018, 19, 1336. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Saha, P.; Kornbluth, S.; Dynlacht, B.D.; Dutta, A. Cyclin-Binding motifs are essential for the function of p21CIP1. Mol. Cell. Biol. 1996, 16, 4673–4682. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Harashima, H.; Dissmeyer, N.; Schnittger, A. Cell cycle control across the eukaryotic kingdom. Trends Cell. Biol. 2013, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.S.; Landers, J.E.; Engle, L.J.; George, D.L. Physical and functional interaction between wild-Type p53 and mdm2 proteins. Mol. Cell. Biol. 1994, 14, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Resnitzky, D.; Hengst, L.; Reed, S.I. Cyclin A-Associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol. Cell. Biol. 1995, 15, 4347–4352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Xiong, Y. Why are there so many CDK inhibitors? Biochim. Biophys. Acta 1996, 1288, O1–O5. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88 (Suppl. 13), E51–E60. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Elabd, C.; Delteil, F.; Astier, J.; Vernochet, C.; Saint-Marc, P.; Guesnet, J.; Guezennec, A.; Amri, E.Z.; Dani, C.; et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 2004, 315, 255–263. [Google Scholar] [CrossRef]
- Bai, Y.; McCoy, J.G.; Levin, E.J.; Sobrado, P.; Rajashankar, K.R.; Fox, B.G.; Zhou, M. X-Ray structure of a mammalian stearoyl-CoA desaturase. Nature 2015, 524, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boord, J.B.; Fazio, S.; Linton, M.F. Cytoplasmic fatty acid-Binding proteins: Emerging roles in metabolism and atherosclerosis. Curr. Opin. Lipidol. 2015, 13, 141–147. [Google Scholar] [CrossRef]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-Activated receptor (PPAR) gamma: Adipose-Predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-Binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug. Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Tsao, J.; Vernet, D.A.; Gelfand, R.; Kovanecz, I.; Nolazco, G.; Bruhn, K.W.; Gonzalez-Cadavid, N.F. Myostatin genetic inactivation inhibits myogenesis by muscle-Derived stem cells in vitro but not when implanted in the mdx mouse muscle. Stem. Cell Res. Ther. 2013, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, B.L.; Olson, E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998, 14, 167–196. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Black, B.L.; Martin, J.F.; Olson, E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 1995, 83, 1125–1136. [Google Scholar] [CrossRef] [Green Version]

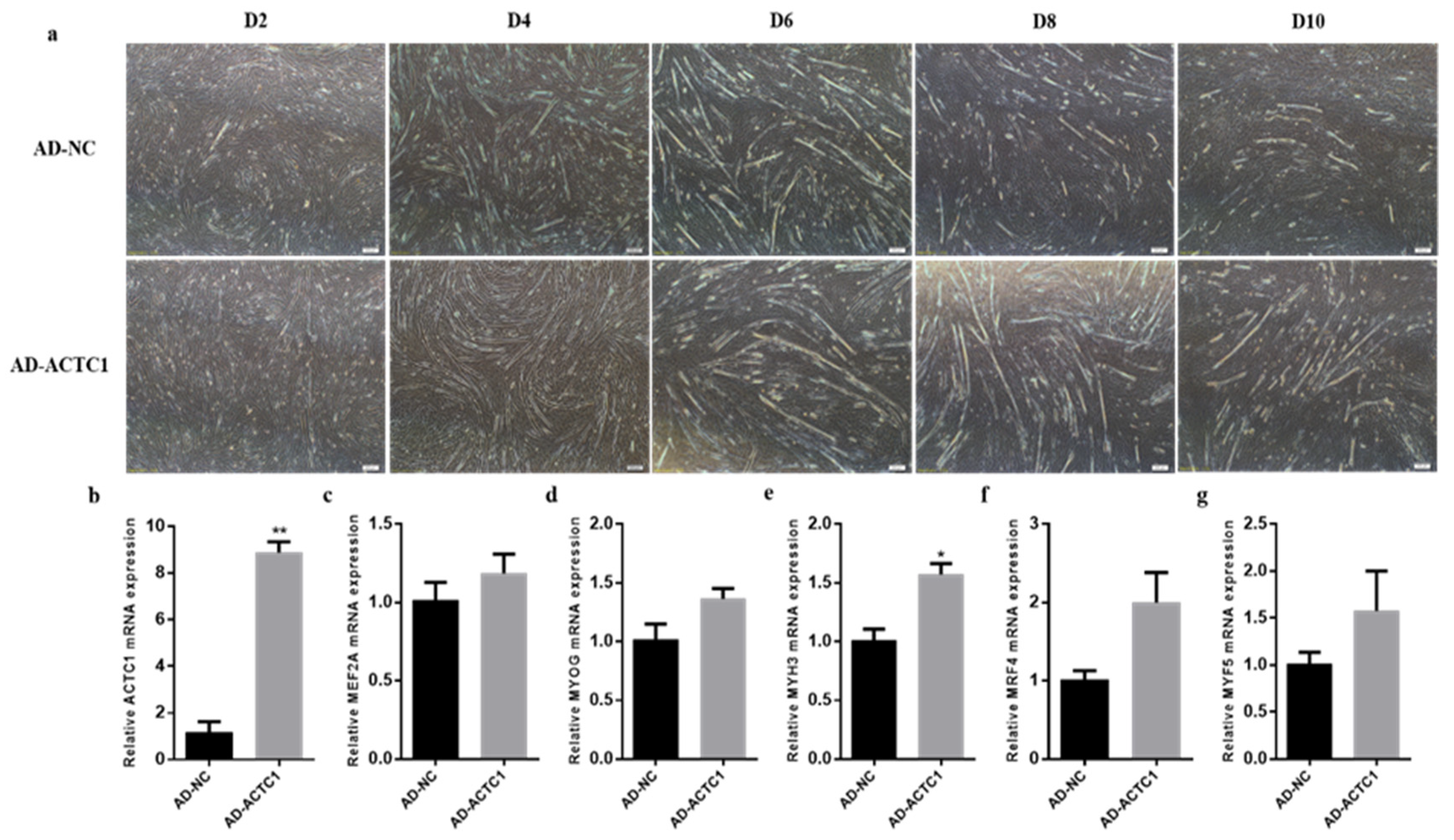


 and
and  respectively represent myoblasts and preadipocytes (all infected with control adenovirus);
respectively represent myoblasts and preadipocytes (all infected with control adenovirus);  represents the myoblasts after knocking down ACTC1;
represents the myoblasts after knocking down ACTC1;  represents the myoblasts after overexpression of ACTC1;
represents the myoblasts after overexpression of ACTC1;  are preadipocytes after ectopic expression ACTC1; “−” means inhibition; and “+” means promotion.
are preadipocytes after ectopic expression ACTC1; “−” means inhibition; and “+” means promotion.
 and
and  respectively represent myoblasts and preadipocytes (all infected with control adenovirus);
respectively represent myoblasts and preadipocytes (all infected with control adenovirus);  represents the myoblasts after knocking down ACTC1;
represents the myoblasts after knocking down ACTC1;  represents the myoblasts after overexpression of ACTC1;
represents the myoblasts after overexpression of ACTC1;  are preadipocytes after ectopic expression ACTC1; “−” means inhibition; and “+” means promotion.
are preadipocytes after ectopic expression ACTC1; “−” means inhibition; and “+” means promotion.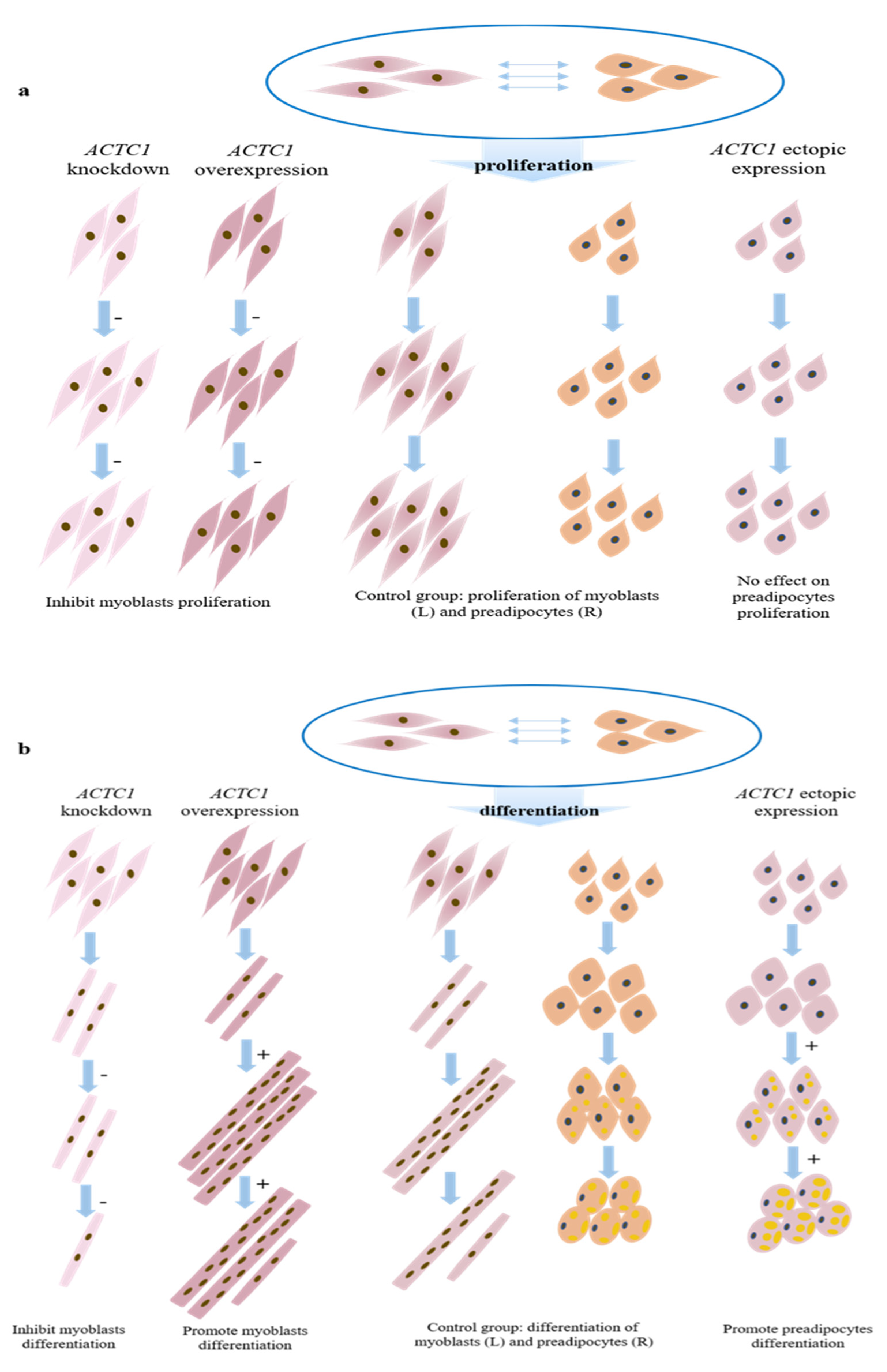
| Gene Name | Accession Numbers | Primer Sequence (5′ > 3′) | Fragments Size (bp) |
|---|---|---|---|
| ACTC1 | NM_001034585.2 | Forward: ATGTGCGACGATGAGGAGAC Reverse: TTAGAAGCACTTGCGGTGGA | 1134 |
| Gene Name | Accession Numbers | Primer Sequence (5′ > 3′) | Primer Length (bp) |
|---|---|---|---|
| GAPDH | NM_001034034 | Forward: AGTTCAACGGCACAGTCAAGG Reverse: ACCACATACTCAGCACCAGCA | 124 |
| ACTC1 | NM_001034585.2 | Forward: CAGTGCTGTCCCTGTATGCT Reverse: AAGCAGCCGTAGCCATTTCA | 282 |
| PPARγ | NM_181024 | Forward: TGAAGAGCCTTCCAACTCCC Reverse: GTCCTCCGGAAGAAACCCTTG | 117 |
| SCD1 | NM_173959 | Forward: TCCGACCTAAGAGCCGAGAA Reverse: TGGGCAGCACTATTCACCAG | 200 |
| FASN | NM_001012669 | Forward: GGCAAACGGAAAAACGGTGA Reverse: CTTGGTATTCCGGGTCCGAG | 183 |
| FABP4 | NM_174314 | Forward: TGAGATTTCCTTCAAATTGGG Reverse: CTTGTACCAGAGCACCTTCATC | 101 |
| MYOD1 | NM_001040478 | Forward: AACCCCAACCCGATTTACC Reverse: CACAACAGTTCCTTCGCCTCT | 196 |
| MYOG | NM_001111325 | Forward: GGCGTGTAAGGTGTGTAAG Reverse: CTTCTTGAGTCTGCGCTTCT | 85 |
| MYH3 | NM_001101835.1 | Forward: AAATGAGGGATGACCGCCTG Reverse: GCACTCTTGAGAAGGGGCTT | 205 |
| MYF5 | NM_174116 | Forward: CCTCTAGTTCCAGGCTCATCTA Reverse: ACCTCCTTCCTCCTGTGTAATA | 90 |
| MRF4 | NM_181811 | Forward: GTGATAACTGCCAAGGAAGGAG Reverse: CGAGGAAATGCTGTCCACGA | 93 |
| MEF2A | NM_001083638 | Forward: AATGAACCTCACGAAAGCAGAAC Reverse: TTAGCACATAGGAAGTATCAGGGTC | 106 |
| CKM | NM_174773 | Forward: GTGGCTGGTGATGAGGAGTC Reverse: TTTCCCCTTGAACTCACCCG | 270 |
| PCNA | NM_001034494.1 | Forward: TGAACCTCACCAGCATGTCCAAAAT Reverse: TTCAAATACTAGTGCCAACGTGTCCG | 104 |
| P21 | NM_001098958.2 | Forward: GACCAGCATGACAGATTTCTACCA Reverse: TGAAGGCCCAAGGCAAAAG | 144 |
| P27 | NM_001271625.3 | Forward: AGATGTCAAACGTGCGAGTG Reverse: GCCAAAGAGGTTTCTGCAAG | 104 |
| CCND2 | NM_001076372.1 | Forward: GGGCAAGTTGAAATGGAA Reverse: TCATCGACGGCGGGTAC | 173 |
| CCNA2 | NM_001075123.1 | Forward: GCCACTGGCACCTCTTGATTA Reverse: TCCACGAGGATAGCCCTCATA | 231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Su, X.; Tian, Y.; Song, G.; Zan, L.; Wang, H. Effect of Actin Alpha Cardiac Muscle 1 on the Proliferation and Differentiation of Bovine Myoblasts and Preadipocytes. Animals 2021, 11, 3468. https://doi.org/10.3390/ani11123468
Li A, Su X, Tian Y, Song G, Zan L, Wang H. Effect of Actin Alpha Cardiac Muscle 1 on the Proliferation and Differentiation of Bovine Myoblasts and Preadipocytes. Animals. 2021; 11(12):3468. https://doi.org/10.3390/ani11123468
Chicago/Turabian StyleLi, Anqi, Xiaotong Su, Yuan Tian, Guibing Song, Linsen Zan, and Hongbao Wang. 2021. "Effect of Actin Alpha Cardiac Muscle 1 on the Proliferation and Differentiation of Bovine Myoblasts and Preadipocytes" Animals 11, no. 12: 3468. https://doi.org/10.3390/ani11123468
APA StyleLi, A., Su, X., Tian, Y., Song, G., Zan, L., & Wang, H. (2021). Effect of Actin Alpha Cardiac Muscle 1 on the Proliferation and Differentiation of Bovine Myoblasts and Preadipocytes. Animals, 11(12), 3468. https://doi.org/10.3390/ani11123468






