1. Introduction
As an essential trace element, manganese (Mn) is a component or activator of many essential enzymes, such as arginase, pyruvate carboxylase, hydrolases, etc., which are involved in carbohydrate, lipid, and protein metabolism and many crucial biochemical reactions [
1,
2]. It is also an essential cofactor of chondroitin sulfate synthesis that is closely related to the bone formation of broilers [
3]. Otherwise, Mn plays a vital role in antioxidant and immune systems of animals [
4,
5,
6]. As recommended by the National Research Council (NRC), the content of Mn in the diet for broilers should be at least 60 mg/kg [
7]. However, the content of Mn is just around 30 mg/kg in the diet mainly consisting of corn and soybean meal and its utilization rate of Mn is very low [
8]. In order to meet the requirement of Mn in broiler production, the additives of Mn sources are usually added into the diet for broilers.
At present, additives of Mn source which is usually used in broiler diets include inorganic Mn (such as Mn sulfate, Mn carbonate, and Mn oxide) and organic Mn (such as amino acid-chelated Mn and protein Mn). For traditional inorganic sources of Mn, they are easy to deliquescence and the utilization rate is relatively low, although they are cheaper [
9,
10]. Organic sources of Mn have shown excellent chemical stability and high absorption efficiency, but they have not been widely applied in production because of their uneven product quality, inconsistent effect, and high cost [
9,
11,
12]. Thus, it is very important to develop new sources of Mn with better efficacy and relatively low cost.
Manganese hydroxychloride (MHC), also known as basic Mn chloride or tribasic Mn chloride, is a category of hydroxy trace minerals. The solubility of MHC in water is minimal, but it becomes more soluble under acidic conditions in intestine [
13]. MHC contains strong covalent bonds that are similar to that of organic minerals and have a special crystalline structure, which may be beneficial for the stability in the diet and better absorption in the intestine. MHC has been approved as a feed additive by the European Food Safety Authority (EFSA) [
14]. Previous studies showed that dietary supplementation with MHC could increase egg yolk and shell Mn levels of laying hens, and improve feed conversion of broilers [
15,
16]. A study conducted with pigs also showed that dietary inclusion of MHC improved the growth rate and feed intake compared with that of MnSO
4 [
17]. However, there is limited literature about the suitable addition level of MHC for broilers, and the relative effect compared with typical inorganic Mn has not been reported. Therefore, the main aim of this study was to explore the suitable level of Mn as MHC in the broiler diet by investigating the effects of dietary MHC supplementation on growth performance, slaughter traits, antioxidant capacity, tibial quality, and tissue Mn level of broilers.
2. Materials and Methods
2.1. Experimental Material
Manganese hydroxychloride (MHC) was supplied by Changsha Xingjia Bio-Engineering Co., Ltd. (Changsha, China). The molecular formula of MHC is Mn2(OH)3Cl and the analyzed content of Mn was 55.64%. The feed grade MnSO4 was purchased from Beijing Tongli Xingke Agricultural Technology Co., Ltd. (Beijing, China), the analyzed Mn concentration was 31.94%.
2.2. Animals, Experimental Design and Management
This study was performed on the Fengning Research Unit of China Agricultural University (Chengde, Hebei, China). A total of 756 one-day-old Arbor Acres male broiler chicks with an average body weight of 44.28 ± 1.74 g were obtained from Arbor Acres Poultry Breeding Company (Beijing, China). All birds were weighed and randomly assigned to 7 treatments with 6 replicates and 18 chicks in each replicate. The diets consisted of a corn and soybean diet with 0 (control group), 20, 40, 60, 80, or 100 mg /kg Mn as MHC, and 100 mg/kg Mn as MnSO
4 (positive control), respectively. The recommended Mn content for broilers is 120 mg/kg during 1–3 weeks and 100 mg/kg during 4–6 weeks by the Feeding Standard of Chicken in China. Additionally, we searched recent related articles on broiler experiments in
Animals and also found that the amount of added Mn in the diet was around 100 mg/kg [
18,
19,
20,
21,
22]. So we added 100 mg/kg Mn in the form of MnSO
4 as the positive control. This experiment lasted for 42 d, and all broilers were fed with starter diets from day 0 to day 21, and then fed with grower diets until the end of the trial. All diets were fed in mash form. Except for Mn, the corn-soybean meal basal diet was formulated according to the recommendations of the nutritional requirements of broilers (NRC, 1994) [
7]. The ingredients compositions and nutritional levels of basal diets are shown in
Table 1.
The experiment was performed on the Fengning Research Base of China Agricultural University (Chengde, Hebei, China). All broilers were raised in 3-layer cages (0.093 m2 per bird) with six birds per cage in an environmentally controlled room. Feed and water were offered ad libitum throughout the experiment. The lighting program was 23 h light: 1 h dark per day. The room temperature was maintained at 35 °C for the first 3 day and gradually reduced by 3 °C each week until it reached to final temperature of 24 °C. All chicks were inoculated with Newcastle disease vaccine on day 7 and day 21, and infectious bursal disease vaccine on day 14 and day 28.
2.3. Growth Performance
Body weight and feed intake per replicate were recorded on days 21 and 42 of the experiment. Average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F:G) were calculated from days 0 to 21, 22 to 42, and 0 to 42.
2.4. Sample Collection
On day 21 and 42 after fasting for 12 h, 6 broilers approximating the average weight from each treatment (one bird per replicate) were selected to collect blood samples from the wing vein of broilers. Blood samples were allowed to stand for 30 min at room temperature, followed by centrifugation at 3600× g for 15 min. Then, serum samples were collected and stored at −20 °C for further analysis. The selected broilers were euthanized by jugular vein bleeding after stunning using 60% concentration of CO2 gas. The tibia, liver, heart, kidney, and breast muscle samples were collected and stored at −20 °C for subsequent analysis. In addition, live weight, carcass weight, eviscerated weight, breast weight, thigh muscle weight, and abdominal fat weight of broilers were measured on day 42. Dressing percentage and eviscerated percentage were expressed as a percentage of its initial live weight, while breast meat percentage, leg meat percentage, and abdominal fat percentage were expressed as a percentage of the eviscerated weight.
2.5. Sample Analysis
2.5.1. Nutrients Level of Diets
Diets were analyzed according to the methods of the Association of Official Analytical Chemists (AOAC, 2000) for total phosphorus (method 995.11), calcium (method 927.02), crude protein (method 988.05), and amino acids (method 994.12). For dietary methionine determination, performic acid oxidation was performed prior to acid hydrolysis with 6 M HCl. The Mn concentration of MHC, MnSO4, and diets was determined by Z-2000 flame atomic absorption spectrometry (Hitachi, Tokyo, Japan).
2.5.2. Antioxidant Capacity
Total antioxidant capacity (T-AOC), Mn containing superoxide dismutase (MnSOD) and catalase (CAT) activities, and malondialdehyde (MDA) contents in serum and liver of broilers were measured with commercial kits (Sino-UK Institute of Biological Technology, Beijing, China) according to the manufacturer’s protocol.
2.5.3. Tibia Indicator
After taking the left tibia, muscles, cartilage, and membranes of it were removed. The length and diameter of tibia were measured using a vernier caliper and then weighed. The density index of the tibia was measured using a dual-energy X-ray absorptiometry bone densitometer (Hologic, Bedford, MA, USA), and the breaking strength was measured using a TA.XT plus texture analyser (Stable Microsystems, Surrey, UK).
2.5.4. Manganese Contents
The contents of Mn in heart, liver, kidney, tibia, and breast muscles samples of broilers on day 42 were determined by inductively coupled plasma mass spectrometry (Agilent 7500, Agilent Technologies, Tokyo, Japan) after microwave digestions with nitric acid.
2.6. Statistical Analysis
All data were subjected to one-way ANOVA using the general linear model (GLM) procedure of SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Differences among treatments were further compared using Duncan’s multiple range test. Orthogonal polynomial contrasts were used to analyze the linear and quadratic responses to MHC levels. Also, a quadratic regression fitting curve model [y = ax2 + bx + c, the best addition level x = −b/(2a)] was performed using GraphPad Prism 7 (GraphPad Software Inc.; San Diego, CA, USA) to evaluate the optimal MHC addition levels. A p-value of less than 0.05 was considered to be statistically significant.
4. Discussion
The reports related to the effects of Mn on growth performance of broilers were inconsistent. Many studies have shown that dietary supplementation with different chemical forms of Mn such as Mn propionate [
12], Mn proteinates [
23], Mn oxide [
24], Mn sulfate [
25], Mn fumarate [
26], or Mn amino acid chelate [
27] did not significantly affect ADG, ADFI, and F:G of broilers. However, Meng et al. [
28] found dietary inclusion of 50 mg/kg Mn as Mn methionine hydroxyl analog chelated could improve ADG and ADFI of broilers. Otherwise, Ognik et al. [
29] reported that diet supplemented with 50 or 100 mg/kg Mn in the form of Mn oxide nanoparticles decreased F:G of turkeys. At present, there are a few studies on the effect of MHC on broiler, and the results are inconsistent. Conly et al. [
30] showed that diet (45 mg/kg Mn) supplemented with 30–130 mg/kg Mn in the form of MHC had no significant effect on feed intake, body weight, and F:G of Cobb 500 broilers. The present study also showed that dietary (37 mg/kg Mn) supplementation of 20–100 mg/kg Mn as MHC had no significant effect on the ADG, ADFI, and F:G of AA broilers. However, Jasek et al. [
16] reported dietary (40 mg/kg Mn) inclusion of 40–160 mg/kg Mn as MHC decreased F:G of Ross 708 broilers. These disparities in results among studies may be due to the difference in experiment broiler breed, Mn content in basal diet, source and addition level of Mn.
Carcass characteristics are important parameters for evaluating the meat production performance of broilers. Studies have shown that dietary supplementation of 100 mg/kg Mn as MnO and Mn
2O
3 nanoparticles improved the carcass yield of turkeys [
31], and supplementation of 100 mg/kg Mn in the form of MnSO
4 or amino acid chelated Mn reduced the abdominal fat rate of broilers [
25,
32,
33]. The present study showed that dietary inclusion of MHC did not significantly affect slaughter characteristics of broilers, and there were no differences with MnSO
4 were detected. Matuszewski et al. [
34] also reported that dietary supplementation with Mn
2O
3 and Mn
2O
3 nanoparticles (21–70 mg/kg) did not significantly affect any slaughter characteristics of broilers. Further experiments need to be conducted with several Mn sources and levels under the same condition, especially in large-scale commercial farm conditions to confirm the results.
Parameter CAT, T-AOC, and MDA are usually used to evaluate the antioxidant ability of animals. As a component of MnSOD, Mn can improve broiler’s antioxidant ability by catalyzing the reduction of superoxide anion to hydrogen peroxide [
35]. An in vitro study indicated that MnSOD activity and mRNA expression level in chick embryonic myocardial cells were improved by 1.0 mmol/L of Mn as MnCl
2 treatment [
36]. Studies also have shown that dietary inclusion of Mn as MnSO
4, Mn methionine, or Mn oxide improved MnSOD, CAT, and GSH-Px activities and T-AOC, while reducing MDA level in serum, liver, and leg muscle of broilers [
25,
37]. In this study, dietary inclusion of MHC increased MnSOD and CAT activities and T-AOC, while decreasing MDA content in serum and liver of broilers. Therefore, dietary supplementation with MHC can improve the antioxidant capacity and reduce oxidative damage of broilers by improving antioxidative enzyme activities and reducing peroxidation products content. The increase of MnSOD activity may be due to that Mn activating protein kinase C and protein tyrosine kinase [
35], altering specificity protein 1 and activating protein-2 DNA-binding activities, and enhancing MnSOD binding protein RNA-binding activity at the translational level [
38]. The increase of other antioxidant enzymes may be related to the activation of Nrf2 signaling pathway by Mn treatment [
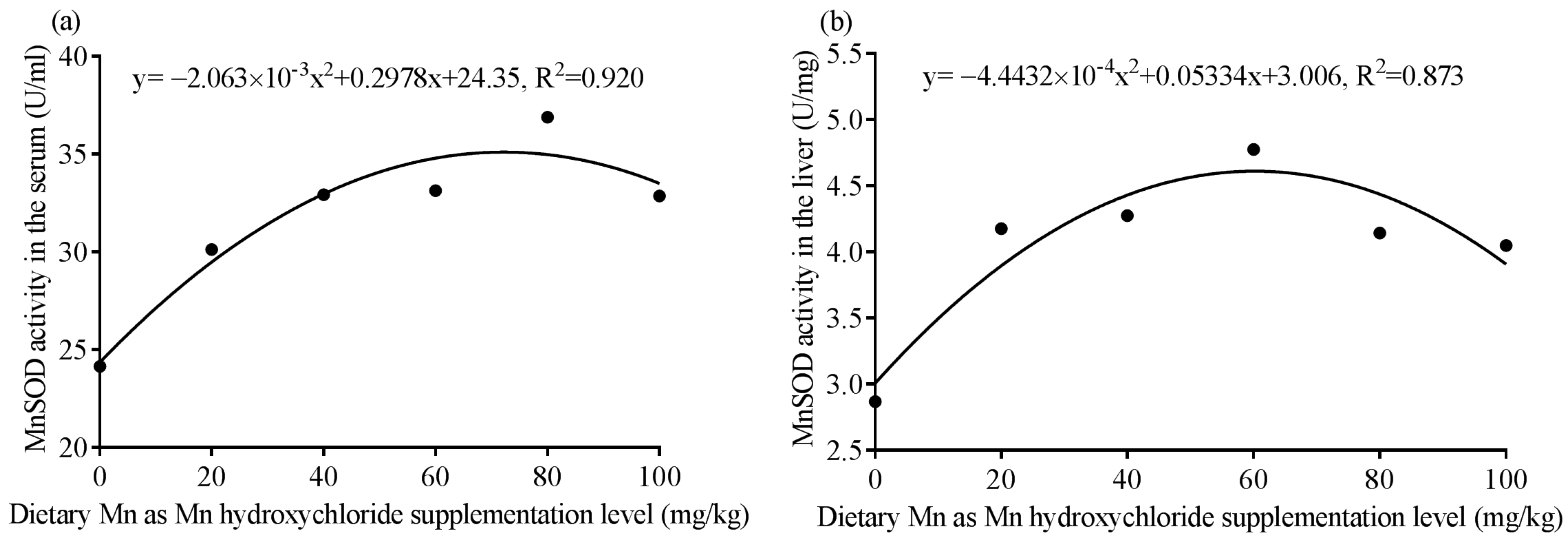
39]. Additionally, due to the MnSOD activities in serum and liver showing significant quadratic response to dietary MHC addition level, it can be also concluded that the optimal dietary supplementation level of Mn in the form of MHC is 50–90 mg/kg according to the quadratic regression curve of MnSOD activities in the serum and liver. The broken-line models are not shown here because the data better fitted the quadratic model (R
2 = 0.920 and 0.873 for the serum and liver, respectively) than a broken line (R
2 = 0.820 and 0.844 for the serum and liver, respectively).
Studies showed that Mn deficiency experimental model of broilers was successfully established at the dose of 40 mg/kg which can affect the normal development of tibia by inhibiting the vitality of osteoblasts and chondrocyte proliferation and promoting chondrocyte apoptosis in the tibia [
40,
41], disordering the level of bone regulatory hormones and enzymes of bone metabolism in the serum [
42], and leading to metaphyseal osteoporosis [
40]. The length, weight, diameter, breaking strength, or density index of tibia are usual parameters to be used for evaluating development of tibia. Studies reported that dietary supplementation of Mn as MnSO
4, MnCO
3, and MnO could increase the length, weight, diameter, breaking strength, and density index of tibia in broilers [
42,
43,
44], reduce the incidence of leg abnormalities [
25,
32]. However, Bozkurt et al. [
37] reported that dietary supplementation of Mn as Mn-methionine and MnO with levels 12.5, 25, and 50 mg/kg has no effect on the weight, length, diameter, and density index of tibia in broilers. It is assumed that the different bioavailability of different chemical forms in Mn sources may be the reason which resulted in these inconsistent results. In this study, it is shown that dietary supplementation of Mn as MHC increased length and density index of tibia in the early growth stage of broilers, which may be due to that broilers had low feed intake, and rapid bone growth and development, especially during the first two week of post-hatch age when the bone is not completely formed [
45]; whereas, broilers can obtain sufficient Mn for bone growth due to the increase of feed intake at the late growth stage.
The source and addition level of Mn in diet may directly affect the Mn content of broiler tissue. Dietary supplementation of Mn in the form of MnSO
4, MnO, or Mn fumarate could improve Mn levels in tibia, liver, and kidney of broilers [
26,
46]. In the present study, dietary supplementation with MHC improved Mn levels in the heart, liver, kidney, and tibia, which was agreed with the study conducted by Conly et al. [
30]. However, it is also found that dietary Mn level has no significant effect on the Mn content in serum and breast muscle. European Food Safety Authority (2016) also reported that dietary MHC or MnSO
4 increased Mn levels in the liver and tibia but did not significantly affect Mn level in breast muscle [
14]. This may be due to the weak ability to deposit Mn in breast muscle, where mitochondria are not abundant. And most of Mn in serum was transferred to other organizations.
According to the results of the present study, it seems that the efficacy of MHC is a little higher than that of MnSO
4 on the basis of some measured indicators including antioxidant capacity, tibial parameters, and Mn contents in liver and tibia. So it is assumed that MHC maybe have higher relative bioavailability than that of MnSO
4. MHC was combined by covalent bonds between Mn, hydroxy groups as well as chloride ions, creating a stronger chemical bond than traditional sulfate minerals [
14]. The covalent bonds possessed by hydroxychloride minerals can also reduce the reaction of minerals with other components in feeds [
47], so its bioavailability could be potentially improved. However, the accurate relative biological availability of Mn as MHC to Mn sulfate has not been reported, which needs to be further studied.