Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval, Animals and Experimental Design
2.2. Determination of the H/L Ratio
2.3. Salmonella Infection and Sampling
2.4. Ileum and Caecum Goblet Cells Count, and Ileal Villi Morphological Analysis
2.5. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (RT-QPCR)
2.6. Statistical Analysis
3. Results
3.1. Salmonella enteritidis Infection Decreases the Number of Goblet Cells
3.2. Association of H/L Ratio with the Number of Goblet Cells in the Ileum and Caecum
3.3. Effects of Salmonella enteritidis Infection on the Ileal Villi Integrity
3.4. Association between H/L Ratio and Important Ileal Villi Morphometry Parameters
3.5. Effects of Salmonella Infection on the Expression of Immune Response-Related Genes
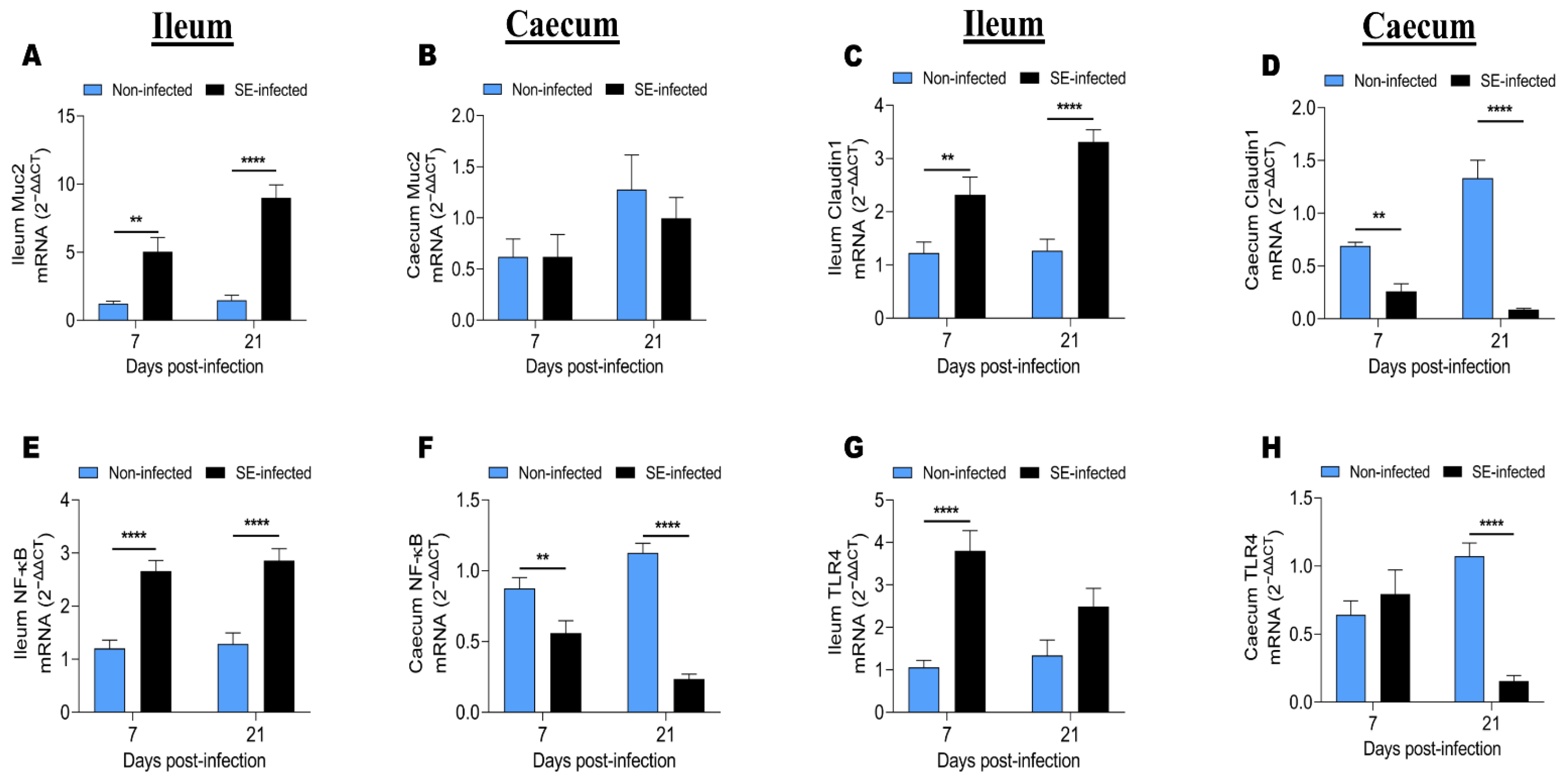
3.6. Effects of Salmonella Infection on the Expression of Genes Related to Intestinal Barrier Functions
3.7. Correlation between H/L Ratio and Intestinal Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Mon, K.K.Z.; Zhu, Y.; Chanthavixay, G.; Kern, C.; Zhou, H. Integrative analysis of gut microbiome and metabolites revealed novel mechanisms of intestinal Salmonella carriage in chicken. Sci. Rep. 2020, 10, 4809. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.; Kaiser, P.; Barrow, P.; Jones, M.A.; Johnston, C.; Wigley, P. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 2009, 128, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Davies, R.H. A critical review of Salmonella Typhimurium infection in laying hens. Avian Pathol. 2011, 40, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, P.L.; Kuo, T.; Javanbakht, M.; Shafir, S.; Wang, M.; Sorvillo, F. Salmonellosis Hospitalizations in the United States: Associated Chronic Conditions, Costs, and Hospital Outcomes, 2011, Trends 2000–2011. Foodborne Pathog. Dis. 2016, 13, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Aschenbach, J.R.; Khayal, B.; Hess, C.; Hess, M. Intestinal epithelial responses to Salmonella enterica serovar Enteritidis: Effects on intestinal permeability and ion transport. Poult. Sci. 2012, 91, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Q.; Everaert, N.; Liu, R.; Zheng, M.; Zhao, G.; Wen, J. Effects of inulin supplementation on intestinal barrier function and immunity in specific pathogen-free chickens with Salmonella infection. J. Anim. Sci. 2020, 98, skz396. [Google Scholar] [CrossRef] [PubMed]
- Minias, P.; Włodarczyk, R.; Meissner, W.; Husak, J. Leukocyte profiles are associated with longevity and survival, but not migratory effort: A comparative analysis of shorebirds. Funct. Ecol. 2017, 32, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Tao, W.; Zhu, S. T lymphocytes in the intestinal mucosa: Defense and tolerance. Cell. Mol. Immunol. 2019, 16, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Lentfer, T.L.; Pendl, H.; Gebhardt-Henrich, S.G.; Frohlich, E.K.; Von Borell, E. H/L ratio as a measurement of stress in laying hens—Methodology and reliability. Br. Poult. Sci. 2015, 56, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Al-Murrani, W.K.; Kassab, A.; Al-Sam, H.Z.; Al-Athari, A.M. Heterophil/lymphocyte ratio as a selection criterion for heat resistance in domestic fowls. Br. Poult. Sci. 1997, 38, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Al-Murrani, W.K.; Al-Rawi, A.J.; Al-Hadithi, M.F.; Al-Tikriti, B. Association between heterophil/lymphocyte ratio, a marker of ’resistance’ to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 2006, 47, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.L.; Cloft, S.E.; Wong, E.A. Changes with age in density of goblet cells in the small intestine of broiler chicks. Poult. Sci. 2020, 99, 2342–2348. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal. Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, S.; Szegi, K.; Winkler, Z.; Barna, T.; Kovacs, K.J. Oligomannan Prebiotic Attenuates Immunological, Clinical and Behavioral Symptoms in Mouse Model of Inflammatory Bowel Disease. Sci. Rep. 2016, 6, 34132. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017, 96, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Meyer, D.; Pullens, G.; Faas, M.; Smelt, M.; Venema, K.; Ramasamy, U.; Schols, H.A.; De Vos, P. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 2015, 55, 414–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, K. Probiotics and prebiotics in the management of irritable bowel syndrome: A review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Al-Murrani, W.K.; Al-Rawi, I.K.; Raof, N.M. Genetic resistance to Salmonella typhimurium in two lines of chickens selected as resistant and sensitive on the basis of heterophil/lymphocyte ratio. Br. Poult. Sci. 2002, 43, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Fidan, E.D.; Nazlıgül, A.; Türkyılmaz, M.K.; Aypak, S.Ü.; Kilimci, F.S.; Karaarslan, S.; Kaya, M. Effect of photoperiod length and light intensity on some welfare criteria, carcass, and meat quality characteristics in broilers. Rev. Bras. Zootec. 2017, 46, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Li, Q.; Li, P.; Liu, R.; Cui, H.; Zheng, M.; Everaert, N.; Zhao, G.; Wen, J. The effects of inulin on the mucosal morphology and immune status of specific pathogen-free chickens. Poult. Sci. 2018, 97, 3938–3946. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Wang, Q.; Zhang, N.; Zheng, J.; Zheng, M.; Liu, R.; Cui, H.; Wen, J.; Zhao, G. SPOP promotes ubiquitination and degradation of MyD88 to suppress the innate immune response. PLoS Pathog. 2020, 16, e1008188. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Xing, Z.; Li, J.; Cardona, C.J. Immune-related gene expression in response to H11N9 low pathogenic avian influenza virus infection in chicken and Pekin duck peripheral blood mononuclear cells. Mol. Immunol. 2009, 46, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ayansola, H.; Hou, Q.; Liao, C.; Lei, J.; Lai, Y.; Jiang, Q.; Masatoshi, H.; Zhang, B. Genistein Inhibits Colonic Goblet Cell Loss and Colorectal Inflammation Induced by Salmonella Typhimurium Infection. Mol. Nutr. Food Res. 2021, 65, e2100209. [Google Scholar] [CrossRef]
- Schultz, B.M.; Salazar, G.A.; Paduro, C.A.; Pardo-Roa, C.; Pizarro, D.P.; Salazar-Echegarai, F.J.; Torres, J.; Riedel, C.A.; Kalergis, A.M.; Alvarez-Lobos, M.M.; et al. Persistent Salmonella enterica serovar Typhimurium Infection Increases the Susceptibility of Mice to Develop Intestinal Inflammation. Front. Immunol. 2018, 9, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasina, Y.O.; Hoerr, F.J.; McKee, S.R.; Conner, D.E. Influence of Salmonella enterica serovar Typhimurium infection on intestinal goblet cells and villous morphology in broiler chicks. Avian Dis. 2010, 54, 841–847. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Hansson, G.C.; Johansson, M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010, 1, 51–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, A.N.; Rigby, N.M.; Garcia de la Torre, A.; Macierzanka, A.; Mills, E.N.; Mackie, A.R. Lamellar structures of MUC2-rich mucin: A potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules 2012, 13, 3253–3261. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e688. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ruan, T.; Ji, X.; Ran, D.; Sun, J.; Shi, H.; Prinz, R.A.; Sun, J.; Pan, Z.; Jiao, X.; et al. The Gli1-Snail axis contributes to Salmonella Typhimurium-induced disruption of intercellular junctions of intestinal epithelial cells. Cell. Microbiol. 2020, 22, e13211. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ye, L.; Lu, X.; Xie, S.; Yang, Q.; Yu, Q. Lactobacillus acidophilus Alleviated Salmonella-Induced Goblet Cells Loss and Colitis by Notch Pathway. Mol. Nutr. Food Res. 2018, 62, 1800552. [Google Scholar] [CrossRef]
- Gong, J.; Yu, H.; Liu, T.; Gill, J.J.; Chambers, J.R.; Wheatcroft, R.; Sabour, P.M. Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. J. Appl. Microbiol. 2008, 104, 1372–1382. [Google Scholar] [CrossRef]
- Revolledo, L.; Ferreira, A.J.P.; Mead, G.C. Prospects in Salmonella Control: Competitive Exclusion, Probiotics, and Enhancement of Avian Intestinal Immunity. J. Appl. Poult. Res. 2006, 15, 341–351. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Zhou, D. Organoid and Enteroid Modeling of Salmonella Infection. Front. Cell. Infect. Microbiol. 2018, 8, 102. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Hussein, E.O.S.; Ali, M.H.; Al-Ghadi, M.Q. The effect of some natural alternative to antibiotics on growth and changes in intestinal histology in broiler exposed to Salmonella challenge. Poult. Sci. 2019, 98, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Zhang, H.; Xie, Y.; Xiong, L.; Liu, H.; Wang, F. Effects of a probiotic on the growth performance, intestinal flora, and immune function of chicks infected with Salmonella pullorum. Poult. Sci. 2020, 99, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.C.; Bounous, D.I.; Lee, M.D. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 1999, 67, 3580–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, M.H. Characterization of the pattern of inflammatory cell influx in chicks following the intraperitoneal administration of lie Salmonella enteritidis and Salmonella enteritidis-immune lymphokines. Poult. Sci. 1995, 74, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. IFN-gamma priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. J. Interferon. Cytokine Res. 2005, 25, 73–81. [Google Scholar] [CrossRef]
- Kogut, M.H.; Tellez, G.I.; McGruder, E.D.; Hargis, B.M.; Williams, J.D.; Corrier, D.E.; DeLoach, J.R. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 1994, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Bohm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Urwat, U.; Ahmad, S.M.; Ahmad, R.; Kashoo, Z.A.; Dar, T.A.; Bhat, S.A.; Mumtaz, P.T.; Shabir, N.; Shah, R.A.; et al. Gene expression and antibody response in chicken against Salmonella Typhimurium challenge. Poult. Sci. 2019, 98, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Kaiser, P.; StÄHeli, P. 10—Avian Cytokines and Chemokines. In Avian Immunology; Davison, F., Kaspers, B., Schat, K.A., Eds.; Academic Press: London, UK, 2008; pp. 203–222. [Google Scholar]
- Fasina, Y.O.; Holt, P.S.; Moran, E.T.; Moore, R.W.; Conner, D.E.; McKee, S.R. Intestinal cytokine response of commercial source broiler chicks to Salmonella typhimurium infection. Poult. Sci. 2008, 87, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Imagawa, T.; Uehara, M. The apical caecal diverticulum of the chicken identified as a lymphoid organ. J. Anat. 1996, 189 Pt 3, 667–672. [Google Scholar] [PubMed]
- Beal, R.K.; Powers, C.; Wigley, P.; Barrow, P.A.; Smith, A.L. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 2004, 33, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.K.; Wigley, P.; Powers, C.; Hulme, S.D.; Barrow, P.A.; Smith, A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004, 100, 151–164. [Google Scholar] [CrossRef]
- Kaiser, P.; Rothwell, L.; Galyov, E.E.; Barrow, P.A.; Burnside, J.; Wigley, P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 2000, 146 Pt 12, 3217–3226. [Google Scholar] [CrossRef] [Green Version]
- Withanage, G.S.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon. Cytokine Res. 2003, 23, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef]
- Withanage, G.S.; Sasai, K.; Fukata, T.; Miyamoto, T.; Lillehoj, H.S.; Baba, E. Increased lymphocyte subpopulations and macrophages in the ovaries and oviducts of laying hens infected with Salmonella enterica serovar Enteritidis. Avian Pathol. 2003, 32, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.J.; Charrier, K.; Vogel, S.N. Exogenous tumor necrosis factor alpha and interleukin-1 alpha increase resistance to Salmonella typhimurium: Efficacy is influenced by the Ity and Lps loci. Infect. Immun. 1995, 63, 3196–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakolev, R.; Gospodinova, K.; Sotirov, L.; Nikolov, D.; Angelov, A.; Koynarski, T. Blood Serum Interferon-Alpha and -Gamma Concentrations in Broiler Chickens Treated with the Immunomodulator Helpankar. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 296–299. [Google Scholar]
- Rosenberger, C.M.; Scott, M.G.; Gold, M.R.; Hancock, R.E.; Finlay, B.B. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J. Immunol. 2000, 164, 5894–5904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Seo, K.H.; Holt, P.S.; Brackett, R.E.; Gast, R.K.; Stone, H.D. Mucosal humoral immunity to experimental Salmonella enteritidis infection in the chicken crop. Avian Dis. 2002, 46, 1015–1020. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarepour, M.; Bhullar, K.; Montero, M.; Ma, C.; Huang, T.; Velcich, A.; Xia, L.; Vallance, B.A. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 2013, 81, 3672–3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furter, M.; Sellin, M.E.; Hansson, G.C.; Hardt, W.D. Mucus Architecture and Near-Surface Swimming Affect Distinct Salmonella Typhimurium Infection Patterns along the Murine Intestinal Tract. Cell Rep. 2019, 27, 2665–2678.e2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecher, B. Establishing causality in Salmonella-microbiota-host interaction: The use of gnotobiotic mouse models and synthetic microbial communities. Int. J. Med Microbiol. IJMM 2021, 311, 151484. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef]
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Jiao, L.F.; Xiao, K.; Luan, Z.S.; Hu, C.H.; Shi, B.; Zhan, X.A. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed. Sci. Technol. 2013, 185, 175–181. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- de Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front. Immunol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef]
- Tellez, G.I.; Kogut, M.H.; Hargis, B.M. Immunoprophylaxis of Salmonella enteritidis infection by lymphokines in Leghorn chicks. Avian Dis. 1993, 37, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A. Salmonella infections: Immune and non-immune protection with vaccines. Avian Pathol. 2007, 36, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beal, R.K.; Powers, C.; Davison, T.F.; Barrow, P.A.; Smith, A.L. Clearance of enteric Salmonella enterica serovar Typhimurium in chickens is independent of B-cell function. Infect. Immun. 2006, 74, 1442–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolowschiak, T.; Mueller, A.A.; Pisan, L.J.; Feigelman, R.; Felmy, B.; Sellin, M.E.; Namineni, S.; Nguyen, B.D.; Wotzka, S.Y.; Heikenwalder, M.; et al. IFN-gamma Hinders Recovery from Mucosal Inflammation during Antibiotic Therapy for Salmonella Gut Infection. Cell Host Microbe 2016, 20, 238–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, H.; Askar, B.; Barrow, P.; Foster, N. Dysregulation of JAK/STAT genes by vasoactive intestinal peptide (VIP) in Salmonella-infected monocytes may inhibit its therapeutic potential in human sepsis. Cytokine 2018, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, Z.W. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 2018, 15, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Traits | Mean ± SEM | SD | Min | Max | CV (%) |
|---|---|---|---|---|---|
| Monocytes (M) | 8.46 ± 0.45 | 6.49 | 1 | 39 | 76.68 |
| Heterophils (H) | 18.2 ± 0.54 | 7.77 | 3 | 47 | 42.69 |
| Lymphocytes (L) | 73.35 ± 0.67 | 9.56 | 40 | 90 | 13.03 |
| H/L ratio | 0.26 ± 0.01 | 0.16 | 0.035 | 1.04 | 50.04 |
| Genes | Forward (F) and Reverse (R) Primers 5′ to 3′ | Accession no./Reference |
|---|---|---|
| IL-1β | F: GCATCAAGGGCTACAAGCTCT | [26] |
| R: CCAGGCGGTAGAAGATGAAG | ||
| IL-8 | F: TCCTCCTGGTTTCAGCTGCT | [26] |
| R: GTGGATGAACTTAGAATGAGTG | ||
| IFN-γ | F: CAAGTCAAAGCCGCACATC | [27] |
| R: CGCTGGATTCTCAAGTCGTT | ||
| IL-6 | F: AATCCCTCCTCGCCAATCT | NM_204628.1 |
| R: TCACGGTCTTCTCCATAAACG | ||
| LITAF | F: TGTGTATGTGCAGCAACCCGTAGT | [9] |
| R: GGCATTGCAATTTGGACAGAAGT | ||
| SOCS3 | F: TGCGCCTCAAGACGTTCA | NM_204600.1 |
| R: GTACTCGCTCTTAGAGCT | ||
| Muc2 | F: ACTCCTCCTTTGTATGCGTGA | NM_001318434.1 |
| R: GTTAACGCTGCATTCAACCTT | ||
| Claudin-1 | F: GGTGTACGACTCGCTGCTTA | NM-001013611.2 |
| R: CGAGCCACTCTGTTGCCATA | ||
| NF-κB | F: CAATGGACCAGCTCATGGGAAT | NM_205134.1 |
| R: CTTCGCATACGTATCGGAATCG | ||
| TLR4 | F: ACGGCATTTCAGAACGGACT | NM_001030693.1 |
| R: ACAGCTTCTCAGCAGGCAAT | ||
| GAPDH | F: GGAGAAACCAGCCAAGTAT | NM_204305.1 |
| R: CCATTGAAGTCACAGGAGA |
| Gut- Segment | Items | Age 1 | Non-Infected | SE-Infected | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H/L | GCs (n) | Pearson r | p-Values | H/L | GCs (n) | Pearson r | p-Values | |||
| Ileum | Villus | 14 | 0.21 | 183 | −0.5 | 0.154 | 0.38 | 136.3 | 0.6 | 0.077 |
| 28 | 0.18 | 191.7 | −0.78 * | 0.019 | 0.3 | 173 | 0.06 | 0.452 | ||
| Crypt | 14 | 0.21 | 40.25 | 0.42 | 0.206 | 0.38 | 31.02 | −0.35 | 0.222 | |
| 28 | 0.18 | 34.43 | −0.81 * | 0.013 | 0.3 | 30.42 | −0.64 | 0.085 | ||
| Caecum | Fold | 14 | 0.24 | 210.9 | −0.84 | 0.081 | 0.39 | 150.5 | −0.38 | 0.232 |
| 28 | 0.17 | 129 | −0.27 | 0.300 | 0.32 | 88 | 0.35 | 0.249 | ||
| Crypt | 14 | 0.24 | 21.93 | 0.38 | 0.266 | 0.39 | 15.43 | −0.41 | 0.183 | |
| 28 | 0.17 | 13.46 | −0.28 | 0.272 | 0.32 | 8.65 | 0.08 | 0.423 | ||
| Age 1 | Items | Non-Infected | SE-Infected | p-Values |
|---|---|---|---|---|
| 14 | VH (µm) | 585.82 ± 43.26 | 512.35 ± 42.20 | 0.013 |
| CD (µm) | 110.69 ± 36.4 | 89.79 ± 8.09 | 0.121 | |
| VW (µm) | 95.32 ± 9.44 | 84.29 ± 7.16 | 0.035 | |
| VSA (µm3) | 173,774.42 ± 20,247.01 | 135,183.81 ± 13,676.20 | 0.004 | |
| VH/CD ratio | 5.82 ± 1.76 | 5.92 ± 0.57 | 0.453 | |
| EPT (µm) | 32.75 ± 3.71 | 27.33 ± 3.26 | 0.020 | |
| LPT (µm) | 29.05 ± 4.91 | 30.22 ± 6.68 | 0.380 | |
| 28 | VH (µm) | 703.75 ± 61.19 | 554.42 ± 53.99 | 0.002 |
| CD (µm) | 77.22 ± 15.54 | 72.95 ± 0.19 | 0.278 | |
| VW (µm) | 129.33 ± 13.14 | 147.83 ± 27.76 | 0.107 | |
| VSA (µm3) | 285,496.87 ± 27,153.06 | 253,325.51 ± 29,288.02 | 0.055 | |
| VH/CD ratio | 9.62 ± 1.57 | 7.74 ± 0.80 | 0.022 | |
| EPT (µm) | 46.26 ± 5.18 | 49.82 ± 10.92 | 0.265 | |
| LPT (µm) | 27.18 ± 2.93 | 41.97 ± 13.84 | 0.024 |
| Genes | Gut- Segment | 14 Days-Old 1 | 28 Days-Old 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Infected | SE-Infected | Non-Infected | SE-Infected | ||||||
| Pearson r | p-Values | Pearson r | p-Values | Pearson r | p-Values | Pearson r | p-Values | ||
| IL-1β | Ileum | −0.42 | 0.113 | −0.62 * | 0.028 | 0.3 | 0.201 | 0.68 * | 0.015 |
| Caecum | −0.43 | 0.110 | −0.19 | 0.282 | −0.49 | 0.055 | −0.17 | 0.334 | |
| IL-8 | Ileum | −0.12 | 0.367 | −0.58 * | 0.039 | −0.35 | 0.161 | 0.70 * | 0.012 |
| Caecum | −0.4 | 0.100 | −0.41 | 0.095 | −0.25 | 0.216 | −0.04 | 0.464 | |
| IFN-γ | Ileum | 0.38 | 0.142 | −0.67 * | 0.017 | −0.19 | 0.304 | 0.34 | 0.172 |
| Caecum | 0.24 | 0.273 | 0.27 | 0.260 | −0.48 | 0.056 | 0.37 | 0.164 | |
| IL-6 | Ileum | −0.3 | 0.198 | −0.51 | 0.067 | 0.63 * | 0.026 | 0.64 * | 0.024 |
| Caecum | −0.51 * | 0.044 | −0.38 | 0.111 | −0.47 | 0.060 | 0.47 | 0.100 | |
| LITAF | Ileum | 0.58 * | 0.041 | −0.41 | 0.120 | −0.80 ** | 0.004 | 0.07 | 0.425 |
| Caecum | 0.27 | 0.200 | 0.17 | 0.301 | 0.15 | 0.316 | −0.41 | 0.138 | |
| SOCS3 | Ileum | 0.23 | 0.294 | −0.65 * | 0.021 | −0.43 | 0.107 | 0.22 | 0.268 |
| Caecum | −0.32 | 0.154 | −0.37 | 0.117 | −0.68 ** | 0.007 | 0.25 | 0.255 | |
| MUC2 | Ileum | 0.36 | 0.152 | −0.44 | 0.102 | −0.42 | 0.116 | −0.5 | 0.072 |
| Caecum | −0.63 * | 0.014 | 0.1 | 0.391 | −0.32 | 0.158 | 0.26 | 0.256 | |
| Claudin1 | Ileum | 0.02 | 0.482 | −0.27 | 0.228 | −0.17 | 0.321 | −0.23 | 0.261 |
| Caecum | 0.38 | 0.159 | −0.05 | 0.464 | −0.44 | 0.078 | 0.18 | 0.321 | |
| NF-κB | Ileum | −0.44 | 0.102 | −0.22 | 0.268 | −0.27 | 0.224 | −0.43 | 0.106 |
| Caecum | −0.59 * | 0.022 | 0.15 | 0.318 | −0.16 | 0.310 | 0.36 | 0.168 | |
| TLR4 | Ileum | −0.44 | 0.102 | −0.5 | 0.071 | 0.22 | 0.272 | 0.15 | 0.341 |
| Caecum | −0.38 | 0.113 | −0.04 | 0.451 | −0.44 | 0.079 | 0.23 | 0.276 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiam, M.; Barreto Sánchez, A.L.; Zhang, J.; Zheng, M.; Wen, J.; Zhao, G.; Wang, Q. Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals 2021, 11, 3498. https://doi.org/10.3390/ani11123498
Thiam M, Barreto Sánchez AL, Zhang J, Zheng M, Wen J, Zhao G, Wang Q. Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals. 2021; 11(12):3498. https://doi.org/10.3390/ani11123498
Chicago/Turabian StyleThiam, Mamadou, Astrid Lissette Barreto Sánchez, Jin Zhang, Maiqing Zheng, Jie Wen, Guiping Zhao, and Qiao Wang. 2021. "Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken" Animals 11, no. 12: 3498. https://doi.org/10.3390/ani11123498
APA StyleThiam, M., Barreto Sánchez, A. L., Zhang, J., Zheng, M., Wen, J., Zhao, G., & Wang, Q. (2021). Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals, 11(12), 3498. https://doi.org/10.3390/ani11123498






