Effects of Cyclic Thermal Stress at Later Age on Production Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Sample Collection
2.3. Meat Quality Determination
2.4. Statistical Analyses
3. Results
3.1. Growth Performances
3.2. Carcass Composition
3.3. Meat Quality
3.3.1. Chemical Composition
3.3.2. Surface Color
3.3.3. pH, Water-Holding Capacity and Texture
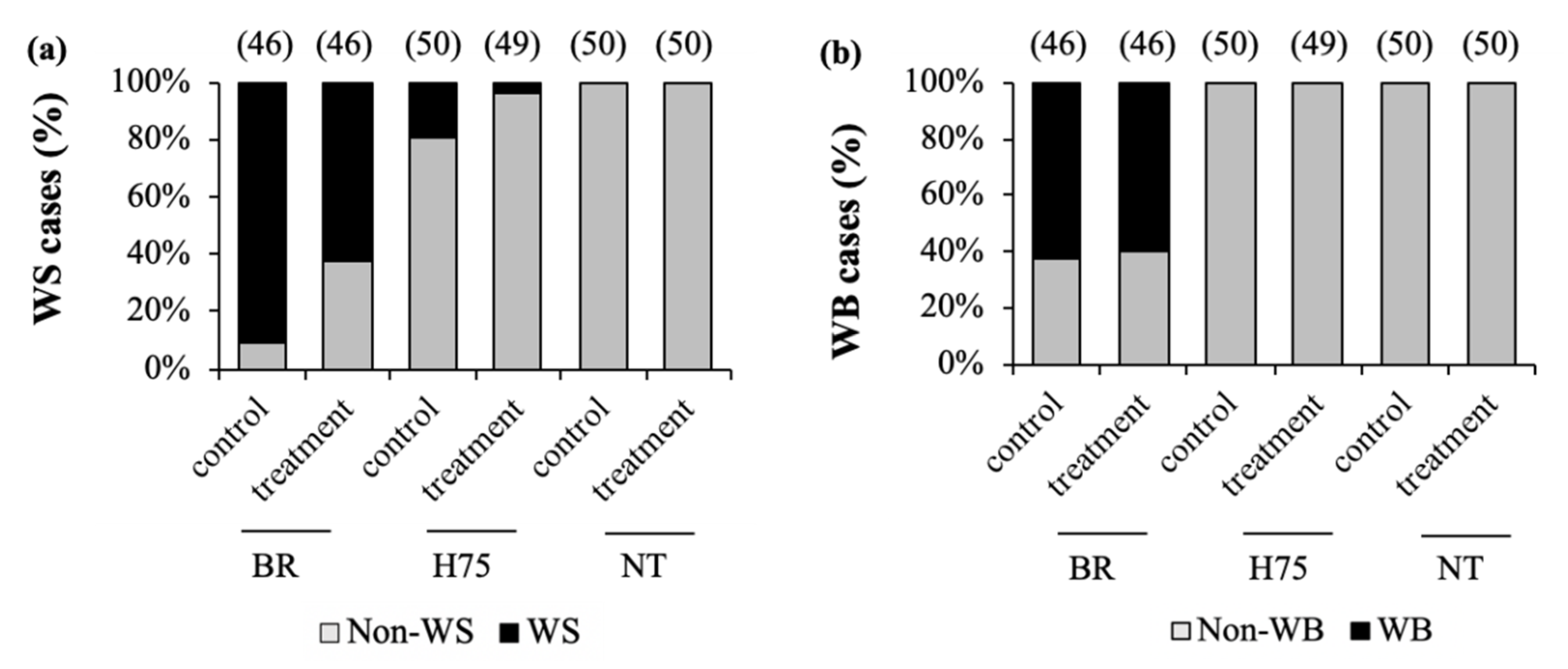
3.3.4. Cases of Growth-Related Myopathies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1509–1518. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Lin, X.; Liu, H.-C.; Odle, J.; Luo, X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015, 94, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.F.A.d.; Espinha, L.P.; Almeida, E.A.d.; Lunedo, R.; Furlan, R.L.; Macari, M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest. Sci. 2016, 192, 39–43. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef]
- Awad, E.A.; Najaa, M.; Zulaikha, Z.A.; Zulkifli, I.; Soleimani, A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas J. Anim. Sci. 2020, 33, 778–787. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Salah, A.S.; Nassan, M.A.; El-Tarabany, M.S. Effects of Chronic Thermal Stress on Performance, Energy Metabolism, Antioxidant Activity, Brain Serotonin, and Blood Biochemical Indices of Broiler Chickens. Animals 2021, 11, 2554. [Google Scholar] [CrossRef]
- Emami, N.K.; Greene, E.S.; Kogut, M.H.; Dridi, S. Heat Stress and Feed Restriction Distinctly Affect Performance, Carcass and Meat Yield, Intestinal Integrity, and Inflammatory (Chemo) Cytokines in Broiler Chickens. Front. Physiol. 2021, 12, 707757. [Google Scholar] [CrossRef] [PubMed]
- Akşit, M.; Yalçin, S.; Özkan, S.; Metin, K.; Özdemir, D. Effects of Temperature During Rearing and Crating on Stress Parameters and Meat Quality of Broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wen, J.; Zhang, H. Effect of Chronic Heat Exposure on Fat Deposition and Meat Quality in Two Genetic Types of Chicken1. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic Heat Stress Impairs the Quality of Breast-Muscle Meat in Broilers by Affecting Redox Status and Energy-Substance Metabolism. J. Agric. Food Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef]
- Andretta, I.; Kipper, M.; Schirmann, G.D.; Franceschina, C.S.; Ribeiro, A.M.L. Modeling the performance of broilers under heat stress. Poult. Sci. 2021, 100, 101338. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jiao, H.C.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Tirawattanawanich, C.; Chantakru, S.; Nimitsantiwong, W.; Tongyai, S. The effects of tropical environmental conditions on the stress and immune responses of commercial broilers, Thai indigenous chickens, and crossbred chickens. J. Appl. Poult. Res. 2011, 20, 409–420. [Google Scholar] [CrossRef]
- Abu-Dieyeh, Z.H.M. Effect of Chronic Heat Stress and Long-Term Feed Restriction on Broiler Performance. Int. J. Poult. Sci. 2006, 5, 185–190. [Google Scholar] [CrossRef]
- Laganá, C.; Ribeiro, A.M.L.; Kessler, A.M.; Kratz, L.R.; Pinheiro, C.C. Effects of the reduction of dietary heat increment on the performance, carcass yield, and diet digestibility of broilers submitted to heat stress. Braz. J. Poult. Sci. 2007, 9, 45–51. [Google Scholar] [CrossRef]
- Abdelhameed Salah Abdelhameed Mohamed; Alexander Robertovich Lozovskiy; Ali, A.M.A. Nutritional strategies to alleviate heat stress effects through feed restrictions and feed additives (vitamins and minerals) in broilers under summer conditions. J. Anim. Behav. Biometeorol. 2019, 7, 123–131. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Washburn, K.W.; Peavey, R.; Renwick, G.M. Relationship of Strain Variation and Feed Restriction to Variation in Blood Pressure and Response to Heat Stress. Poult. Sci. 1980, 59, 2586–2588. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P.J.; Farias Filho, R.V.; Souza, T.M.; Oliveira, E.R.d.; Oliveira, E.B.d.; Nascimento, C.S.d.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS One 2017, 12, e0186083. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. Recent myopathies in broiler’s breast meat fillets. Worlds Poult. Sci. J. 2019, 75, 559–582. [Google Scholar] [CrossRef]
- Yunis, R.; Cahaner, A. The effects of the naked neck (Na) and frizzle (F) genes on growth and meat yield of broilers and their interactions with ambient temperatures and potential growth rate. Poult. Sci. 1999, 78, 1347–1352. [Google Scholar] [CrossRef]
- Aengwanich, W. Comparative ability to tolerate heat between thai indigenous chickens, thai indigenous chickens crossbred and broilers by using heterophil/lymphocyte ratio. Pak. J. Biol. Sci. 2007, 10, 1840–1844. [Google Scholar] [CrossRef] [Green Version]
- Azoulay, Y.; Druyan, S.; Yadgary, L.; Hadad, Y.; Cahaner, A. The viability and performance under hot conditions of featherless broilers versus fully feathered broilers. Poult. Sci. 2011, 90, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Lee, Y.S.; Erf, G.F.; Meullenet, J.F.C.; McKee, S.R.; Owens, C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012, 91, 1240–1247. [Google Scholar] [CrossRef]
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016, 95, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W., Jr. Official Methods of analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- U-chupaj, J.; Malila, Y.; Gamonpilas, C.; Kijroongrojana, K.; Petracci, M.; Benjakul, S.; Visessanguan, W. Differences in textural properties of cooked caponized and broiler chicken breast meat. Poult. Sci. 2017, 96, 2491–2500. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Color difference delta E- a survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Duangjinda, M.; Tunim, S.; Duangdaen, C.; Boonkum, W. Hsp70 Genotypes and Heat Tolerance of Commercial and Native Chickens Reared in Hot and Humid Conditions. Rev. Bras. Cienc. 2017, 19, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Duangduen, C.; Duangjinda, M.; Katawatin, S.; Aengwanich, W. Effects of heat stress on growth performance and physiological response in Thai indigenous chickens (Chee) and broilers. J. Kasetsart Vet. 2007, 17, 122–133. [Google Scholar]
- Shao, D.; Wang, Q.; Hu, Y.; Shi, S.; Tong, H. Effects of cyclic heat stress on the phenotypic response, meat quality and muscle glycolysis of breasts and thighs of yellow-feather broilers. Ital. J. Anim. Sci. 2019, 18, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.F.; Wang, L.K.; Wen, A.Y.; Wang, L.X.; Jin, G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009, 50, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Fernandez, X.; Santé, V.; Baeza, E.; Lebihan-Duval, E.; Berri, C.; Rémignon, H.; Babilé, R.; Le Pottier, G.; Astruc, T. Effects of the rate of muscle post mortem pH fall on the technological quality of turkey meat. Br. Poult. Sci. 2002, 43, 245–252. [Google Scholar] [CrossRef]
- Yunianto, V.D.; Hayashit, K.; Kaiwda, S.; Ohtsuka, A.; Tomita, Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 1997, 77, 897–909. [Google Scholar] [CrossRef]
- Malila, Y.; J, U.C.; Srimarut, Y.; Chaiwiwattrakul, P.; Uengwetwanit, T.; Arayamethakorn, S.; Punyapornwithaya, V.; Sansamur, C.; Kirschke, C.P.; Huang, L.; et al. Monitoring of white striping and wooden breast cases and impacts on quality of breast meat collected from commercial broilers (Gallus gallus). Asian-Australas J. Anim. Sci. 2018, 31, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strains | Thermal Stress | Initial BW (g) | Final BW (g) | BWG (%) | ADG (g/d) | FI (g/d) | F/G | Mortality (%) |
|---|---|---|---|---|---|---|---|---|
| BR | Control | 519.8 ± 91.0 | 1740.5 ± 283.8 | 238.8 ± 50.1 | 57.4 ± 12.3 | 99.5 ± 27.0 | 1.7 ± 0.2 | 8 |
| Treatment | 539.0 ± 80.9 | 1620.4 ± 231.5 | 203.9 ± 41.3 | 51.2 ± 9.6 | 93.6 ± 26.6 | 1.8 ± 0.1 | 8 | |
| p-value 1 | 0.31 | 0.04 | 0.001 | 0.01 | 0.14 | 0.45 | 0.96 | |
| H75 | Control | 671.5 ± 133.3 | 1428.3 ± 277.2 | 114.0 ± 23.4 | 36.0 ± 8.3 | 85.0 ± 20.0 | 2.4 ± 0.3 | 0 |
| Treatment | 666.3 ± 122.1 | 1346.6 ± 281.8 | 102.2 ± 22.6 | 32.4 ± 9.0 | 77.2 ± 28.6 | 2.6 ± 0.7 | 2 | |
| p-value | 0.85 | 0.17 | 0.02 | 0.05 | 0.04 | 0.62 | 0.56 | |
| NT | Control | 745.2 ± 148.6 | 1131.4 ± 191.2 | 54.1 ± 18.4 | 18.4 ± 4.5 | 60.9 ± 14.6 | 3.3 ± 0.5 | 0 |
| Treatment | 684.2 ± 198.5 | 902.3 ± 261.3 | 47.7 ± 21.3 | 14.7 ± 4.8 | 61.5 ± 15.0 | 4.2 ± 0.8 | 0 | |
| p-value | 0.18 | 0.02 | 0.22 | 0.003 | 0.83 | 0.16 | 1.00 |
| Strains | Thermal Stress | Whole Carcass (g) | Visceral Organs (%) | Breast (%) | Thigh (%) | Abdominal Fat (%) | Liver (%) |
|---|---|---|---|---|---|---|---|
| BR | Control (n = 46) | 1526.8 ± 283.2 | 12.7 ± 2.4 | 24.3 ± 2.8 | 10.3 ± 0.7 | 1.7 ± 0.7 | 2.5 ± 0.4 |
| Treatment (n = 46) | 1472.5 ± 215.8 | 12.9 ± 1.7 | 23.8 ± 2.8 | 10.5 ± 0.7 | 1.6 ± 0.9 | 2.4 ± 0.4 | |
| p-value 1 | 0.31 | 0.66 | 0.46 | 0.23 | 0.41 | 0.36 | |
| H75 | Control (n = 50) | 1267.3 ± 234.7 | 13.6 ± 2.5 | 17.4 ± 2.0 | 10.7 ± 1.2 | 2.2 ± 1.2 | 2.3 ± 0.4 |
| Treatment (n = 49) | 1218.9 ± 224.4 | 13.8 ± 2.8 | 16.8 ± 2.1 | 11.0 ± 0.7 | 2.3 ± 1.1 | 2.3 ± 0.3 | |
| p-value | 0.33 | 0.72 | 0.15 | 0.26 | 0.96 | 0.85 | |
| NT | Control (n = 50) | 993.0 ± 176.8 | 13.8 ± 2.5 | 15.2 ± 1.9 | 12.1 ± 1.6 | nd | 2.2 ± 0.3 |
| Treatment (n = 50) | 920.5 ± 226.9 | 13.8 ± 2.4 | 14.9 ± 1.8 | 11.5 ± 0.8 | nd | 2.1 ± 0.3 | |
| p-value | 0.18 | 0.93 | 0.51 | 0.12 | na | 0.44 |
| Strains | Thermal Stress | Moisture (%) | Protein (%) | Fat (%) | Carbohydrate (%) | Ash (%) |
|---|---|---|---|---|---|---|
| BR | Control | 75.73 ± 0.43 | 21.31 ± 0.46 | 0.99 ± 0.17 | 0.75 ± 0.25 | 1.22 ± 0.09 |
| Treatment | 74.92 ± 0.61 | 22.43 ± 0.52 | 0.78 ± 0.17 | 0.46 ± 0.24 | 1.41 ± 0.11 | |
| p-value 1 | 0.001 | <0.001 | 0.004 | 0.02 | 0.01 | |
| H75 | Control | 74.42 ± 1.28 | 22.60 ± 1.07 | 0.70 ± 0.21 | 0.57 ± 0.30 | 1.76 ± 0.24 |
| Treatment | 74.24 ± 0.69 | 22.67 ± 0.69 | 0.60 ± 0.18 | 0.85 ± 0.35 | 1.63 ± 0.25 | |
| p-value | 0.66 | 0.84 | 0.29 | 0.05 | 0.20 | |
| NT | Control | 73.15 ± 0.49 | 23.96 ± 0.62 | 0.30 ± 0.07 | 0.65 ± 0.33 | 1.94 ± 0.06 |
| Treatment | 73.37 ± 0.24 | 23.64 ± 0.47 | 0.40 ± 0.12 | 0.67 ± 0.30 | 1.85 ± 0.08 | |
| p-value | 0.23 | 0.23 | 0.002 | 0.25 | 0.05 |
| Strains | Thermal Stress | L*-Value | a*-Value | b*-Value | ΔE 2 |
|---|---|---|---|---|---|
| BR | Control | 40.87 ± 3.38 | –1.15 ± 0.68 | 1.18 ± 1.12 | 3.23 |
| Treatment | 43.76 ± 2.31 | –1.11 ± 0.50 | 2.63 ± 1.74 | ||
| p-value 1 | 0.02 | 0.86 | 0.03 | ||
| H75 | Control | 43.18 ± 3.74 | –0.68 ± 0.86 | 1.99 ± 1.83 | 0.56 |
| Treatment | 43.29 ± 2.54 | –1.13 ± 0.42 | 1.67 ± 2.06 | ||
| p-value | 0.94 | 0.13 | 0.69 | ||
| NT | Control | 44.05 ± 2.45 | –0.97 ± 0.40 | 1.51 ± 1.29 | 0.71 |
| Treatment | 44.72 ± 2.60 | –1.15 ± 0.55 | 1.64 ± 1.32 | ||
| p-value | 0.58 | 0.42 | 0.83 |
| Strains | Thermal Stress | pH | Drip Loss (%) | Cook Loss (%) | Total Processing Loss (%) |
|---|---|---|---|---|---|
| BR | Control | 6.01 ± 0.10 | 1.67 ± 0.53 | 14.64 ± 1.83 | 16.07 ± 2.00 |
| Treatment | 5.97 ± 0.13 | 1.29 ± 0.53 | 13.85 ± 2.75 | 14.96 ± 2.87 | |
| p-value 1 | 0.92 | 0.09 | 0.41 | 0.28 | |
| H75 | Control | 5.81 ± 0.08 | 2.25 ± 0.52 | 15.20 ± 3.70 | 17.20 ± 3.57 |
| Treatment | 5.84 ± 0.09 | 2.48 ± 0.78 | 17.15 ± 2.91 | 19.20 ± 3.14 | |
| p-value | 0.29 | 0.40 | 0.18 | 0.16 | |
| NT | Control | 5.77 ± 0.11 | 2.72 ± 0.79 | 15.20 ± 3.47 | 17.50 ± 3.60 |
| Treatment | 5.78 ± 0.82 | 3.15 ± 0.84 | 15.68 ± 1.64 | 18.33 ± 2.23 | |
| p-value | 0.82 | 0.28 | 0.71 | 0.57 |
| Strains | Thermal Stress | Shear Force (N) | Shear Energy (N.s) | Hardness (N) | Springiness | Cohesiveness | Chewiness (N) |
|---|---|---|---|---|---|---|---|
| BR | Control | 24.4 ± 7.0 | 100.6 ± 26.2 | 10.5 ± 1.9 | 0.59 ± 0.04 | 0.57 ± 0.04 | 3.6 ± 0.7 |
| Treatment | 30.1 ± 8.9 | 121.3 ± 38.5 | 10.7 ± 2.8 | 0.60 ± 0.03 | 0.60 ± 0.03 | 4.0 ± 0.6 | |
| p-value 1 | 0.04 | 0.04 | 0.77 | 0.28 | 0.05 | 0.10 | |
| H75 | Control | 20.4 ± 10.1 | 87.2 ± 42.5 | 8.8 ± 1.1 | 0.63 ± 0.03 | 0.57 ± 0.05 | 3.2 ± 0.7 |
| Treatment | 29.8 ± 16.6 | 122.5 ± 60.9 | 8.7 ± 2.0 | 0.61 ± 0.03 | 0.58 ± 0.04 | 3.1 ± 0.9 | |
| p-value | 0.08 | 0.11 | 0.89 | 0.19 | 0.39 | 0.64 | |
| NT | Control | 29.1 ± 14.0 | 122.2 ± 52.3 | 8.3 ± 1.5 | 0.62 ± 0.03 | 0.61 ± 0.02 | 3.2 ± 0.5 |
| Treatment | 26.6 ± 10.6 | 121.7 ± 36.9 | 8.0 ± 2.1 | 0.62 ± 0.02 | 0.59 ± 0.03 | 3.1 ± 0.8 | |
| p-value | 0.68 | 0.98 | 0.74 | 0.71 | 0.24 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malila, Y.; Jandamuk, A.; Uopasai, T.; Buasook, T.; Srimarut, Y.; Sanpinit, P.; Phasuk, Y.; Kunhareang, S. Effects of Cyclic Thermal Stress at Later Age on Production Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens. Animals 2021, 11, 3532. https://doi.org/10.3390/ani11123532
Malila Y, Jandamuk A, Uopasai T, Buasook T, Srimarut Y, Sanpinit P, Phasuk Y, Kunhareang S. Effects of Cyclic Thermal Stress at Later Age on Production Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens. Animals. 2021; 11(12):3532. https://doi.org/10.3390/ani11123532
Chicago/Turabian StyleMalila, Yuwares, Anuwat Jandamuk, Thanawan Uopasai, Thongsa Buasook, Yanee Srimarut, Pornnicha Sanpinit, Yupin Phasuk, and Sajee Kunhareang. 2021. "Effects of Cyclic Thermal Stress at Later Age on Production Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens" Animals 11, no. 12: 3532. https://doi.org/10.3390/ani11123532
APA StyleMalila, Y., Jandamuk, A., Uopasai, T., Buasook, T., Srimarut, Y., Sanpinit, P., Phasuk, Y., & Kunhareang, S. (2021). Effects of Cyclic Thermal Stress at Later Age on Production Performance and Meat Quality of Fast-Growing, Medium-Growing and Thai Native Chickens. Animals, 11(12), 3532. https://doi.org/10.3390/ani11123532







