Influence of Microalgae Diets on the Biological and Growth Parameters of Oithona nana (Copepoda: Cyclopoida)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Microalgae and Copepods
2.2. Experimental Design
2.3. Population and Individual Growth and Ingestion Rate
2.4. Number of Spawning and Fertility
2.5. Development Time by Stage and Sex Ratio
2.6. Analysis of Data
3. Results
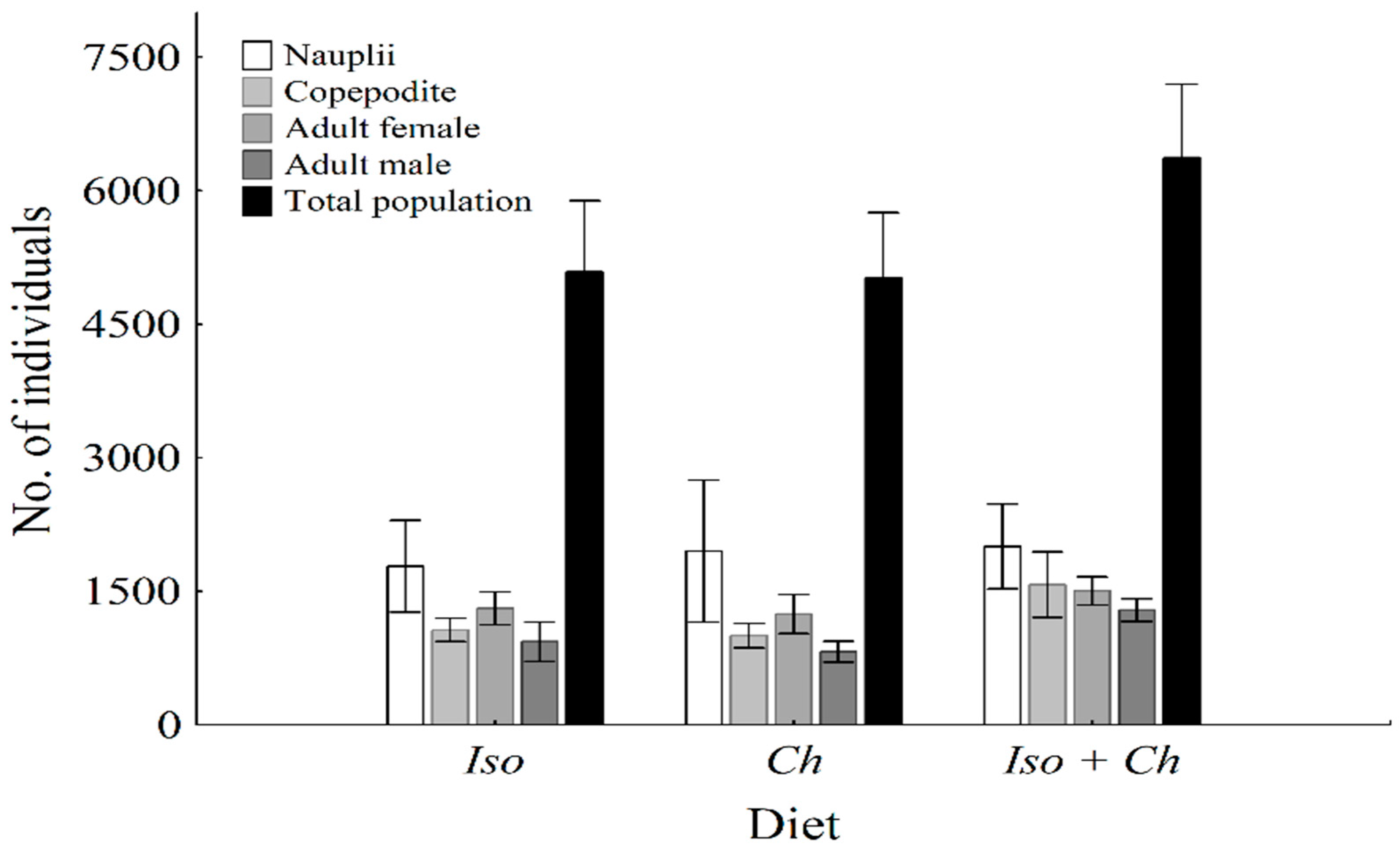
3.1. Population and Individual Growth and Ingestion Rate
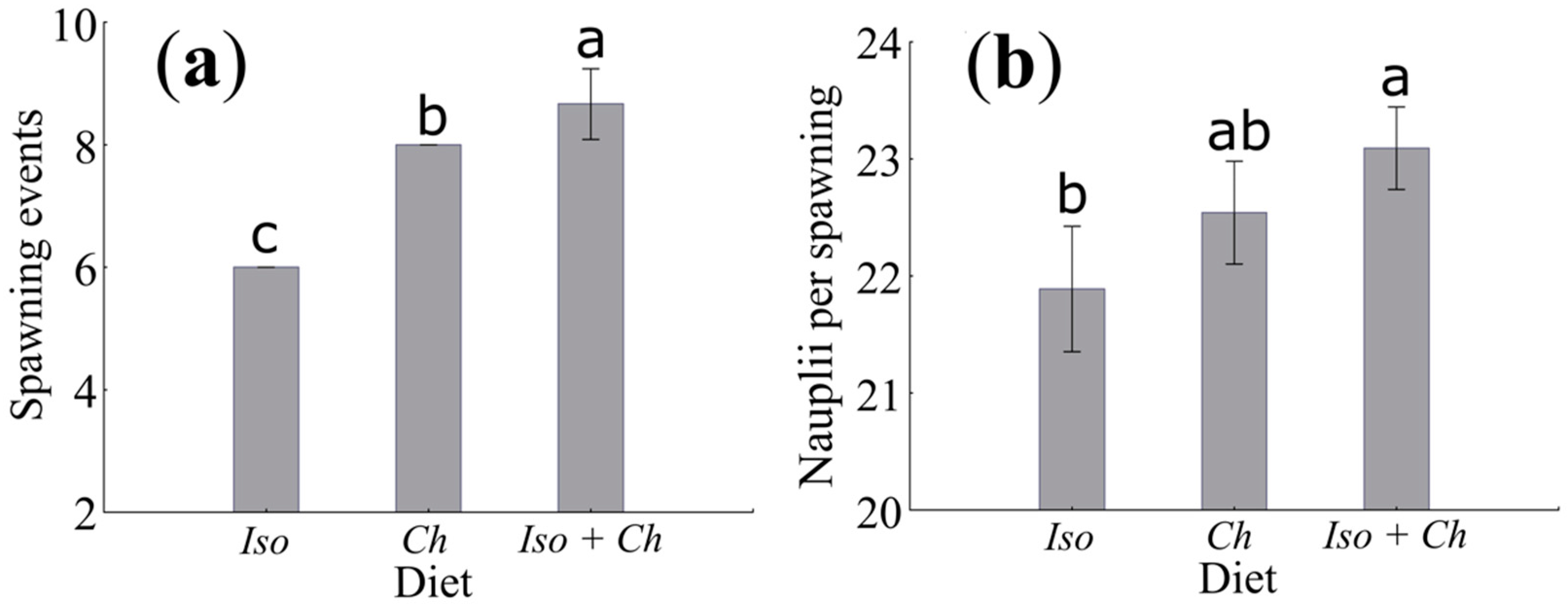
3.2. Number of Spawning and Fertility
3.3. Development Time by Stage and Sex Ratio
4. Discussion
4.1. Population Growth
4.2. Individual Growth
4.3. Ingestion Rate
4.4. Number of Spawning
4.5. Fecundity
4.6. Development Time Per Stage
4.7. Sex Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandathil Radhakrishnan, D.; AkbarAli, I.; Schmidt, B.V.; John, E.M.; Sivanpillai, S.; Thazhakot Vasunambesan, S. Improvement of nutritional quality of live feed for aquaculture: An overview. Aquac. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Jepsen, P.M.; van Someren Gréve, H.; Jørgensen, K.N.; Kjær, K.G.W.; Hansen, B.W. Evaluation of high-density tank cultivation of the live-feed cyclopoid copepod Apocyclops royi (Lindberg 1940). Aquaculture 2021, 533, 736125. [Google Scholar] [CrossRef]
- Chilmawati, D.; Suminto. The Effect of Different Diet of Phytoplankton Cells on Growth Performance of Copepod, Oithona sp. in Semi-mass Culture. Aquat. Procedia 2016, 7, 39–45. [Google Scholar] [CrossRef]
- Samat, N.A.; Yusoff, F.M.; Rasdi, N.W.; Karim, M. Enhancement of live food nutritional status with essential nutrients for improving aquatic animal health: A review. Animals 2020, 10, 2457. [Google Scholar] [CrossRef]
- Azani, N.; Rasdi, N.W. Effect of entiched copepods on the growth, survival and colouration of angelfish (Pterophyllum scalare). J. Undergrad. Res. 2021, 3, 25–36. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Ghonim, A.Z.; El-Khodary, G.M.; El-Sheikh, M.A.; Khairy, H.M. Application of enriched Cyclops abyssorum divergens with mixed algal diet compared to Artemia franciscana for improving larval growth and body composition of Dicentrarchus labrax. Aquac. Rep. 2021, 20, 100715. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; El-Shafei, A.; Mansour, A.T.; Mahmoud, S.m.; Ashour, M. Effect of extended feeding with live copepods, Oithona nana, and Artemia franciscana on the growth performance, intestine histology, and economic viability of european seabass (Dicentrarchus labrax) postlarvae. Fresenius Environ. Bull. 2021, 30, 7106–7116. [Google Scholar]
- Matsui, H.; Sasaki, T.; Kobari, T.; Waqalevu, V.; Kikuchi, K.; Ishikawa, M.; Kotani, T. DHA Accumulation in the Polar Lipids of the Euryhaline Copepod Pseudodiaptomus inopinus and Its Transfer to Red Sea Bream Pagrus major Larvae. Front. Mar. Sci. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Shao, L.; Zeng, C. Survival, growth, ingestion rate and foraging behavior of larval green mandarin fish (Synchiropus splendidus) fed copepods only versus co-fed copepods with rotifers. Aquaculture 2020, 520, 734958. [Google Scholar] [CrossRef]
- Mauchline, J.; Blaxter, J.H.S.; Southward, A.J.; Tyler, P.A. The Biology of Calanoid Copepods, 1st ed.; Mauchline, J., Ed.; Elsevier Academic Press: San Diego, CA, USA, 1998; Volume 33, ISBN 9780080579566. [Google Scholar]
- Nogueira, N.; Sumares, B.; Nascimento, F.A.; Png-Gonzalez, L.; Afonso, A. Effects of mixed diets on the reproductive success and population growth of cultured Acartia grani (Calanoida). J. Appl. Aquac. 2021, 33, 1–14. [Google Scholar] [CrossRef]
- Wilson, J.M.; Ignatius, B.; Sawant, P.B.; Santhosh, B.; Chadha, N.K. Productivity of the calanoid copepod Acartia tropica in response to different salinities and multigenerational acclimatization. Aquaculture 2021, 531, 735818. [Google Scholar] [CrossRef]
- Torres, G.A.; Merino, G.E.; Prieto-Guevara, M.J.; Acosta Portillo, J.E.; Gamboa, J.H.; Imués, M.A.; Chapman, F.A. Spawning of calanoid copepod Acartia tonsa at low temperature and high salinity improves hatch success for cold-stored egg production. Aquaculture 2021, 530, 735725. [Google Scholar] [CrossRef]
- Svendheim, L.H.; Jager, T.; Olsvik, P.A.; Øverjordet, I.B.; Ciesielski, T.M.; Nordtug, T.; Kristensen, T.; Hansen, B.H.; Kvæstad, B.; Altin, D.; et al. Effects of marine mine tailing exposure on the development, growth, and lipid accumulation in Calanus finmarchicus. Chemosphere 2021, 282, 131051. [Google Scholar] [CrossRef] [PubMed]
- Toxværd, K.; Dinh, K.V.; Henriksen, O.; Hjorth, M.; Nielsen, T.G. Delayed effects of pyrene exposure during overwintering on the Arctic copepod Calanus hyperboreus. Aquat. Toxicol. 2019, 217, 105332. [Google Scholar] [CrossRef]
- Hatlebakk, M.; Graeve, M.; Boissonnot, L.; Søreide, J.E. Lipid storage consumption and feeding ability of Calanus glacialis Jaschnov, 1955 males. J. Exp. Mar. Biol. Ecol. 2019, 521, 151226. [Google Scholar] [CrossRef]
- Yusuf, M.B.; Suminto, S.; Herawati, V.E. Penngaruh Pemberian Pakan Alami yang Berbeda dari Jenis Zooplankton (Artemia salina, Brachionus rotundiformis dan Oithona similis) terhadap Performa Pertumbuhan Phronima sp. Sains Akuakultur Trop. 2020, 4, 109–118. [Google Scholar] [CrossRef]
- Samara, S.H.; Santanumurti, M.B.; Widyantoro, A.G.; Putri, B.; Hudaidah, S. The effect of spirulina meals on Oithona sp. (Claus, 1866) production through growth analysis. IOP Conf. Ser Earth Environ. Sci. 2021, 718, 012045. [Google Scholar] [CrossRef]
- Santanumurti, M.B.; Samara, S.H.; Wiratama, A.; Putri, B.; Hudaidah, S. The effect of fishmeal on the density and growth of Oithona sp. (Claus, 1866). IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012042. [Google Scholar] [CrossRef]
- Contreras-Sánchez, W.M.; Hernández-Vidal, U.; Perez-Urbiola, J.C. Population growth of a generational cohort of the copepod Apocyclops panamensis (Marsh, 1913) under different temperatures and salinities. Ecosistemas Recur. Agropecu. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Nielsen, B.L.H.; Gréve, H.V.S.; Hansen, B.W. Cultivation success and fatty acid composition of the tropical copepods Apocyclops royi and Pseudodiaptomus annandalei fed on monospecific diets with varying PUFA profiles. Aquac. Res. 2021, 52, 1127–1138. [Google Scholar] [CrossRef]
- Wang, K.; Li, K.; Shao, J.; Hu, W.; Li, M.; Yang, W.; Tian, J.; Lin, Q. Yeast and Corn Flour Supplement to Enhance Large-scale Culture Efficiency of Marine Copepod Tisbe furcata, a Potential Live Food for Fish Larvae. Isr. J. Aquac.-Bamidgeh. 2017, 69, 20892. [Google Scholar] [CrossRef]
- Hutchins, D.A. Research Methods of Environmental Physiology in Aquatic Sciences; Gao, K., Hutchins, D.A., Beardall, J., Eds.; Springer: Singapore, 2021; ISBN 978-981-15-5353-0. [Google Scholar]
- Santhanam, P.; Begum, A.; Pachiappan, P. Basic and Applied Zooplankton Biology; Springer: Singapore, 2018; ISBN 9789811079535. [Google Scholar]
- Lee, M.C.; Choi, B.S.; Kim, M.S.; Yoon, D.S.; Park, J.C.; Kim, S.; Lee, J.S. An improved genome assembly and annotation of the Antarctic copepod Tigriopus kingsejongensis and comparison of fatty acid metabolism between T. kingsejongensis and the temperate copepod T. japonicus. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2020, 35, 100703. [Google Scholar] [CrossRef] [PubMed]
- Bock, A. RNA-seq Analysis of Tigriopus Californicus under Multiple Dietary Conditions; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2021. [Google Scholar]
- Baker, B. Short-Term Continuous Cultures of Gammarus sp., Tigriopus Californicus, and Nannochloropsis sp.; Oakland University: Oakland County, MI, USA, 2019. [Google Scholar]
- Drillet, G.; Jørgensen, N.O.G.; Sørensen, T.F.; Ramløv, H.; Hansen, B.W. Biochemical and technical observations supporting the use of copepods as live feed organisms in marine larviculture. Aquac. Res. 2006, 37, 756–772. [Google Scholar] [CrossRef]
- Berraho, A.; Abdelouahab, H.; Larissi, J.; Baibai, T.; Charib, S.; Idrissi, M.; Belbchir, Y.; Ettahiri, O.; Hilmi, K. Biodiversity and spatio-temporal variability of copepods community in Dakhla Bay (southern Moroccan coast). Reg. Stud. Mar. Sci. 2019, 28, 100437. [Google Scholar] [CrossRef]
- Berraho, A.; Abdelouahab, H.; Baibai, T.; Charib, S.; Larissi, J.; Agouzouk, A.; Makaoui, A. Short-term variation of zooplankton community in Cintra Bay (Northwest Africa). Oceanologia 2019, 61, 368–383. [Google Scholar] [CrossRef]
- Flores, G.; Morón, O.; Bances, S.; Solís, J.; Guzmán, M.; Correa, J.; Gómez, E.; Crispín, M.; Anculle, T. Characterization of Physical, Chemical, Biological and Sedimentological Processes. Lobos de Tierra Island. March 2014. Inf.-Inst. Mar. Perú. 2017, 44, 212–244. [Google Scholar]
- Leitão, S.N.; Junior, M.d.M.; Porto Neto, F.d.F.; Silva, A.P.; Garcia Diaz, X.F.; e Silva, T.d.A.; Vieira, D.A.d.N.; Figueiredo, L.G.P.; da Costa, A.E.S.F.; de Santana, J.R.; et al. Connectivity between coastal and oceanic zooplankton from rio grande do Norte in the Tropical Western Atlantic. Front. Mar. Sci. 2019, 6, 1–19. [Google Scholar] [CrossRef]
- Villalba, W.; Marquez-Rojas, B.; Troccoli, L.; Alzolar, M.; López, J. Composición y abundancia del zooplancton en la laguna El Morro, Isla de Margarita, Venezuela. Rev. Peru. Biol. 2017, 24, 343. [Google Scholar] [CrossRef] [Green Version]
- Baird, W. Notes on British Entomostraca. Zoologist 1843, 1, 193–197. [Google Scholar]
- Gallienne, C.P.; Robins, D.B. Is Oithona the most important copepod in the world’s oceans? J. Plankton. Res. 2001, 23, 1421–1432. [Google Scholar] [CrossRef]
- Wang, L.; Du, F.; Wang, X.; Li, Y.; Ning, J. Distribution and role of the genus Oithona (Copepoda: Cyclopoida) in the South China Sea. Oceanologia 2017, 59, 300–310. [Google Scholar] [CrossRef]
- Medina, M.; Herrera, L.; Castillo, J.; Jaque, J.; Pizarro, N. Alimentación de la anchoveta (Engraulis ringens) en el norte de Chile(18°25′-25°40′ S) en diciembre de 2010. Lat. Am. J. Aquat. Res. 2015, 43, 46–58. [Google Scholar] [CrossRef]
- Hernández-Molejón, O.G.; Alvarez-Lajonchère, L. Culture experiments with Oithona oculata Farran, 1913 (Copepoda: Cyclopoida), and its advantages as food for marine fish larvae. Aquaculture 2003, 219, 471–483. [Google Scholar] [CrossRef]
- Giesbrecht, W. Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der Angrenzenden Meeres-Abschnitte, von d’ Wilhelm Giesbrecht. Mit 54 Tafeln in Lithographie. Hrsg. von der Zoologischen Station zu Neapel; R. Friedlander & Sohn: Berlin, Germany, 1892. [Google Scholar]
- Annabi-Trabelsi, N.; Guermazi, W.; Karam, Q.; Ali, M.; Uddin, S.; Leignel, V.; Ayadi, H. Concentrations of trace metals in phytoplankton and zooplankton in the Gulf of Gabès, Tunisia. Mar. Pollut. Bull. 2021, 168, 112392. [Google Scholar] [CrossRef] [PubMed]
- Annabi-Trabelsi, N.; Daly-Yahia, M.N.; Romdhane, M.S.; Ben Maïz, N. Seasonal variability of planktonic copepods in Tunis North Lagoon (Tunisia, North Africa). Cah. Biol. Mar. 2005, 46, 325–333. [Google Scholar]
- Fernandes, L.F.L.; Paiva, T.R.M.; Longhini, C.M.; Pereira, J.B.; Ghisolfi, R.D.; Lázaro, G.C.S.; Demoner, L.E.; Laino, P.d.S.; Conceição, L.R.d.; Sá, F.; et al. Marine zooplankton dynamics after a major mining dam rupture in the Doce River, southeastern Brazil: Rapid response to a changing environment. Sci. Total Environ. 2020, 736, 139621. [Google Scholar] [CrossRef]
- Sathishkumar, R.S.; Sundaramanickam, A.; Sahu, G.; Mohanty, A.K.; Ramesh, T.; Khan, S.A. Intense bloom of the diatom Hemidiscus hardmanianus (Greville) in relation to water quality and plankton communities in Tuticorin coast, Gulf of Mannar, India. Mar. Poll. Bull. 2021, 163, 111757. [Google Scholar] [CrossRef] [PubMed]
- Isinibilir, M.; Svetlichny, L. Small oithonid copepods in the northeastern marmara sea. In Proceedings of the 41 st CIESM Congress, Kiel, Germany, 12–16 September 2016; p. 20. [Google Scholar]
- Lavaniegos, B.E.; Heckel, G.; Ladrón de Guevara, P. Variabilidad estacional de copépodos y cladóceros de bahía de los Ángeles (golfo de California) e importancia de Acartia clausi como alimento del tiburón ballena. Cienc. Mar. 2012, 38, 11–30. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Tageldein Mansour, A.; Alkafafy, M.; Ashour, M. Population Dynamics, Fecundity and Fatty Acid Composition of Oithona nana (Cyclopoida, Copepoda), Fed on Different Diets. Animals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Lampitt, R.S.; Gamble, J.C. Diet and respiration of the small planktonic marine copepod Oithona nana. Mar. Biol. 1982, 66, 185–190. [Google Scholar] [CrossRef]
- Murphy, H.E. The life cycle of Oithona nana reared experimentally. Contrib. Scripps Inst. Biol. Res. 1923, 22, 449–453. [Google Scholar]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Ashour, M. Evaluation of the population growth and fatty acid composition of copepoda, Oithona nana, fed on different diets. Int. J. Aquat. Biol. 2021, 9, 167–176. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Mansour, A.T.; Gaber, A.; Ashour, M. Effect of Different Salinity Levels on Population Dynamics and Growth of the Cyclopoid Copepod Oithona nana. Diversity 2021, 13, 190. [Google Scholar] [CrossRef]
- Drillet, G.; Frouël, S.; Sichlau, M.H.; Jepsen, P.M.; Højgaard, J.K.; Joardeer, A.K.; Hansen, B.W. Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture 2011, 315, 155–166. [Google Scholar] [CrossRef]
- Kaviyarasan, M.; Santhanam, P.; Ananth, S.; Dinesh Kumar, S.; Raju, P.; Kandan, S. Population growth, nauplii production and post-embryonic development of Pseudodiaptomus annandalei (Sewell, 1919) in response to temperature, light intensity, pH, salinity and diets. Indian J. Geo-Mar. Sci. 2020, 49, 1000–1009. [Google Scholar]
- Puello-Cruz, A.C.; Mezo-Villalobos, S.; González-Rodríguez, B.; Voltolina, D. Culture of the calanoid copepod Pseudodiaptomus euryhalinus (Johnson 1939) with different microalgal diets. Aquaculture 2009, 290, 317–319. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Garland, C.D. Nutritional Aspects of Microalgae Used in Mariculture: A Literature Review; CSIRO Marine Laboratories: Hobart, Australia, 1989. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Santoyo-Flores, J.A. Efecto de las Dietas Microalgales en la Alimentación y Producción de Huevos de Parvocalanus Crassirostris (Copepoda, Calanoidea); Centro Interdisciplinario de Ciencias Marinas: La Paz, Mexico, 2020. [Google Scholar]
- Flores-Santana, R.E. Variación en el Contenido de Ácidos Grasos del Copépodo Calanoide Pseudodiaptomus Euryhalinus (Johnson, 1939) Alimentado con las Microalgas Chaetoceros Calcitrans e Isochrysis Galbana; Centro de Investigaciones Biológicas del Noroeste, S.C.: La Paz, Mexico, 2008. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanog. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, F.D.; Bowman, T.E. Pelagic copepods of the family Oithonidae (Cyclopoida) from the east coasts of Central and South America. Smithson. Contr. Zool. 1980, 312, 1–27. [Google Scholar] [CrossRef]
- Frost, B.W. Effects of size and concentration of food particles on feeding behavior of marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 9780131008465. [Google Scholar]
- Holm, M.W.; Rodríguez-Torres, R.; Hansen, B.W.; Almeda, R. Influence of behavioral plasticity and foraging strategy on starvation tolerance of planktonic copepods. J. Exp. Mar. Biol. Ecol. 2019, 511, 19–27. [Google Scholar] [CrossRef]
- López-Peralta, R.H.; Mojica-López, L.H. Especies de Oithona (Crustacea: Copepoda) en el Pacífico Colombiano en el Segundo Periodo Lluvioso de 2001. Rev. Fac. Cien. Básicas 2016, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Ben Ltaief, T.; Drira, Z.; Hannachi, I.; Bel Hassen, M.; Hamza, A.; Pagano, M.; Ayadi, H. What are the factors leading to the success of small planktonic copepods in the Gulf of Gabes, Tunisia? J. Mar. Biol. Assoc. U. K. 2015, 95, 747–761. [Google Scholar] [CrossRef]
- Temperoni, B.; Viñas, M.D.; Diovisalvi, N.; Negri, R. Seasonal production of Oithona nana Giesbrecht, 1893 (Copepoda: Cyclopoida) in temperate coastal waters of Argentina. J. Plankton. Res. 2011, 33, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, N.; Mariotto, L. Seasonal variations in copepod body length: A comparison between different species in the lagoon of Venice. Aquat. Ecol. 2000, 34, 243–252. [Google Scholar] [CrossRef]
- El-Maghraby, A.M. The Seasonal Variations in Length of Some Marine Planktonic Copepods From the Eastern Mediterranean At Alexandria. Crustaceana 1965, 8, 37–47. [Google Scholar] [CrossRef]
- Haq, S.M. The larval development of Oithonina nana. Proc. Zool. Soc. Lond. 1965, 146, 555–566. [Google Scholar] [CrossRef]
- Shmeleva, A.A. Weight characteristics of the zooplankton of the Adriatic Sea. Bull. Inst. Océanog. 1965, 65, 1–24. [Google Scholar]
- Cornwell, L.E.; Findlay, H.S.; Fileman, E.S.; Smyth, T.J.; Hirst, A.G.; Bruun, J.T.; McEvoy, A.J.; Widdicombe, C.E.; Castellani, C.; Lewis, C.; et al. Seasonality of Oithona similis and Calanus helgolandicus reproduction and abundance: Contrasting responses to environmental variation at a shelf site. J. Plankton. Res. 2018, 40, 295–310. [Google Scholar] [CrossRef] [Green Version]
- Dayras, P.; Bialais, C.; Lee, J.S.; Souissi, S. Effects of microalgal diet on the population growth and fecundity of the cyclopoid copepod Paracyclopina nana. J. World Aquac. Soc. 2020, 51, 1386–1401. [Google Scholar] [CrossRef]
- Pan, Y.J.; Sadovskaya, I.; Hwang, J.S.; Souissi, S. Assessment of the fecundity, population growth and fatty acid composition of Apocyclops royi (Cyclopoida, Copepoda) fed on different microalgal diets. Aquac. Nutr. 2018, 24, 970–978. [Google Scholar] [CrossRef]
- Santhanam, P.; Perumal, P. Effect of temperature, salinity and algal food concentration on population density, growth and survival of marine copepod Oithona rigida Giesbrecht. Indian J. Mar. Sci. 2012, 41, 369–376. [Google Scholar]
- De Troch, M.; Boeckx, P.; Cnudde, C.; Van Gansbeke, D.; Vanreusel, A.; Vincx, M.; Caramujo, M. Bioconversion of fatty acids at the basis of marine food webs: Insights from a compound-specific stable isotope analysis. Mar. Ecol. Prog. Ser. 2012, 465, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Helenius, L.; Budge, S.M.; Johnson, C.L. Stable isotope labeling reveals patterns in essential fatty acid growth efficiency in a lipid-poor coastal calanoid copepod. Mar. Biol. 2020, 167, 178. [Google Scholar] [CrossRef]
- Ravet, J.L.; Brett, M.T.; Arhonditsis, G.B. The effects of seston lipids on zooplankton fatty acid composition in Lake Washington, Washington, USA. Ecology 2010, 91, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Lee, M.-C.; Puthumana, J.; Park, J.C.; Kang, S.; Hwang, D.-S.; Shin, K.-H.; Park, H.G.; Souissi, S.; Om, A.-S.; et al. Effects of salinity on growth, fatty acid synthesis, and expression of stress response genes in the cyclopoid copepod Paracyclopina nana. Aquaculture 2017, 470, 182–189. [Google Scholar] [CrossRef]
- Nielsen, B.L.H.; Gøtterup, L.; Jørgensen, T.S.; Hansen, B.W.; Hansen, L.H.; Mortensen, J.; Jepsen, P.M. n-3 PUFA biosynthesis by the copepod Apocyclops royi documented using fatty acid profile analysis and gene expression analysis. Biol. Open 2019, 8, bio038331. [Google Scholar] [CrossRef] [Green Version]
- Veloza, A.J.; Chu, F.L.E.; Tang, K.W. Trophic modification of essential fatty acids by heterotrophic protists and its effects on the fatty acid composition of the copepod Acartia tonsa. Mar. Biol. 2006, 148, 779–788. [Google Scholar] [CrossRef]
- Boyen, J.; Fink, P.; Mensens, C.; Hablützel, P.I.; De Troch, M. Fatty acid bioconversion in harpacticoid copepods in a changing environment: A transcriptomic approach. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190645. [Google Scholar] [CrossRef] [PubMed]
- Horne, C.R.; Hirst, A.G.; Atkinson, D.; Almeda, R.; Kiørboe, T. Rapid shifts in the thermal sensitivity of growth but not development rate causes temperature–size response variability during ontogeny in arthropods. Oikos 2019, 128, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Alajmi, F.; Zeng, C. The effects of stocking density on key biological parameters influencing culture productivity of the calanoid copepod, Parvocalanus crassirostris. Aquaculture 2014, 434, 201–207. [Google Scholar] [CrossRef]
- Kiørboe, T. Mate finding, mating, and population dynamics in a planktonic copepod Oithona davisae: There are too few males. Limnol. Oceanogr. 2007, 52, 1511–1522. [Google Scholar] [CrossRef]
- Lee, K.W.; Rhee, J.S.; Han, J.; Park, H.G.; Lee, J.S. Effect of culture density and antioxidants on naupliar production and gene expression of the cyclopoid copepod, Paracyclopina nana. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 161, 145–152. [Google Scholar] [CrossRef]
- Chintada, B.; Ranjan, R.; Santhosh, B.; Megarajan, S.; Ghosh, S.; Babitha Rani, A.M. Effect of stocking density and algal concentration on production parameters of calanoid copepod Acartia bilobata. Aquac. Rep. 2021, 21, 100909. [Google Scholar] [CrossRef]
- Wilson, J.M.; Ignatius, B.; Santhosh, B.; Sawant, P.B.; Soma, S.A. Effect of adult density on egg production, egg hatching success, adult mortality, nauplii cannibalism and population growth of the tropical calanoid copepod Acartia tropica. Aquaculture 2022, 547, 737508. [Google Scholar] [CrossRef]
- Drillet, G.; Maguet, R.; Mahjoub, M.S.; Roullier, F.; Fielding, M.J. Egg cannibalism in Acartia tonsa: Effects of stocking density, algal concentration, and egg availability. Aquac. Int. 2014, 22, 1295–1306. [Google Scholar] [CrossRef]
- Holm, M.W.; Rodríguez-Torres, R.; Van Someren Gréve, H.; Hansen, B.W.; Almeda, R. Sex-specific starvation tolerance of copepods with different foraging strategies. J. Plankton. Res. 2018, 40, 284–294. [Google Scholar] [CrossRef]
- Hansen, B.W.; Rayner, T.A.; Hwang, J.S.; Højgaard, J.K. To starve or not to starve: Deprivation of essential fatty acids and change in escape behavior during starvation by nauplii of the tropical calanoid copepod Pseudodiaptomus annandalei. J. Exp. Mar. Biol. Ecol. 2020, 524, 151287. [Google Scholar] [CrossRef]
- Harris, R.P.; Paffenhöfer, G.-A. Feeding, growth and reproduction of the marine planktonic copepod Temora longicornis Müller. J. Mar. Biol. Assoc. United Kingd. 1976, 56, 675–690. [Google Scholar] [CrossRef]
- Dayras, P.; Bialais, C.; Sadovskaya, I.; Lee, M.-C.; Lee, J.; Souissi, S. Microalgal Diet Influences the Nutritive Quality and Reproductive Investment of the Cyclopoid Copepod Paracyclopina nana. Front. Mar. Sci. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Besiktepe, S.; Dam, H.G. Effect of diet on the coupling of ingestion and egg production in the ubiquitous copepod, Acartia tonsa. Prog. Oceanogr. 2020, 186, 102346. [Google Scholar] [CrossRef]
- Dagg, M.J. A method for the determination of copepod feeding rates during short time intervals. Mar. Biol. 1983, 75, 63–67. [Google Scholar] [CrossRef]
- Petipa, T.; Mironov, G.; Pavlova, E.V. The food web structure, utilization and transport of energy by trophic levels in the planktonic communities. In Marine Food Chains; Steele, J.H., Ed.; Oliver & Boyd: Edimburgo, UK, 1970; pp. 142–167. ISBN 3874290476. [Google Scholar]
- Lampitt, R.S. Carnivorous feeding by a small marine copepod 1. Limnol. Oceanogr. 1978, 23, 1228–1231. [Google Scholar] [CrossRef]
- Siqwepu, O.; Richoux, N.B.; Vine, N.G. The effect of different dietary microalgae on the fatty acid profile, fecundity and population development of the calanoid copepod Pseudodiaptomus hessei (Copepoda: Calanoida). Aquaculture 2017, 468, 162–168. [Google Scholar] [CrossRef]
- Abdullahi, B.A. Effects of diet on growth and development of three species of cyclopoid copepods. Hydrobiologia 1992, 232, 233–241. [Google Scholar] [CrossRef]
- Piña, P.; Voltolina, D.; Nieves, M.; Robles, M. Survival, development and growth of the Pacific white shrimp Litopenaeus vannamei protozoea larvae, fed with monoalgal and mixed diets. Aquaculture 2006, 253, 523–530. [Google Scholar] [CrossRef]
- Lora-Vilchis, M.C.; Cordero-Esquivel, B.; Voltolina, D. Growth of Artemia franciscana fed Isochrysis sp. and Chaetoceros muelleri during its early life stages. Aquac. Res. 2004, 35, 1086–1091. [Google Scholar] [CrossRef]
- Temperoni, B. Variación Estacional de la Producción Secundaria de Oithona nana (Copepoda: Cyclopoida) en Aguas Costeras Bonaerenses; Universidad Nacional de Mar Del Plata: Mar del Plata, Argentina, 2008. [Google Scholar]
- Støttrup, J.G.; Jensen, J. Influence of algal diet on feeding and egg-production of the calanoid copepod Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 1990, 141, 87–105. [Google Scholar] [CrossRef]
- Lauritano, C.; Carotenuto, Y.; Miralto, A.; Procaccini, G.; Ianora, A. Copepod Population-Specific Response to a Toxic Diatom Diet. PLoS ONE 2012, 7, e47262. [Google Scholar] [CrossRef] [Green Version]
- Suminto, S.; Chilmawati, D.; Harwanto, D. Effect of Different Doses of Fermented Organic Feed on the Growth Performance of Oithona sp. in Semi-Mass Culture Condition. Omni-Akuatika 2018, 14, 53–59. [Google Scholar] [CrossRef]
- Chilmawati, D.; Hutabarat, J.; Anggoro, S.; Suminto, S. Organic Feed Enrichment Effects toward Growth Performance and Egg Production of Oithona similis. Omni-Akuatika 2020, 16, 128. [Google Scholar] [CrossRef]
- Torres, G.A.; Merino, G.E.; Prieto-Guevara, M.J.; Acosta Portillo, J.E.; López Arboleda, J.E.; Chapman, F.A. Spawning Parvocalanus crassirostris at a high adult density: Explaining low adult population numbers and means for improving their intensive culture. Aquaculture 2022, 546, 737347. [Google Scholar] [CrossRef]
- Almeda, R.; Calbet, A.; Alcaraz, M.; Yebra, L.; Saiz, E. Effects of temperature and food concentration on the survival, development and growth rates of naupliar stages of Oithona davisae (Copepoda, Cyclopoida). Mar. Ecol. Prog. Ser. 2010, 410, 97–109. [Google Scholar] [CrossRef]
- Gusmão, L.F.M.; McKinnon, A.D. Sex ratios, intersexuality and sex change in copepods. J. Plankton. R. 2009, 31, 1101–1117. [Google Scholar] [CrossRef]
- Chilmawati, D.; Hutabarat, J.; Anggoro, S.; Suminto, S. Biomolecular identification and optimization of growth performance and egg production in Oithona sp. Under different salinity culture conditions. AACL Bioflux. 2019, 12, 575–585. [Google Scholar]
- Drira, Z.; Belhassen, M.; Ayadi, H.; Hamza, A.; Zarrad, R.; Bouaïn, A.; Aleya, L. Copepod community structure related to environmental factors from a summer cruise in the Gulf of Gabès (Tunisia, eastern Mediterranean Sea). J. Mar. Biol. Assoc. United Kingd. 2010, 90, 145–157. [Google Scholar] [CrossRef]
- Irigoien, X.; Obermüller, B.; Head, R.N.; Harris, R.P.; Rey, C.; Hansen, B.W.; Hygum, B.H.; Heath, M.R.; Durbin, E.G. The effect of food on the determination of sex ratio in Calanus spp.: Evidence from experimental studies and field data. ICES J. Mar. Sci. 2000, 57, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Carotenuto, Y.; Ianora, A.; Miralto, A. Maternal and neonate diatom diets impair development and sex differentiation in the copepod Temora stylifera. J. Exp. Mar. Biol. Ecol. 2011, 396, 99–107. [Google Scholar] [CrossRef]
| Size (µm) 1 | Biovolume (µm3⋅cell–1) 1 | Carbon Content (pg C⋅cell–1) 1 | % ARA 2 | % EPA 2 | % DHA 2 | |
|---|---|---|---|---|---|---|
| I. galbana | 5.7 | 99.5 | 12.0 | 0.11 | 0.32 | 19.55 |
| C. calcitrans | 7.0 × 4.9 | 136.2 | 15.4 | 3.86 | 27.06 | 2.75 |
| Single Diet | Mixed Diet | ||||
|---|---|---|---|---|---|
| Iso | Ch | Iso + Ch | |||
| Production per stage (%) | Nauplii | 35 ± 5 | 38 ± 10 | 31 ± 4 | |
| Copepod | 21 ± 3 | 20 ± 2 | 25 ± 4 | ||
| Adults | Female | 26 ± 4 | 25 ± 6 | 24 ± 4 | |
| Male | 18 ± 4 | 17 ± 3 | 20 ± 3 | ||
| Total production (individual/L) | 6360 ± 998 | 6273 ± 919 | 7966 ± 1032 | ||
| Single Diet | Mixed Diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iso | Ch | Iso + Ch | |||||||||
| Min | Max | Average | Min | Max | Average | Min | Max | Average | |||
| (μm) | Nauplii | 96.3 | 298.7 | 190.8 ± 46.6 | 82.4 | 288.4 | 167.6 ± 49.1 | 96.6 | 288.4 | 195.0 ± 53.7 | |
| Copepodite | 309.0 | 494.4 | 401.8 ± 42.8 | 247.2 | 545.9 | 403.3 ± 62.4 | 245.2 | 544.0 | 380.0 ± 58.3 | ||
| Adults | Female | 535.6 | 690.1 | 620.8 ± 33.9 | 515.0 | 690.1 | 621.3 ± 35.9 | 515.0 | 698.3 | 622.4 ± 36.7 | |
| Male | 506.7 | 700.6 | 613.0 ± 60.6 | 535.6 | 690.1 | 611.5 ± 39.7 | 506.6 | 699.9 | 615.5 ± 61.2 | ||
| I | cells/ind/h | 118.3 ± 61.9 b | 154.2 ± 30.2 a | — | |||||||
| ng C/ind/day | 34.1 | 57.0 | — | ||||||||
| Microalgae (×103/mL) | Nauplii | Copepodite | Female | Male | Source |
|---|---|---|---|---|---|
| I. galbana (200) and C. calcitrans (200) | 82.4–298.7 | 247.2–587.1 | 432.6–772.5 | 515.0–762.2 | Present research |
| Oxhyrris marina (1) + Rhodomonas salina (1) | 352 ± 23 ** | [62] | |||
| N.E. Colombia | - | <1000 | <1000 | [63] | |
| N.E. Tunez | 480–800 | 480–800 | [64] | ||
| N.E. Argentina | C-I: 276 ± 18 C-II: 327 ± 17 C-III: 382 ± 25 C-IV: 448 ± 24 C-V: 531 ± 36 | 609 ± 36 | 608 ± 49 | [65] | |
| N.E. Italy | 569 ± 33 | 539 ± 24 | [66] | ||
| N.E. Central and South America | 580–720 | 470–530 | [59] | ||
| N.E. Mediterranean sea | 400–640 | 400–610 | [67] | ||
| Phaeodactylum tricornutum * | N-I: 50–85 N-II: 85–100 N-III: 100–110 N-IV: 110–125 N-V: 130–145 N-VI: 150–175 | [68] | |||
| N.E. Adriatic sea | 520 | 520 | [69] | ||
| Fresh and decomposed algae + Navicula | N-I: 40 N-II: 75 N-III: 90–97 N-IV:110–120 N-V: 130–150 N-VI: 150–190 | C-I: 190–200 C-II: 260–320 C-III: 340–380 C-IV: 400–480 C-V: 450–520 | 480 | 550 | [48] |
| Single Diet | Mixed Diet | |||
|---|---|---|---|---|
| Iso | Ch | Iso + Ch | ||
| Development | Nauplii | 4.00 ± 0.00 b | 7.00 ± 0.00 a | 5.00 ± 0.00 ab |
| Copepodite | 2.33 ± 0.00 b | 4.67 ± 0.58 a | 2.00 ± 0.00 b | |
| Adults | 13.67 ± 0.58 a | 8.33 ± 0.58 b | 13.67 ± 0.00 a | |
| Sex ratio | (No. male/No. female) (%Male:%Female) | 0.67 ± 0.10 (38:62) | 0.81 ± 0.24 (44:56) | 0.75 ± 0.22 (42:58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huanacuni, J.I.; Pepe-Victoriano, R.; Lora-Vilchis, M.C.; Merino, G.E.; Torres-Taipe, F.G.; Espinoza-Ramos, L.A. Influence of Microalgae Diets on the Biological and Growth Parameters of Oithona nana (Copepoda: Cyclopoida). Animals 2021, 11, 3544. https://doi.org/10.3390/ani11123544
Huanacuni JI, Pepe-Victoriano R, Lora-Vilchis MC, Merino GE, Torres-Taipe FG, Espinoza-Ramos LA. Influence of Microalgae Diets on the Biological and Growth Parameters of Oithona nana (Copepoda: Cyclopoida). Animals. 2021; 11(12):3544. https://doi.org/10.3390/ani11123544
Chicago/Turabian StyleHuanacuni, Jordan I., Renzo Pepe-Victoriano, María C. Lora-Vilchis, Germán E. Merino, Fressia G. Torres-Taipe, and Luis A. Espinoza-Ramos. 2021. "Influence of Microalgae Diets on the Biological and Growth Parameters of Oithona nana (Copepoda: Cyclopoida)" Animals 11, no. 12: 3544. https://doi.org/10.3390/ani11123544






