Mannan Oligosaccharide Enhanced the Growth Rate, Digestive Enzyme Activity, Carcass Composition, and Blood Chemistry of Thinlip Grey Mullet (Liza ramada)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Conditions
2.2. Final Sampling
2.3. Digestive Enzyme Activity
2.4. Blood Analysis
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Composition
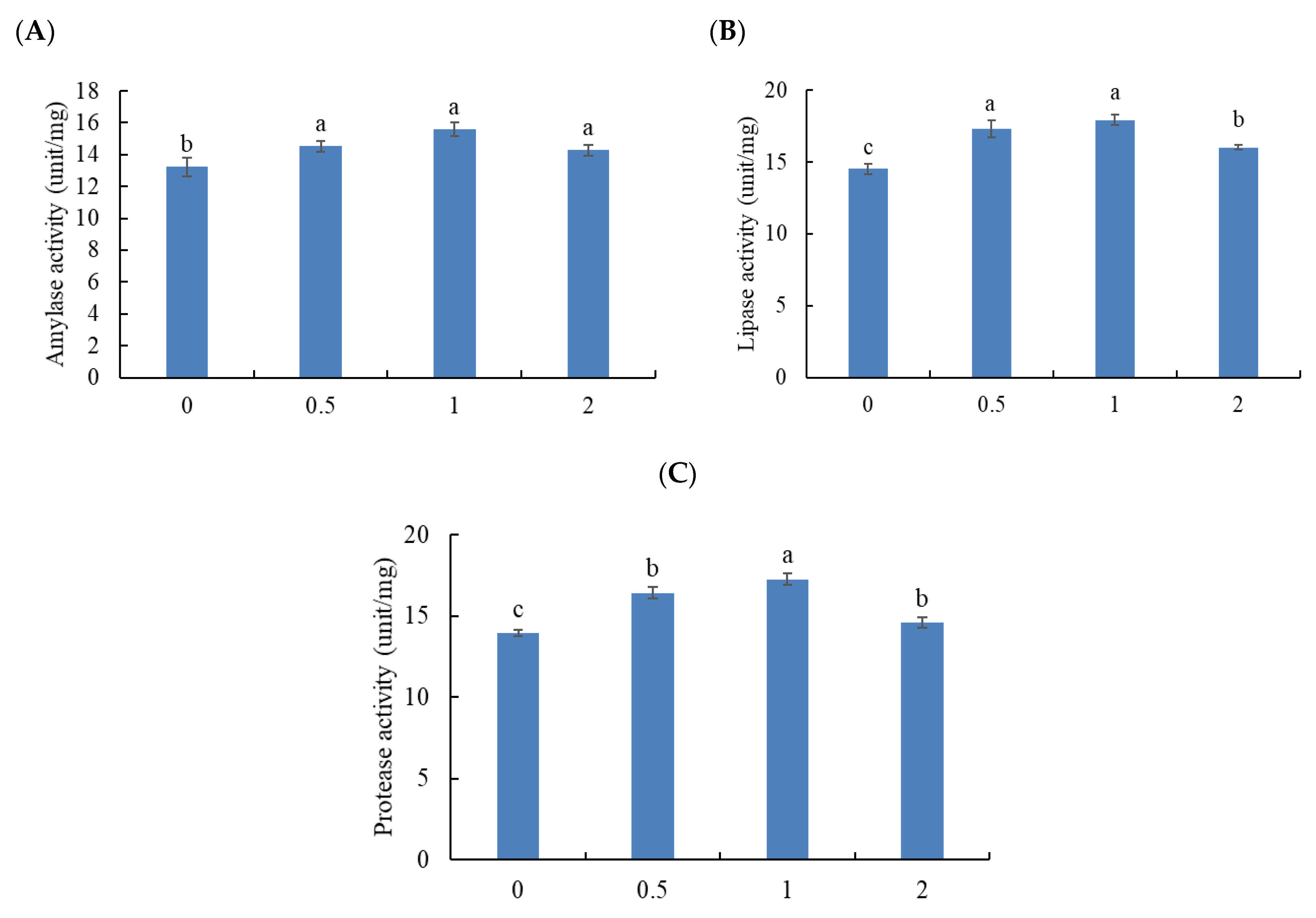
3.2. Digestive Enzyme Activity
3.3. Biochemical Blood Indices
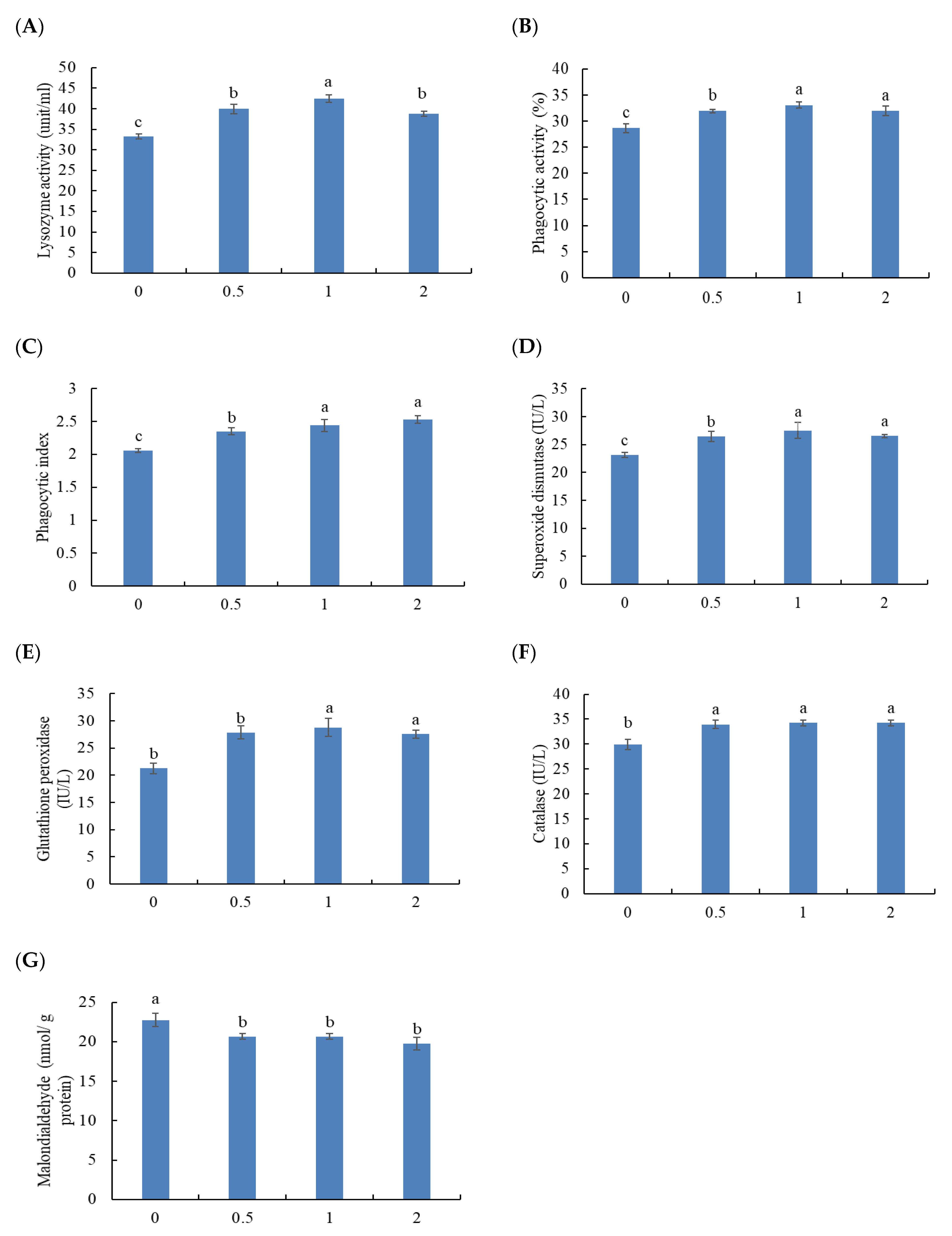
3.4. Immune Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The state of world fisheries and aquaculture. In Sustainability in Action Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2020. [Google Scholar]
- Adel, M.; Dawood, M.A.O. Probiotics application: Implications for sustainable aquaculture. In Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health; Mojgani, N., Dadar, M., Eds.; Microorganisms for Sustainability Series 2; Springer Publishing: New York, NY, USA, 2021; pp. 191–219. [Google Scholar]
- Besbes, R.; Benseddik, A.B.; Kokokiris, L.; Changeux, T.; Hamza, A.; Kammoun, F.; Missaoui, H. Thicklip (Chelon labrosus) and flathead (Mugil cephalus) grey mullets fry production in tunisian aquaculture. Aquac. Rep. 2020, 17, 100380. [Google Scholar] [CrossRef]
- Froese, R.J. Fish Base Online. Available online: www.fishbase.org (accessed on 1 May 2021).
- El-Bokhty, E.-A.; Amin, A. Current status of Liza ramada (risso, 1810) (mugilidae) caught by trammel net (ballah) at El-gamil region, Manzala lake, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 281–308. [Google Scholar] [CrossRef] [Green Version]
- Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A.O. Yucca schidigera usage for healthy aquatic animals: Potential roles for sustainability. Animals 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Won, S.; Choi, W.; Lim, S.-G.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Bai, S.C. Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 2020, 525, 735333. [Google Scholar] [CrossRef]
- Ringø, E.; Song, S.K. Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24. [Google Scholar] [CrossRef]
- Soltani, M.; Ghosh, K.; Hoseinifar, S.H.; Kumar, V.; Lymbery, A.J.; Roy, S.; Ringø, E. Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev. Fish. Sci. Aquac. 2019, 27, 331–379. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Cavalcante, R.B.; Telli, G.S.; Tachibana, L.; de Carla Dias, D.; Oshiro, E.; Natori, M.M.; da Silva, W.F.; Ranzani-Paiva, M.J. Probiotics, prebiotics and synbiotics for Nile tilapia: Growth performance and protection against Aeromonas hydrophila infection. Aquac. Rep. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Mesbah, M. Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: An experimental infection with Aeromonas hydrophila. Aquaculture 2019, 511, 634197. [Google Scholar]
- Sohn, K.; Kim, M.; Kim, J.; Han, I.K. The role of immunostimulants in monogastric animal and fish-review. Asian-Australas. J. Anim. Sci. 2000, 13, 1178–1187. [Google Scholar] [CrossRef]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.E.; Gifstad, T.Ø.; Dalmo, R.A.; Amlund, H.; Hemre, G.I.; Bakke, A.M. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Gültepe, N.; Salnur, S.; HoŞSu, B.; Hisar, O. Dietary supplementation with mannanoligosaccharides (MOS) from Bio-MOS enhances growth parameters and digestive capacity of gilthead sea bream (Sparus aurata). Aquac. Nutr. 2011, 17, 482–487. [Google Scholar] [CrossRef]
- Hisano, H.; Soares, M.P.; Luiggi, F.G.; Arena, A.C. Dietary β-glucans and mannanoligosaccharides improve growth performance and intestinal morphology of juvenile pacu Piaractus mesopotamicus (holmberg, 1887). Aquac. Int. 2018, 26, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Wang, S.; Cai, Y.; Wu, Y.; Tian, L.; Wang, S.; Jiang, L.; Guo, W.; Sun, Y.; Zhou, Y. Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, non-specific immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Aquaculture 2020, 523, 735195. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; GinÉS, R.; Sweetman, J.; Izquierdo, M. Improved feed utilization, intestinal mucus production and immune parameters in sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Aquac. Nutr. 2011, 17, 223–233. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Sewid, A.H.; Nada, H.S.; Kamel, M.A.; El-Mandrawy, S.A.M.; Abdelhakim, T.M.N.; El-Murr, A.E.I.; Nahhas, N.E.; Hozzein, W.N.; Ibrahim, D. Mannanoligosaccharides as a carbon source in biofloc boost dietary plant protein and water quality, growth, immunity and Aeromonas hydrophila resistance in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-Y.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Mannan oligosaccharides improved growth performance and antioxidant capacity in the intestine of on-growing grass carp (Ctenopharyngodon idella). Aquac. Rep. 2020, 17, 100313. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Fadl, S.E.; Ahmed, H.A.; El Asely, A.; Abdel-Daim, M.M.; Alkahtani, S. The modulatory effect of mannanoligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquac. Rep. 2020, 16, 100278. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Montero, D.; Izquierdo, M. Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish Shellfish Immunol. 2014, 36, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Gewaily, M.S.; Soliman, A.A.; Shukry, M.; Amer, A.A.; Younis, E.M.; Abdel-Warith, A.-W.A.; Van Doan, H.; Saad, A.H.; Aboubakr, M.; et al. Marine-derived chitosan nanoparticles improved the intestinal histo-morphometrical features in association with the health and immune response of grey mullet (Liza ramada). Mar. Drugs 2020, 18, 611. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; AOAC: Washington, DC, USA, 1998. [Google Scholar]
- NRC. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993; 114p. [Google Scholar]
- Zampacavallo, G.; Parisi, G.; Mecatti, M.; Lupi, P.; Giorgi, G.; Poli, B.M. Evaluation of different methods of stunning/killing sea bass (Dicentrarchus labrax) by tissue stress/quality indicators. J. Food Sci. Technol. 2015, 52, 2585–2597. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C. Activity Measuring for Implemental Enzyme; Science and Technology Press: Shanghai, China, 1982. [Google Scholar]
- Worthington, V. Worthington Enzyme Manual: Enzymes and Related Biochemicals Worthingthon Chemical; Worthington Biochemical Corporation: Freehold, NJ, USA, 1993; p. 399. [Google Scholar]
- Borlongan, I.G. Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 1990, 89, 315–325. [Google Scholar] [CrossRef]
- Jin, Z. The Avaluation Principle and Method of Functional Food; Beijing Publishers: Beijing, China, 1995. [Google Scholar]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Dumas, B.T.; Biggs, H.G. Standard Methods of Clinical Chemistry; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Cai, W.-Q.; Li, S.-F.; Ma, J.-Y. Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia×male blue tilapia) to Aeromonas sobria. Aquaculture 2004, 229, 79–87. [Google Scholar] [CrossRef]
- Kawahara, E.; Ueda, T.; Nomura, S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Ellis, A.; Stolen, J.; Fletcher, T.; Anderson, D.; Robertson, B.; Van Muiswinkel, W. Lysozyme assay. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Marín-Rincón, A.; Martínez-Rodríguez, G.; Ruiz-Jarabo, I.; Mancera, J.M. A natural additive in the diet to improve growth and reduce energy expenditure of gilthead seabream (Sparus aurata L.): Attenuation of high stocking density stress responses. Aquaculture 2020, 524, 735263. [Google Scholar] [CrossRef]
- Rossi, W.; Allen, K.M.; Habte-Tsion, H.-M.; Meesala, K.-M. Supplementation of glycine, prebiotic, and nucleotides in soybean meal-based diets for largemouth bass (Micropterus salmoides): Effects on production performance, whole-body nutrient composition and retention, and intestinal histopathology. Aquaculture 2021, 532, 736031. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Chai, Y.H.; Marsh, T.L.; Nor, S.A.M. Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquaculture 2016, 460, 59–68. [Google Scholar] [CrossRef]
- Piccolo, G.; Centoducati, G.; Marono, S.; Bovera, F.; Tudisco, R.; Nizza, A. Effects of the partial substitution of fish meal by soy bean meal with or without mannanoligosaccharide and fructooligosaccharide on the growth and feed utilization of sharpsnout seabream, Diplodus puntazzo (cetti, 1777): Preliminary results. Ital. J. Anim. Sci. 2011, 10, e37. [Google Scholar] [CrossRef] [Green Version]
- Dimitroglou, A.; Merrifield, D.L.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Leclercq, E.; Pontefract, N.; Rawling, M.; Valdenegro, V.; Aasum, E.; Andujar, L.V.; Migaud, H.; Castex, M.; Merrifield, D. Dietary supplementation with a specific mannan-rich yeast parietal fraction enhances the gut and skin mucosal barriers of Atlantic salmon (Salmo salar) and reduces its susceptibility to sea lice (Lepeophtheirus salmonis). Aquaculture 2020, 529, 735701. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.-C.; Buentello, J.A.; Gatlin, D.M. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture 2010, 309, 253–257. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.A.M.; Król, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.N.; Sutriana, A.; Talpur, A.D.; Hashim, R. Dietary supplementation with mannan oligosaccharide influences growth, digestive enzymes, gut morphology, and microbiota in juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Int. 2016, 24, 127–144. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Yu, Y.-M.; Chen, X.; Liu, H.; Yuan, J.-F.; Shi, Y.; Chen, X.-X. Effect of prebiotic konjac mannanoligosaccharide on growth performances, intestinal microflora, and digestive enzyme activities in yellow catfish, Pelteobagrus fulvidraco. Fish Physiol. Biochem. 2014, 40, 763–771. [Google Scholar] [CrossRef]
- Casanovas, P.; Walker, S.P.; Johnston, H.; Johnston, C.; Symonds, J.E. Comparative assessment of blood biochemistry and haematology normal ranges between chinook salmon (Oncorhynchus tshawytscha) from seawater and freshwater farms. Aquaculture 2021, 537, 736464. [Google Scholar] [CrossRef]
- Bao, J.-W.; Qiang, J.; Tao, Y.-F.; Li, H.-X.; He, J.; Xu, P.; Chen, D.-J. Responses of blood biochemistry, fatty acid composition and expression of micrornas to heat stress in genetically improved farmed tilapia (Oreochromis niloticus). J. Therm. Biol. 2018, 73, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef]
- Manera, M.; Britti, D. Assessment of blood chemistry normal ranges in rainbow trout. J. Fish Biol. 2006, 69, 1427–1434. [Google Scholar] [CrossRef]
- Yuji-Sado, R.; Raulino-Domanski, F.; de Freitas, P.F.; Baioco-Sales, F. Growth, immune status and intestinal morphology of nile tilapia fed dietary prebiotics (mannan oligosaccharides-mos). Lat. Am. J. Aquat. Res. 2015, 43, 944–952. [Google Scholar] [CrossRef]
- Dotta, G.; de Andrade, J.I.A.; Tavares Gonçalves, E.L.; Brum, A.; Mattos, J.J.; Maraschin, M.; Martins, M.L. Leukocyte phagocytosis and lysozyme activity in Nile tilapia fed supplemented diet with natural extracts of propolis and aloe barbadensis. Fish Shellfish Immunol. 2014, 39, 280–284. [Google Scholar] [CrossRef]
- Luo, C.; Gwekwe, B.; Choto, P.; Miao, W.; Chen, M.; Xue, C.; Xu, Y.; Yin, X.; Magawa, G.; Wu, D.; et al. Bitter peptides from enzymatically hydrolyzed protein increase the number of leucocytes and lysozyme activity of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2018, 81, 130–134. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Angulo, C. Yarrowia lipolytica n6-glucan protects goat leukocytes against Escherichia coli by enhancing phagocytosis and immune signaling pathway genes. Microb. Pathog. 2021, 150, 104735. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Qi, C.; Limbu, S.M.; Han, F.; Yang, L.; Wang, X.; Qin, J.G.; Chen, L. Dietary mannan oligosaccharide (MOS) improves growth performance, antioxidant capacity, non-specific immunity and intestinal histology of juvenile chinese mitten crabs (Eriocheir sinensis). Aquaculture 2019, 510, 337–346. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Forchino, A.; Rimoldi, S.; Brambilla, F.; Antonini, M.; Saroglia, M. Bio-mos®: An effective inducer of dicentracin gene expression in European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 372–377. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | % | Chemical Composition | % |
|---|---|---|---|
| Fish meal | 15 | Crude protein | 34.49 |
| Soybean meal | 40 | Total lipids | 6.29 |
| Yellow corn | 15 | Ash | 7.55 |
| Gluten | 7 | Crude fibers | 5.12 |
| Wheat bran | 12 | Nitrogen free extract | 46.55 |
| Wheat flour | 4.92 | Gross energy (KJ/g) 2 | 18.63 |
| Fish oil | 3 | ||
| Vitamin and mineral mix 1 | 2 | ||
| Dicalcium phosphate | 1 | ||
| Vitamin C | 0.08 |
| Item | 0.0% | 0.5% | 1.0% | 2.0% |
|---|---|---|---|---|
| IBW (g) | 5.13 ± 0.10 | 5.11 ± 0.02 | 5.13 ± 0.04 | 5.16 ± 0.02 |
| FBW (g) | 20.91 ± 0.31 b | 23.93 ± 0.37 a | 23.62 ± 0.53 a | 22.64 ± 0.12 a |
| WG (%) | 307.68 ± 10.03 c | 368.26 ± 6.77 a | 360.10 ± 7.67 a | 339.26 ± 4.26 b |
| SGR (%/day) | 2.34 ± 0.04 c | 2.57 ± 0.02 a | 2.54 ± 0.03 a | 2.47 ± 0.02 b |
| FI (g/fish) | 22.67 ± 1.84 | 22.89 ± 0.02 | 22.42 ± 0.44 | 21.64 ± 0.02 |
| FCR | 1.43 ± 0.10 a | 1.22 ± 0.02 b | 1.21 ± 0.03 b | 1.24 ± 0.01 b |
| PER | 2.32 ± 0.15 c | 2.71 ± 0.05 a | 2.75 ± 0.09 a | 2.66 ± 0.03 b |
| PG | 131.60 ± 5.00 b | 143.73 ± 1.15 a | 143.39 ± 2.51 a | 143.92 ± 1.53 a |
| PR | 19.49 ± 2.17 b | 20.71 ± 0.18 a | 21.36 ± 0.61 a | 21.87 ± 0.24 a |
| Survival (%) | 97.78 ± 2.22 | 100.00 ± 0.00 | 100.00 ± 0.00 | 97.78 ± 2.22 |
| Item | Initial Body Composition | 0.0% | 0.5% | 1.0% | 2.0% |
|---|---|---|---|---|---|
| Moisture | 80.21 ± 0.55 | 78.18 ± 0.55 | 76.97 ± 0.41 | 77.08 ± 0.13 | 76.75 ± 0.12 |
| Crude protein | 12.12 ± 0.38 | 13.42 ± 0.38 | 14.34 ± 0.09 | 14.31 ± 0.04 | 14.35 ± 0.12 |
| Total lipid | 3.83 ± 0.05 | 4.13 ± 0.05 | 4.61 ± 0.03 | 4.84 ± 0.14 | 4.88 ± 0.13 |
| Ash | 3.35 ± 0.09 | 3.69 ± 0.09 | 3.56 ± 0.06 | 3.60 ± 0.14 | 3.94 ± 0.09 |
| Item | 0.0% | 0.5% | 1.0% | 2.0% |
|---|---|---|---|---|
| ALT (U/I) | 3.35 ± 0.12 | 3.25 ± 0.13 | 3.25 ± 0.08 | 3.27 ± 0.24 |
| AST (U/I) | 74.81 ± 1.72 | 74.07 ± 1.15 | 73.82 ± 1.32 | 74.92 ± 1.46 |
| Total protein (g/dl) | 4.13 ± 0.12 c | 4.46 ± 0.21 a | 4.55 ± 0.18 a | 4.30 ± 0.11 b |
| Albumin (g/dl) | 2.17 ± 0.08 c | 2.53 ± 0.11 a | 2.43 ± 0.13 a | 2.37 ± 0.14 b |
| Globulin (g/dl) | 1.96 ± 0.09 b | 1.93 ± 0.05 b | 2.12 ± 0.08 a | 1.93 ± 0.04 b |
| Creatinine (mg/dl) | 0.27 ± 0.02 | 0.25 ± 0.01 | 0.24 ± 0.02 | 0.23 ± 0.01 |
| Urea (mg/dl) | 4.87 ± 0.21 | 4.71 ± 0.11 | 4.61 ± 0.12 | 4.52 ± 0.21 |
| Total cholesterol (mg/dl) | 87.33 ± 2.72 | 92.00 ± 2.15 | 97.00 ± 2.69 | 90.18 ± 2.66 |
| Triglycerides (mg/dl) | 133.83 ± 3.24 | 145.33 ± 4.88 | 142.00 ± 4.23 | 136.67 ± 3.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magouz, F.I.; Bassuini, M.I.; Khalafalla, M.M.; Abbas, R.; Sewilam, H.; Aboelenin, S.M.; Soliman, M.M.; Amer, A.A.; Soliman, A.A.; Van Doan, H.; et al. Mannan Oligosaccharide Enhanced the Growth Rate, Digestive Enzyme Activity, Carcass Composition, and Blood Chemistry of Thinlip Grey Mullet (Liza ramada). Animals 2021, 11, 3559. https://doi.org/10.3390/ani11123559
Magouz FI, Bassuini MI, Khalafalla MM, Abbas R, Sewilam H, Aboelenin SM, Soliman MM, Amer AA, Soliman AA, Van Doan H, et al. Mannan Oligosaccharide Enhanced the Growth Rate, Digestive Enzyme Activity, Carcass Composition, and Blood Chemistry of Thinlip Grey Mullet (Liza ramada). Animals. 2021; 11(12):3559. https://doi.org/10.3390/ani11123559
Chicago/Turabian StyleMagouz, Fawzy I., Mohamed I. Bassuini, Malik M. Khalafalla, Ramy Abbas, Hani Sewilam, Salama Mostafa Aboelenin, Mohamed Mohamed Soliman, Asem A. Amer, Ali A. Soliman, Hien Van Doan, and et al. 2021. "Mannan Oligosaccharide Enhanced the Growth Rate, Digestive Enzyme Activity, Carcass Composition, and Blood Chemistry of Thinlip Grey Mullet (Liza ramada)" Animals 11, no. 12: 3559. https://doi.org/10.3390/ani11123559







