Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain, Mus spretus and Apodemus sylvaticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Collection
2.2. Histology and Immunohistological Methods
2.3. Apoptosis
2.4. Serum Testosterone Levels
2.5. Blood-Testis Barrier Permeability Assay
2.6. Morphometrics and Statistics
3. Results
3.1. Sexually Inactive Males Undergo Testis Regression in Apodemus sylvaticus but Not in Mus spretus
3.2. Meiosis Is Arrested in the Inactive Testes of A. sylvaticus but Not in Those of M. spretus
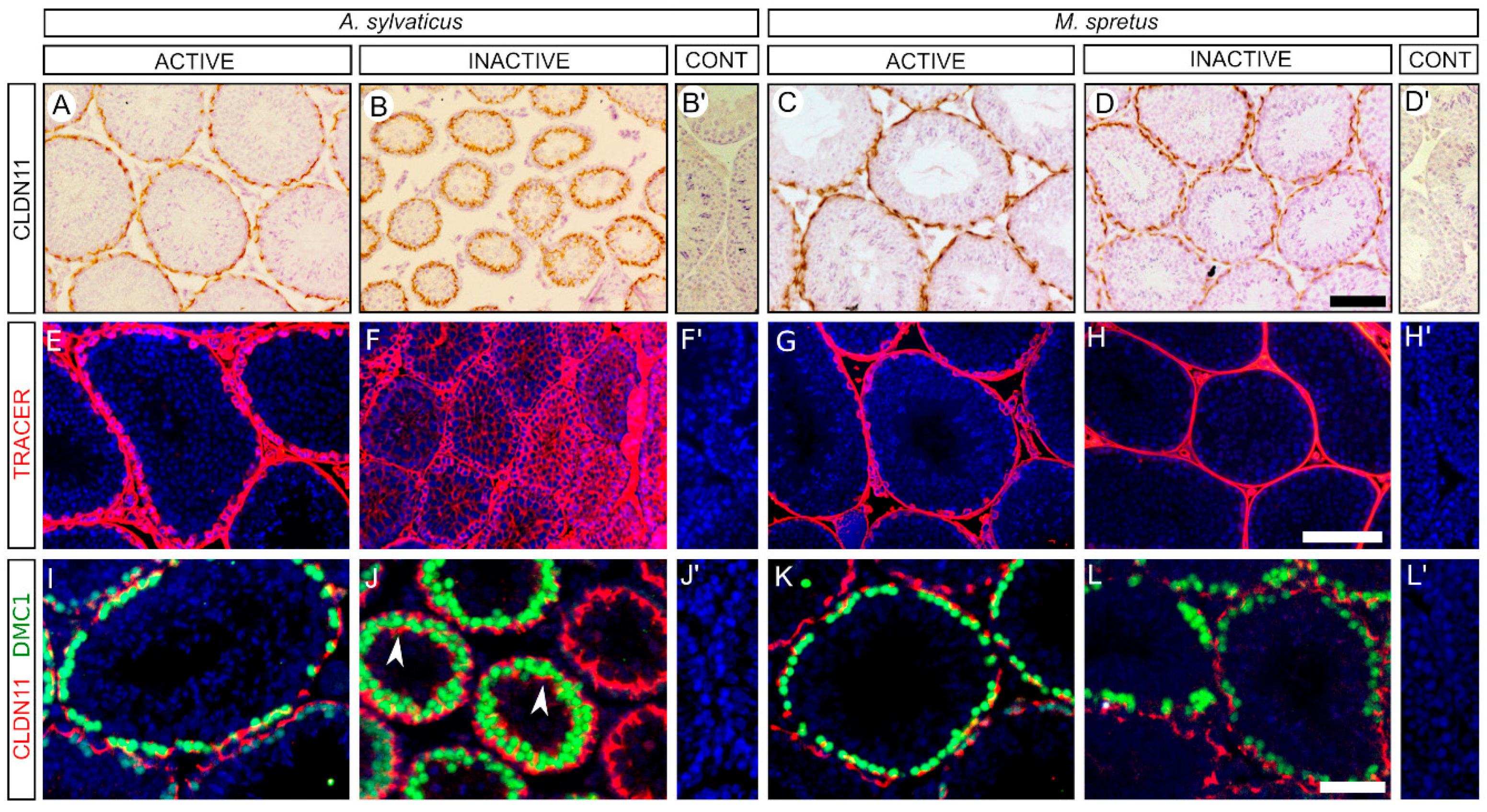
3.3. The Blood Testis Barrier Is Impaired in the Inactive Testes of A. sylvaticus but Not in Those of M. spretus
3.4. Androgen Levels Are Reduced in the Inactive Males of Both A. sylvaticus and M. spretus
3.5. Increased Apoptosis in Inactive Testes of A. sylvaticus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, R.; Burgos, M.; Barrionuevo, F.J. Circannual Testis Changes in Seasonally Breeding Mammals. SXD 2015, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Nelson, R.J. Mediation of Seasonal Testicular Regression by Apoptosis. Reproduction 2001, 122, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Blottner, S.; Hingst, O.; Meyer, H.H.D. Seasonal Spermatogenesis and Testosterone Production in Roe Deer (Capreolus capreolus). Reproduction 1996, 108, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štrbenc, M.; Fazarinc, G.; Bavdek, S.V.; Pogačnik, A. Apoptosis and Proliferation during Seasonal Testis Regression in the Brown Hare (Lepus europaeus L.). Anat. Histol. Embryol. 2003, 32, 48–53. [Google Scholar] [CrossRef]
- Pelletier, R.-M. The Blood-Testis Barrier: The Junctional Permeability, the Proteins and the Lipids. Prog. Histochem. Cytochem. 2011, 46, 49–127. [Google Scholar] [CrossRef]
- Pawlicki, P.; Milon, A.; Zarzycka, M.; Galas, J.; Tworzydlo, W.; Kaminska, A.; Pardyak, L.; Lesniak, K.; Pacwa, A.; Bilinska, B. Does Signaling of Estrogen-Related Receptors Affect Structure and Function of Bank Vole Leydig Cells. J. Physiol. Pharmacol. 2017, 68, 459–476. [Google Scholar]
- Milon, A.; Pawlicki, P.; Rak, A.; Mlyczynska, E.; Płachno, B.J.; Tworzydlo, W.; Gorowska-Wojtowicz, E.; Bilinska, B.; Kotula-Balak, M. Telocytes Are Localized to Testis of the Bank Vole (Myodes glareolus) and Are Affected by Lighting Conditions and G-Coupled Membrane Estrogen Receptor (GPER) Signaling. Gen. Comp. Endocrinol. 2019, 271, 39–48. [Google Scholar] [CrossRef]
- Profaska-Szymik, M.; Galuszka, A.; Korzekwa, A.J.; Hejmej, A.; Gorowska-Wojtowicz, E.; Pawlicki, P.; Kotula-Balak, M.; Tarasiuk, K.; Tuz, R. Implication of Membrane Androgen Receptor (ZIP9) in Cell Senescence in Regressed Testes of the Bank Vole. Int. J. Mol. Sci. 2020, 21, 6888. [Google Scholar] [CrossRef]
- Dadhich, R.K.; Real, F.M.; Zurita, F.; Barrionuevo, F.J.; Burgos, M.; Jiménez, R. Role of Apoptosis and Cell Proliferation in the Testicular Dynamics of Seasonal Breeding Mammals: A Study in the Iberian Mole, Talpa occidentalis1. Biol. Reprod. 2010, 83, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Dadhich, R.K.; Barrionuevo, F.J.; Real, F.M.; Lupiañez, D.G.; Ortega, E.; Burgos, M.; Jiménez, R. Identification of Live Germ-Cell Desquamation as a Major Mechanism of Seasonal Testis Regression in Mammals: A Study in the Iberian Mole (Talpa occidentalis). Biol. Reprod. 2013, 88, 1–12. [Google Scholar] [CrossRef]
- Luaces, J.P.; Rossi, L.F.; Sciurano, R.B.; Rebuzzini, P.; Merico, V.; Zuccotti, M.; Merani, M.S.; Garagna, S. Loss of Sertoli-Germ Cell Adhesion Determines the Rapid Germ Cell Elimination during the Seasonal Regression of the Seminiferous Epithelium of the Large Hairy Armadillo Chaetophractus villosus. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Merico, V.; Luaces, J.P.; Rossi, L.F.; Rebuzzini, P.; Merani, M.S.; Zuccotti, M.; Garagna, S. Sertoli–Immature Spermatids Disengagement during Testis Regression in the Armadillo. Reproduction 2019, 157, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoud, D.; Barrionuevo, F.J.; Ortega, E.; Burgos, M.; Jiménez, R. The Testis of Greater White-Toothed Shrew Crocidura russula in Southern European Populations: A Case of Adaptive Lack of Seasonal Involution? J. Exp. Zool. (Mol. Dev. Evol.) 2014, 322, 304–315. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; Isla, M.L.M.; Vitullo, A.D. The Balance between Apoptosis and Autophagy Regulates Testis Regression and Recrudescence in the Seasonal-Breeding South American Plains Vizcacha, Lagostomus maximus. PLoS ONE 2018, 13, e0191126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoud, D.; Lao-Pérez, M.; Hurtado, A.; Abdo, W.; Palomino-Morales, R.; Carmona, F.D.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Germ Cell Desquamation-Based Testis Regression in a Seasonal Breeder, the Egyptian Long-Eared Hedgehog, Hemiechinus auritus. PLoS ONE 2018, 13, e0204851. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Hernández, J.; Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Canteras, M.; del Mar Sánchez-Huertas, M.; Pastor, L.M. Testicular Histomorphometry and the Proliferative and Apoptotic Activities of the Seminiferous Epithelium in Syrian Hamster during Spontaneous Recrudescence after Exposure to Short Photoperiod. Reprod. Domest. Anim. 2018, 53, 1041–1051. [Google Scholar] [CrossRef]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Sánchez-Huertas, M.M.; Madrid, J.F.; Saez, F.J.; Pastor, L.M. Lectin Histochemistry as a Tool to Identify Apoptotic Cells in the Seminiferous Epithelium of Syrian Hamster (Mesocricetus auratus) Subjected to Short Photoperiod. Reprod. Domest. Anim. 2013, 48, 974–983. [Google Scholar] [CrossRef]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Saez, F.J.; Madrid, J.F.; Canteras, M.; Pastor, L.M. Testicular Histomorphometry and the Proliferative and Apoptotic Activities of the Seminiferous Epithelium in Syrian Hamster (Mesocricetus auratus) during Regression Owing to Short Photoperiod. Andrology 2015, 3, 598–610. [Google Scholar] [CrossRef]
- Martínez-Hernández, J.; Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Serrano-Sánchez, M.I.; Pastor, L.M. Proliferation, Apoptosis, and Number of Sertoli Cells in the Syrian Hamster during Recrudescence after Exposure to Short Photoperiod. Biol. Reprod. 2020, 102, 588–597. [Google Scholar] [CrossRef]
- Pastor, L.M.; Zuasti, A.; Ferrer, C.; Bernal-Mañas, C.M.; Morales, E.; Beltrán-Frutos, E.; Seco-Rovira, V. Proliferation and Apoptosis in Aged and Photoregressed Mammalian Seminiferous Epithelium, with Particular Attention to Rodents and Humans. Reprod. Domest. Anim. 2011, 46, 155–164. [Google Scholar] [CrossRef]
- Nelson, R.J.; Gubernick, D.J.; Blom, J.M.C. Influence of Photoperiod, Green Food, and Water Availability on Reproduction in Male California Mice (Peromyscus californicus). Physiol. Behav. 1995, 57, 1175–1180. [Google Scholar] [CrossRef]
- Martin, G.B.; Tjondronegoro, S.; Blackberry, M.A. Effects of Nutrition on Testicular Size and the Concentrations of Gonadotrophins, Testosterone and Inhibin in Plasma of Mature Male Sheep. Reproduction 1994, 101, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple, J.L. The Musk Shrew (Suncus murinus): A Model Species for Studies of Nutritional Regulation of Reproduction. ILAR J. 2004, 45, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, R.; Burgos, M.; Sánchez, A.; Diaz De La Guardia, R. The Reproductive Cycle of Talpa occidentalis in the Southeastern Iberian Peninsula. Acta. Theriol. 1990, 35, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Fuster, M.J.L. The Natural Communities of Small Mammals (Insectivores and Rodents) of Catalonia (Spain). Miscellània Zoològica 1985, 9, 375–387. [Google Scholar]
- Sánchez-González, B.; Navarro-Castilla, Á.; Hernández González, M.; Barja, I. Ratón de Campo–Apodemus sylvaticus (Linnaeus, 1758). In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2016. [Google Scholar]
- Rood, J.P. Observations on the Life Cycle and Variation of the Long-Tailed Field Mouse Apodemus sylvaticus on the Isles of Scilly and Cornwall. Proc. Zool. Soc. Lond. 1965, 147, 99–107. [Google Scholar] [CrossRef]
- Moreno, S.; Kufner, M.B. Seasonal Patterns in the Wood Mouse Population in Mediterranean Scrubland. Acta Theriol. 1988, 33, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Niethammer, J.; Krapp, F.F. Handbuch Der Säugetiere Europas. Nagetiere, I., Band 1 Wiesbaden; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1978. [Google Scholar]
- Palomo, L.J.; Justo, E.R.; Vargas, J.M. Mus spretus (Rodentia: Muridae). Mamm. Species 2009, 840, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vargas, J.M.; Palomo, L.J.; Palmqvist, P. Reproduction of the Algerian Mouse (Mus spretus Lataste, 1883) in the South of the Iberian Peninsula. Bonner Zool. Beiträge 1991, 42, 1–10. [Google Scholar]
- R Core Team (2020)—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team (accessed on 10 October 2020).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 1.0. 2 (2020). Available online: https://CRAN.R-project.org/package=dplyr (accessed on 10 October 2020).
- Fröjdman, K.; Harley, V.R.; Pelliniemi, L.J. Sox9 Protein in Rat Sertoli Cells Is Age and Stage Dependent. Histochem. Cell Biol. 2000, 113, 31–36. [Google Scholar] [CrossRef]
- Dadhich, R.K.; Barrionuevo, F.J.; Lupiañez, D.G.; Real, F.M.; Burgos, M.; Jiménez, R. Expression of Genes Controlling Testicular Development in Adult Testis of the Seasonally Breeding Iberian Mole. Sex. Dev. 2011, 5, 77–88. [Google Scholar] [CrossRef]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a Gene Related to Worm and Fly Sexual Regulators, Is Required for Mammalian Testis Differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Kondoh, G.; Matsuda, Y.; Habu, T.; Nishimune, Y.; Morita, T. The Mouse RecA-like Gene Dmc1 Is Required for Homologous Chromosome Synapsis during Meiosis. Mol. Cell 1998, 1, 707–718. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. CNS Myelin and Sertoli Cell Tight Junction Strands Are Absent in Osp/Claudin-11 Null Mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.R. The Reproductive Biology of the Bank Vole (Clethrionomys glareolus) and the Wood Mouse (Apodemus sylvaticus). Symp. Zool. Soc. Lond. 1985, 55, 33–59. [Google Scholar]
- Fons, R.; Saint Girons, M.C. Le Cycle Sexuel Chez Le Mulot Sylvestre, Apodemus sylvaticus (L., 1758), (Muridae) En Région Méditerranéenne. Z. Säugetierkd 1993, 58, 38–47. [Google Scholar]
- Bronson, F.H.; Heideman, P.D. Seasonal regulation of reproduction in mammals. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Bronson, F.H. Climate Change and Seasonal Reproduction in Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3331–3340. [Google Scholar] [CrossRef] [Green Version]
- Lynch, G.R.; Heath, H.W.; Johnston, C.M. Effect of Geographical Origin on the Photoperiodic Control of Reproduction in the White-Footed Mouse, Peromyscus leucopus. Biol. Reprod. 1981, 25, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Torre, I.; Arrizabalaga, A. Relative Roles of Density and Rainfall on the Short-Term Regulation of Mediterraneanwood Mouse Apodemus sylvaticus Populations. Acta Theriol. 2010, 55, 251–260. [Google Scholar] [CrossRef]
- Khidas, K.; Khammes, N.; Khelloufi, S.; Lek, S.; Aulagnier, S. Abundance of the Wood Mouse Apodemus Sylvaticus and the Algerian Mouse Mus spretus (Rodentia, Muridae) in Different Habitats of Northern Algeria. Mamm. Biol. 2002, 67, 34–41. [Google Scholar] [CrossRef]
- Palomo, L.J. Características de los desplazamientos del ratón moruno, Mus spretus Lataste, 1883, en cultivos de caña de azúcar de la provincia de Málaga. Ecología 1990, 4, 185–190. [Google Scholar]
- Hansson, L. The Food of Bank Voles, Wood Mice and Yellow-Necked Mice. Symp. Zool. Soc. London 1985, 55, 141–168. [Google Scholar]
- Castién, E.; Gosàlbez, J. Pequeños Mamíferos Forestales: Influencia de Las Actividades Forestales Sobre Las Comunidades de Insectívoros y Roedores. Conservación de la Biodiversidad y Gestión Forestall; Universidad de Barcelona: Barcelona, Spain, 2001; pp. 353–364. [Google Scholar]
- Hosken, D.J.; Stockley, P. Benefits of Polyandry: A Life History Perspective. In Evolutionary Biology; Macintyre, R.J., Clegg, M.T., Eds.; Springer: Boston, MA, USA, 2003; pp. 173–194. ISBN 978-1-4757-5190-1. [Google Scholar]
- Parapanov, R.N.; Nusslé, S.; Crausaz, M.; Senn, A.; Hausser, J.; Vogel, P. Testis Size, Sperm Characteristics and Testosterone Concentrations in Four Species of Shrews (Mammalia, Soricidae). Anim. Reprod. Sci. 2009, 114, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cassaing, J.; Croset, H. Organisation Spatiale, Competition et Dynamique Des Populations Sauvages de Souris (Mus spretus Lataste et Mus musculus domesticus Rutty) Du Midi de La France. Z. Säugetierkd. 1985, 50, 271–284. [Google Scholar]
- Oakberg, E.F. Duration of Spermatogenesis in the Mouse and Timing of Stages of the Cycle of the Seminiferous Epithelium. Am. J. Anat. 1956, 99, 507–516. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. An Intracellular Trafficking Pathway in the Seminiferous Epithelium Regulating Spermatogenesis: A Biochemical and Molecular Perspective. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 245–263. [Google Scholar] [CrossRef] [Green Version]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. A Local Autocrine Axis in the Testes That Regulates Spermatogenesis. Nat Rev. Endocrinol. 2010, 6, 380–395. [Google Scholar] [CrossRef] [Green Version]
- Kopera, I.A.; Bilinska, B.; Cheng, C.Y.; Mruk, D.D. Sertoli–Germ Cell Junctions in the Testis: A Review of Recent Data. Phil. Trans. Roy. Soc. B Biol. Sci. 2010, 365, 1593–1605. [Google Scholar] [CrossRef] [Green Version]
- Erkkilä, K.; Henriksén, K.; Hirvonen, V.; Rannikko, S.; Salo, J.; Parvinen, M.; Dunkel, L. Testosterone Regulates Apoptosis in Adult Human Seminiferous Tubules in Vitro. J. Clin. Endocrinol. Metab. 1997, 82, 2314–2321. [Google Scholar] [CrossRef]
- Nandi, S.; Banerjee, P.P.; Zirkin, B.R. Germ Cell Apoptosis in the Testes of Sprague Dawley Rats Following Testosterone Withdrawal by Ethane 1,2-Dimethanesulfonate Administration: Relationship to Fas? Biol. Reprod. 1999, 61, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiano, L.; Fustini, M.F.; Lovato, E.; Frasoldati, A.; Malorni, W.; Capri, M.; Grassilli, E.; Marrama, P.; Franceschi, C. Apoptosis and Spermatogenesis: Evidence from an in Vivo Model of Testosterone Withdrawal in the Adult Rat. Biochem. Biophys. Res. Comm. 1994, 202, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Greenlee, A.R.; Taub, C.J.; Braun, R.E. Sertoli Cell-Specific Deletion of the Androgen Receptor Compromises Testicular Immune Privilege in Mice. Biol. Reprod. 2011, 85, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gye, M.C. Changes in the Expression of Claudins and Transepithelial Electrical Resistance of Mouse Sertoli Cells by Leydig Cell Coculture. Int. J. Androl. 2003, 26, 271–278. [Google Scholar] [CrossRef]
- Florin, A.; Maire, M.; Bozec, A.; Hellani, A.; Chater, S.; Bars, R.; Chuzel, F.; Benahmed, M. Androgens and Postmeiotic Germ Cells Regulate Claudin-11 Expression in Rat Sertoli Cells. Endocrinology 2005, 146, 1532–1540. [Google Scholar] [CrossRef] [Green Version]
- Tu’uhevaha, J.; Sluka, P.; Foo, C.F.; Stanton, P.G. Claudin-11 Expression and Localisation Is Regulated by Androgens in Rat Sertoli Cells in Vitro. Reproduction 2007, 133, 1169–1179. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoud, D.; Lao-Pérez, M.; Ortega, E.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain, Mus spretus and Apodemus sylvaticus. Animals 2021, 11, 243. https://doi.org/10.3390/ani11020243
Massoud D, Lao-Pérez M, Ortega E, Burgos M, Jiménez R, Barrionuevo FJ. Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain, Mus spretus and Apodemus sylvaticus. Animals. 2021; 11(2):243. https://doi.org/10.3390/ani11020243
Chicago/Turabian StyleMassoud, Diaa, Miguel Lao-Pérez, Esperanza Ortega, Miguel Burgos, Rafael Jiménez, and Francisco J. Barrionuevo. 2021. "Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain, Mus spretus and Apodemus sylvaticus" Animals 11, no. 2: 243. https://doi.org/10.3390/ani11020243
APA StyleMassoud, D., Lao-Pérez, M., Ortega, E., Burgos, M., Jiménez, R., & Barrionuevo, F. J. (2021). Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain, Mus spretus and Apodemus sylvaticus. Animals, 11(2), 243. https://doi.org/10.3390/ani11020243








