Effects of Zinc and Menthol-Based Diets on Co-Selection of Antibiotic Resistance among E. coli and Enterococcus spp. in Beef Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Quantification, Isolation, Identification, Speciation
2.3. Phenotypic Susceptibility Testing
2.4. Statistical Analyses
3. Results
3.1. Bacterial Identification
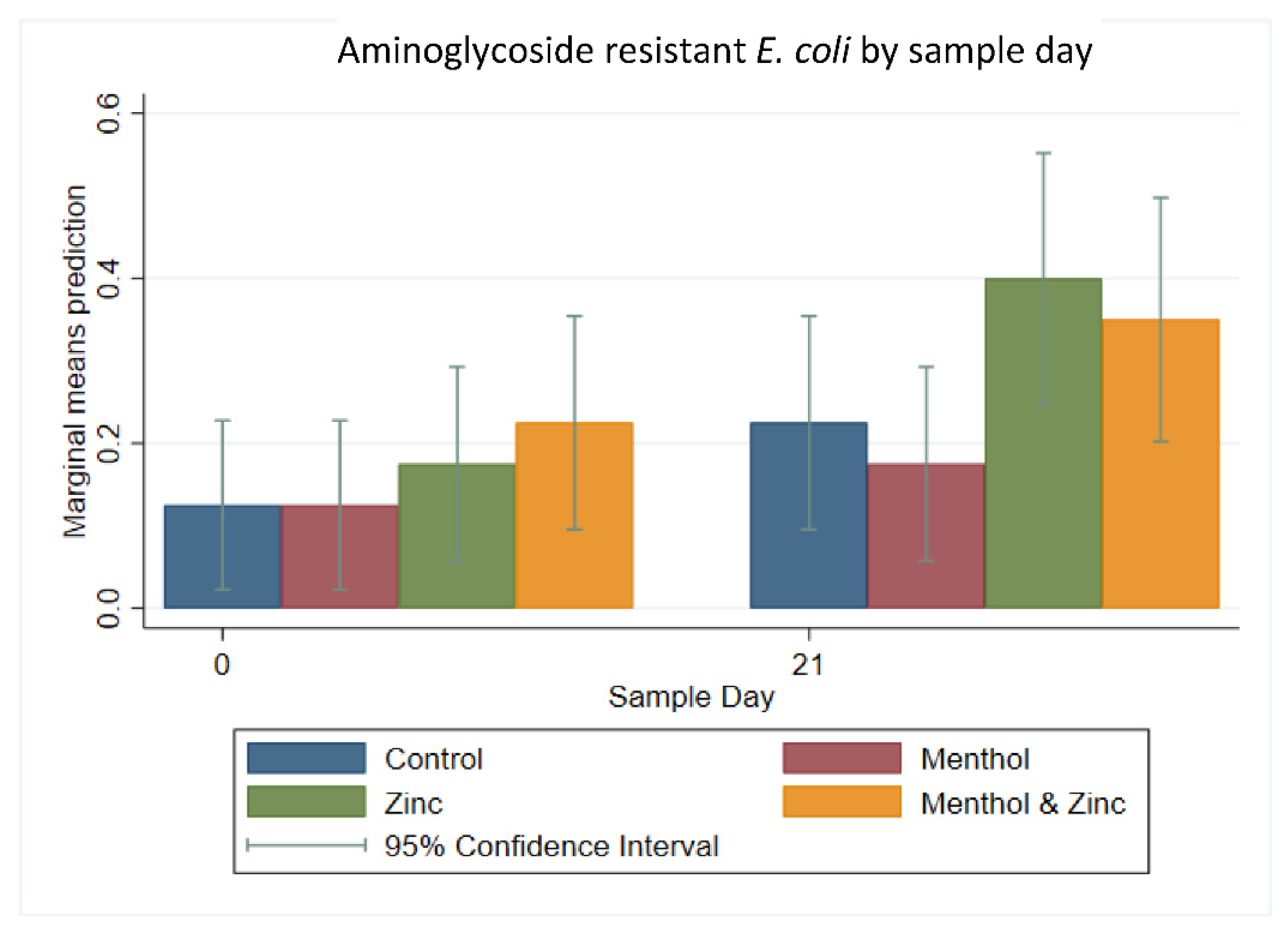
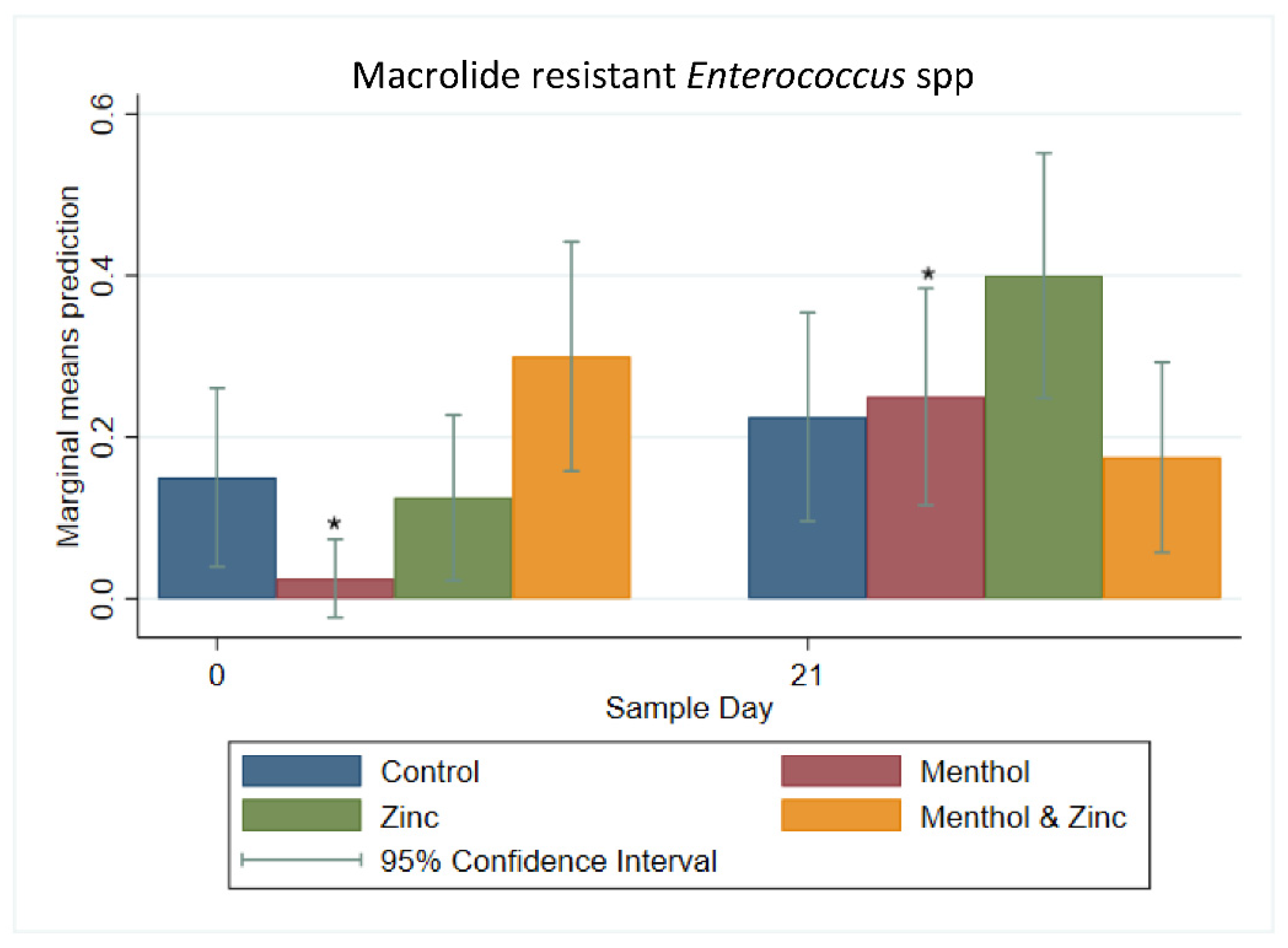
3.2. Multi-Level Mixed Effects Linear Regression Modeling of Plate Quantification
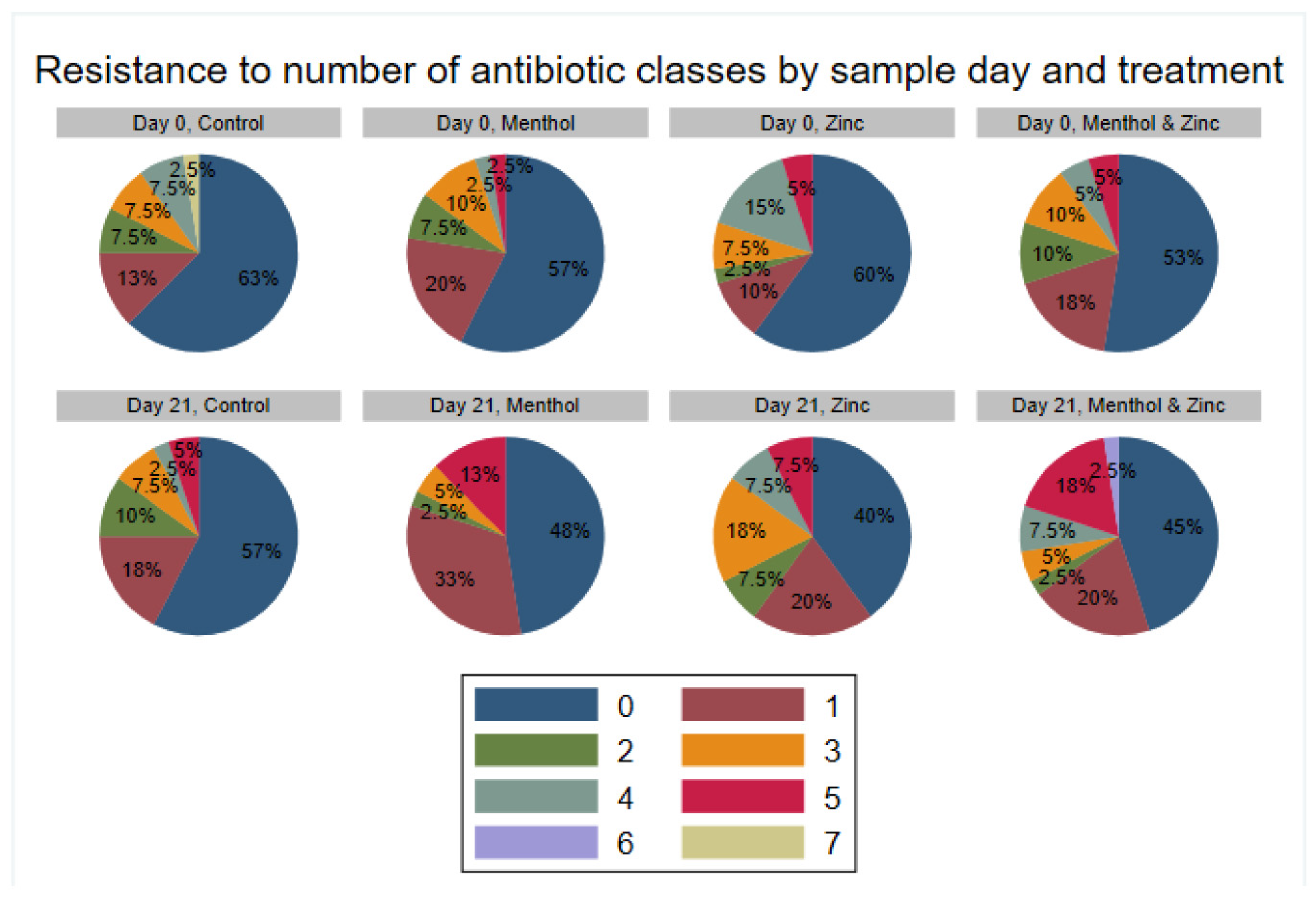
3.3. Descriptive Statistics of Phenotypic Resistance of Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poultry Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.R.; Evenson, A.; Luckey, T.D.; Mccoy, E.; Elvehjem, C.A.; Hart, E.B. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [CrossRef]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, R.H.; Bowen, R.E. Antibiotic use in animal agriculture. J. Appl. Microbiol. 1997, 83, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Stanton, T.B. A call for antibiotic alternatives research. Trends Microbiol. 2013, 21, 111–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, H.K.; Levine, U.Y.; Looft, T.; Bandrick, M.; Casey, T.A. Treatment, promotion, commotion: Antibiotic alternatives in food-producing animals. Trends Microbiol. 2013, 21, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F.V. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Verstegen, M.W.A.; Williams, B.A. Alternatives to the use of antibiotics as growth promoters for monogastric animals. Anim. Biotechnol. 2002, 13, 113–127. [Google Scholar] [CrossRef]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wales, A.D.; Davies, R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Højberg, O.; Canibe, N.; Poulsen, H.D.; Hedemann, M.S.; Jensen, B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl. Environ. Microbiol. 2005, 71, 2267–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabry, S.A.; Ghozlan, H.A.; Abou-Zeid, D.M. Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. J. Appl. Microbiol. 1997, 82, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.O. Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim. Biotechnol. 2006, 17, 125–135. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Tetaz, T.J.; Luke, R.K. Plasmid-controlled resistance to copper in Escherichia coli. J. Bacteriol. 1983, 154, 1263. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R.; Camakaris, J.; Lee, B.T.O.; Luke, R.K.J. Inducible plasmid-mediated copper resistance in Escherichia coli. Microbiology 1985, 131, 939–943. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.L.; Barrett, S.R.; Camakaris, J.; Lee, B.T.; Rouch, D.A. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 1995, 17, 1153–1166. [Google Scholar] [CrossRef]
- Hasman, H.; Aarestrup, F.M. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: Occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob. Agents Chemother. 2002, 46, 1410–1416. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, J.V.; Magnani, D.; Solioz, M. Measurement of cytoplasmic copper, silver, and gold with a lux biosensor shows copper and silver, but not gold, efflux by the CopA ATPase of Escherichia coli. FEBS Lett. 2003, 546, 391–394. [Google Scholar] [CrossRef] [Green Version]
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed—Development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25, 25862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarestrup, F.M.; Cavaco, L.; Hasman, H. Decreased susceptibility to zinc chloride is associated with methicillin resistant Staphylococcus aureus CC398 in Danish swine. Vet. Microbiol. 2010, 142, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.T.; van der Lelie, D.; Springael, D.; Römling, U.; Ahmed, N.; Mergeay, M. Identification of a gene cluster, czr, involved in cadmium and zinc resistance in Pseudomonas aeruginosa. Gene 1999, 238, 417–425. [Google Scholar] [CrossRef]
- Amachawadi, R.G.; Scott, H.M.; Nitikanchana, S.; Vinasco, J.; Tokach, M.D.; Dritz, S.S.; Nelssen, J.L.; Goodband, R.D.; Nagaraja, T.G. Nasal carriage of mecA-positive methicillin-resistant Staphylococcus aureus in pigs exhibits dose-response to zinc supplementation. Foodborne Pathog. Dis. 2015, 12, 159–163. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Ocak, N.; Sivri, F. Liver colourations as well as performance and digestive tract characteristics of broilers may change as influenced by stage and schedule of feed restriction. J. Anim. Physiol. Anim. Nutr. 2008, 92, 546–553. [Google Scholar] [CrossRef]
- Cargill. Essential Oils key to Cargill’s Comprehensive Approach to Reducing Antibiotics in Poultry. Miser, E., Ed.; 2016. Available online: https://www.cargill.com/news/releases/2016/NA31925706.jsp (accessed on 20 January 2021).
- Aperce, C.C.; Amachawadi, R.; Van Bibber-Krueger, C.L.; Nagaraja, T.G.; Scott, H.M.; Vinasco-Torre, J.; Drouillard, J.S. Effects of menthol supplementation in feedlot cattle diets on the fecal prevalence of antimicrobial-resistant Escherichia coli. PLoS ONE 2016, 11, e0168983. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.F.; Erickson, G.E.; Klopfenstein, T.J.; Greenquist, M.A.; Luebbe, M.K.; Williams, P.; Engstrom, M.A. Effect of essential oils, tylosin, and monensin on finishing steer performance, carcass characteristics, liver abscesses, ruminal fermentation, and digestibility. J. Anim. Sci. 2009, 87, 2346–2354. [Google Scholar] [CrossRef] [Green Version]
- Weissend, C.J.; Holzer, K.H.; Huebner, K.L.; Metcalf, J.L.; Geornaras, I.; Parker, J.K.; Belk, K.E.; Morley, P.S.; Martin, J.N. The effect of tylosin supplementation and tylosin alternative control treatments on fecal microbial populations, performance, and liver abscess prevalence in feedlot cattle. J. Anim. Sci. 2017, 95, 130–131. [Google Scholar] [CrossRef] [Green Version]
- Amachawadi, R.G.; Scott, H.M.; Aperce, C.; Vinasco, J.; Drouillard, J.S.; Nagaraja, T.G. Effects of in-feed copper and tylosin supplementations on copper and antimicrobial resistance in faecal enterococci of feedlot cattle. J. Appl. Microbiol. 2015, 118, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Volume 30. [Google Scholar]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmati, S.; Yang, S.; Davidson, A.L.; Zechiedrich, E.L. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 2002, 43, 677–685. [Google Scholar] [CrossRef]
- Hölzel, C.S.; Müller, C.; Harms, K.S.; Mikolajewski, S.; Schäfer, S.; Schwaiger, K.; Bauer, J. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ. Res. 2012, 113, 21–27. [Google Scholar] [CrossRef]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef]
- Bednorz, C.; Oelgeschläger, K.; Kinnemann, B.; Hartmann, S.; Neumann, K.; Pieper, R.; Bethe, A.; Semmler, T.; Tedin, K.; Schierack, P.; et al. The broader context of antibiotic resistance: Zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int. J. Med. Microbiol. 2013, 303, 396–403. [Google Scholar] [CrossRef]
- Ghazisaeedi, F.; Ciesinski, L.; Bednorz, C.; Johanns, V.; Pieper, L.; Tedin, K.; Wieler, L.H.; Günther, S. Phenotypic zinc resistance does not correlate with antimicrobial multi-resistance in fecal E. coli isolates of piglets. Gut Pathogens. 2020, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Shapiro, S.; Meier, A.; Guggenheim, B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral Microbiol. Immunol. 1994, 9, 202–208. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Becerril, R.; Nerín, C.; Gómez-Lus, R. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathog. Dis. 2012, 9, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.E.; Fox, J.T.; Nagaraja, T.G.; Drouillard, J.S.; Amachawadi, R.G.; Narayanan, S.K. Effects of feeding elevated concentrations of copper and zinc on the antimicrobial susceptibilities of fecal bacteria in feedlot cattle. Foodborne Pathog. Dis. 2010, 7, 643–648. [Google Scholar] [CrossRef] [PubMed]









| Antibiotic | Class | Range | Breakpoint |
|---|---|---|---|
| Gentamicin | Aminoglycoside | 128–1024 | ≥500 |
| Kanamycin | Aminoglycoside | 128–1024 | ≥1024 |
| Streptomycin | Aminoglycoside | 512–2048 | >1000 |
| Vancomycin | Glycopeptide | 0.25–32 | ≥32 |
| Tigecycline | Glycylcycline | 0.015–0.5 | ≥0.5 |
| Lincomycin | Lincosamide | 1–8 | ≥8 |
| Daptomycin | Lipopeptide | 0.25–16 | ≥8 |
| Erythromycin | Macrolide | 0.25–8 | ≥8 |
| Tylosin | Macrolide | 0.25–32 | ≥32 |
| Nitrofurantoin | Nitrofuran | 2–64 | ≥128 |
| Linezolid | Oxazolidinone | 0.5–8 | ≥8 |
| Penicillin | Penicillin | 0.25–16 | ≥16 |
| Chloramphenicol | Phenicol | 2–32 | ≥32 |
| Ciprofloxacin | Quinolone | 0.12–4 | ≥4 |
| Quinupristin/dalfopristin | Streptogramin | 0.5–32 | ≥4 |
| Tetracycline | Tetracycline | 1–32 | ≥16 |
| Antibiotic | Class | Range | Breakpoint |
|---|---|---|---|
| Gentamicin | Aminoglycoside | 0.25–16 | ≥16 |
| Streptomycin | Aminoglycoside | 2–64 | ≥64 |
| Cefoxitin | Cephem | 0.5–32 | ≥32 |
| Ceftiofur | Cephem | 0.12–8 | ≥32 |
| Ceftriaxone | Cephem | 0.25–64 | >4 |
| Sulfisoxazole | Folate Pathway Inhibitor | 16–256 | ≥512 |
| Trimethoprim/sulfamethoxazole | Folate Pathway Inhibitor | 0.12/2.38–4/76 | ≥4/76 |
| Azithromycin | Macrolide | 0.12–16 | ≥32 |
| Ampicillin | Penicillin | 1–32 | ≥32 |
| Chloramphenicol | Phenicol | 2–32 | ≥32 |
| Ciprofloxacin | Quinolone | 0.015–4 | ≥1 |
| Nalidixic Acid | Quinolone | 0.5–32 | ≥32 |
| Tetracycline | Tetracycline | 4–32 | ≥16 |
| Amoxicillin/clavulanic acid | β-Lactam/β-Lactamase inhibitor combination | 1/0.5–32/16 | ≥32/16 |
| Antibiotic | Number Resistant (of 320 Tested) | % Resistant | 95% Confidence Interval | MIC in µg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.02 | 0.03 | 0.06 | 0.13 | 0.25 | 0.50 | 1.00 | 2.00 | 4.00 | 8.00 | 16.00 | 32.00 | 64.00 | 128.00 | 256.00 | |||||
| Amoxicillin/Clavulanic Acid | 3.00 | 0.94 | 0.19 | 2.72 | 4.69 | 18.75 | 60.94 | 14.38 | 0.31 | 0.31 | 0.63 | ||||||||
| Ampicillin | 42.00 | 13.30 | 9.63 | 17.32 | 5.31 | 42.50 | 37.50 | 1.56 | 0.00 | 13.13 | |||||||||
| Azithromycin | 2.00 | 0.63 | 0.08 | 2.24 | 0.00 | 0.00 | 0.94 | 0.94 | 8.13 | 78.75 | 10.31 | 0.31 | 0.63 | ||||||
| Cefoxitin | 3.00 | 0.94 | 0.19 | 2.72 | 0.00 | 0.31 | 7.81 | 61.56 | 28.13 | 1.25 | 0.31 | 0.63 | |||||||
| Ceftiofur | 3.00 | 0.94 | 0.19 | 2.72 | 2.81 | 21.25 | 73.75 | 0.94 | 0.31 | 0.00 | 0.63 | 0.31 | |||||||
| Ceftriaxone | 3.00 | 0.94 | 0.19 | 2.72 | 98.13 | 0.94 | 0.00 | 0.00 | 0.31 | 0.00 | 0.31 | 0.00 | 0.31 | ||||||
| Chloramphenicol | 48.00 | 15.00 | 11.27 | 19.39 | 2.50 | 46.25 | 35.63 | 0.63 | 0.63 | 14.37 | |||||||||
| Ciprofloxacin | 0.00 | 0.00 | 0.00 | 1.14 * | 95.94 | 2.50 | 0.63 | 0.31 | 0.63 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| Gentamicin | 1.00 | 0.31 | 0.01 | 1.73 | 0.94 | 75.31 | 23.44 | 0.00 | 0.00 | 0.00 | 0.31 | ||||||||
| Nalidixic Acid | 3.00 | 0.94 | 0.19 | 2.72 | 0.31 | 4.69 | 76.25 | 17.81 | 0.00 | 0.00 | 0.31 | 0.63 | |||||||
| Streptomycin | 73.00 | 22.84 | 18.33 | 27.81 | 0.00 | 16.56 | 50.94 | 4.06 | 5.63 | 11.56 | 11.25 | ||||||||
| Sulfisoxazole | 67.00 | 20.94 | 16.61 | 25.81 | 73.13 | 5.00 | 0.63 | 0.31 | 20.94 | ||||||||||
| Tetracycline | 148.00 | 46.25 | 40.69 | 51.88 | 44.38 | 9.38 | 2.50 | 4.06 | 39.69 | ||||||||||
| Trimethoprim/sulfamethoxazole | 2.00 | 0.63 | 0.08 | 2.24 | 87.81 | 7.50 | 3.75 | 0.31 | 0.00 | 0.63 | |||||||||
| Antibiotic | Number Resistant (of 320 Tested) | % Resistant | 95% Confidence Interval | MIC in µg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.02 | 0.03 | 0.06 | 0.13 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |||||
| Chloramphenicol | 2.00 | 0.63 | 0.08 | 2.24 | 0.31 | 13.80 | 80.00 | 5.31 | 0.63 | ||||||||||
| Ciprofloxacin | 18.00 | 5.63 | 3.37 | 8.74 | 0.31 | 17.80 | 43.40 | 32.80 | 4.69 | 0.94 | |||||||||
| Daptomycin | 28.00 | 8.75 | 5.89 | 12.40 | 1.25 | 0.31 | 2.50 | 20.60 | 66.60 | 8.75 | 0.00 | ||||||||
| Erythromycin | 57.00 | 17.81 | 13.80 | 22.50 | 50.30 | 7.81 | 2.50 | 8.13 | 13.40 | 3.75 | 14.10 | ||||||||
| Gentamicin | 0.00 | 0.00 | 0.00 | 1.15 * | 100.00 | 0.00 | |||||||||||||
| Kanamycin | 3.00 | 0.94 | 0.19 | 2.72 | 91.90 | 6.56 | |||||||||||||
| Lincomycin | 292.00 | 91.25 | 87.60 | 94.10 | 8.44 | 0.31 | 0.00 | 5.31 | 85.90 | ||||||||||
| Linezolid | 1.00 | 0.31 | 0.01 | 1.73 | 0.31 | 6.88 | 68.10 | 24.40 | 0.31 | ||||||||||
| Nitrofurantoin | 52.00 | 16.25 | 12.40 | 20.80 | 0.00 | 0.00 | 2.19 | 2.18 | 16.30 | 62.50 | 16.30 | ||||||||
| Penicillin | 1.00 | 0.31 | 0.01 | 1.73 | 2.50 | 7.19 | 12.20 | 15.60 | 54.40 | 7.81 | 0.31 | ||||||||
| Streptomycin | 3.00 | 0.94 | 0.19 | 2.72 | |||||||||||||||
| Quinupristin/Dalfopristin | 106.00 | 33.13 | 28.00 | 38.60 | 10.00 | 1.56 | 55.30 | 32.80 | 0.31 | 0.00 | 0.00 | ||||||||
| Tetracycline | 106.00 | 33.13 | 28.00 | 38.60 | 64.10 | 1.25 | 0.00 | 1.56 | 2.50 | 3.75 | 26.90 | ||||||||
| Tigecycline | 0.00 | 0.00 | 0.00 | 1.15 * | 0.00 | 11.30 | 61.90 | 26.90 | 0.00 | 0.00 | |||||||||
| Tylosin | 59.00 | 18.44 | 14.30 | 23.10 | 0.00 | 0.00 | 1.25 | 16.90 | 20.30 | 37.50 | 5.63 | 0.94 | 17.50 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, S.A.; Amachawadi, R.G.; Norman, K.N.; Lawhon, S.D.; Nagaraja, T.G.; Drouillard, J.S.; Scott, H.M. Effects of Zinc and Menthol-Based Diets on Co-Selection of Antibiotic Resistance among E. coli and Enterococcus spp. in Beef Cattle. Animals 2021, 11, 259. https://doi.org/10.3390/ani11020259
Murray SA, Amachawadi RG, Norman KN, Lawhon SD, Nagaraja TG, Drouillard JS, Scott HM. Effects of Zinc and Menthol-Based Diets on Co-Selection of Antibiotic Resistance among E. coli and Enterococcus spp. in Beef Cattle. Animals. 2021; 11(2):259. https://doi.org/10.3390/ani11020259
Chicago/Turabian StyleMurray, Sarah A., Raghavendra G. Amachawadi, Keri N. Norman, Sara D. Lawhon, Tiruvoor G. Nagaraja, James S. Drouillard, and Harvey M. Scott. 2021. "Effects of Zinc and Menthol-Based Diets on Co-Selection of Antibiotic Resistance among E. coli and Enterococcus spp. in Beef Cattle" Animals 11, no. 2: 259. https://doi.org/10.3390/ani11020259
APA StyleMurray, S. A., Amachawadi, R. G., Norman, K. N., Lawhon, S. D., Nagaraja, T. G., Drouillard, J. S., & Scott, H. M. (2021). Effects of Zinc and Menthol-Based Diets on Co-Selection of Antibiotic Resistance among E. coli and Enterococcus spp. in Beef Cattle. Animals, 11(2), 259. https://doi.org/10.3390/ani11020259







