Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Exercise Training and Exercise Test
2.3. Sample Collection
2.4. DNA Isolation
2.5. Real-Time PCR Analysis
2.6. Data Analysis
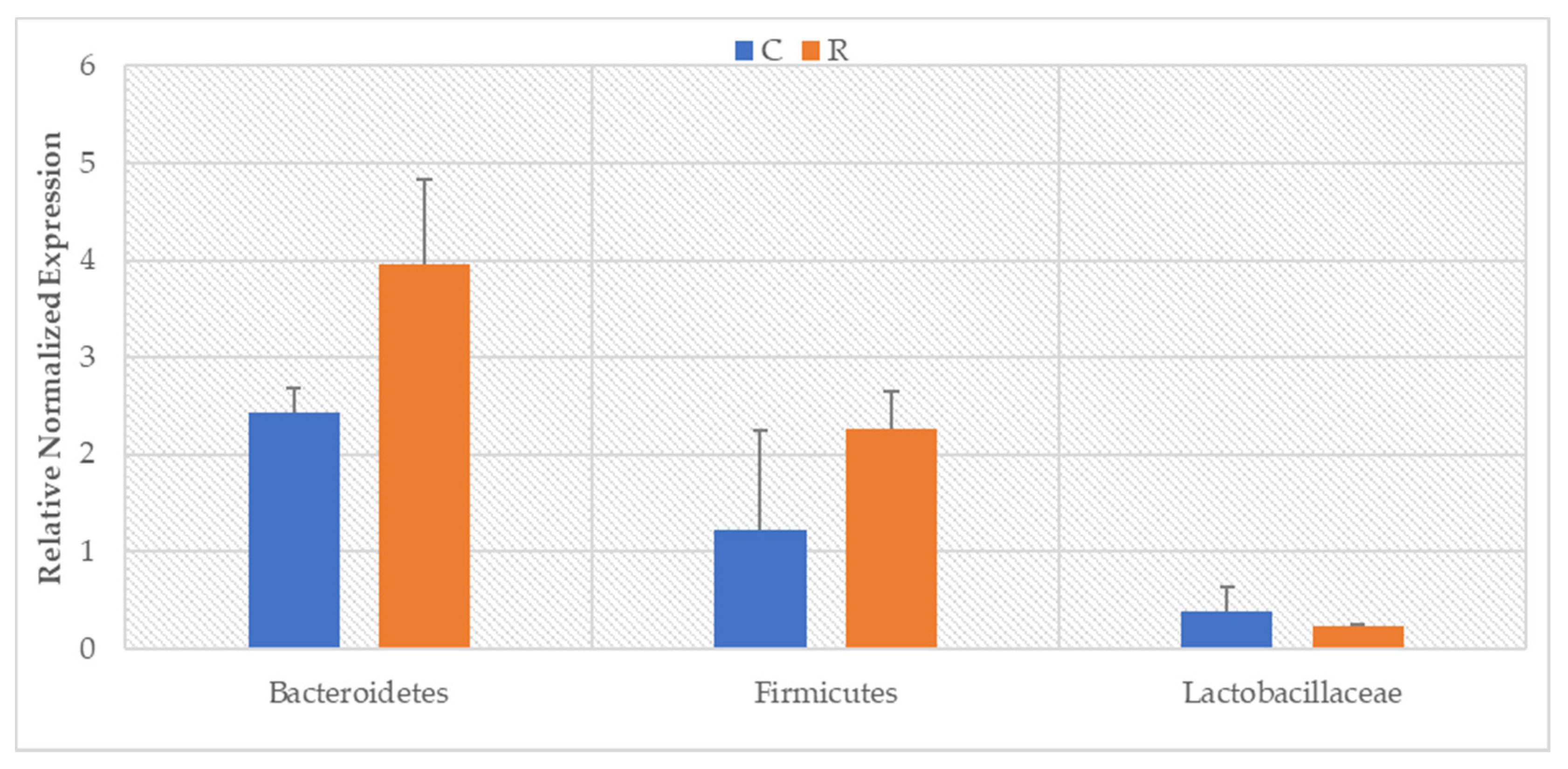
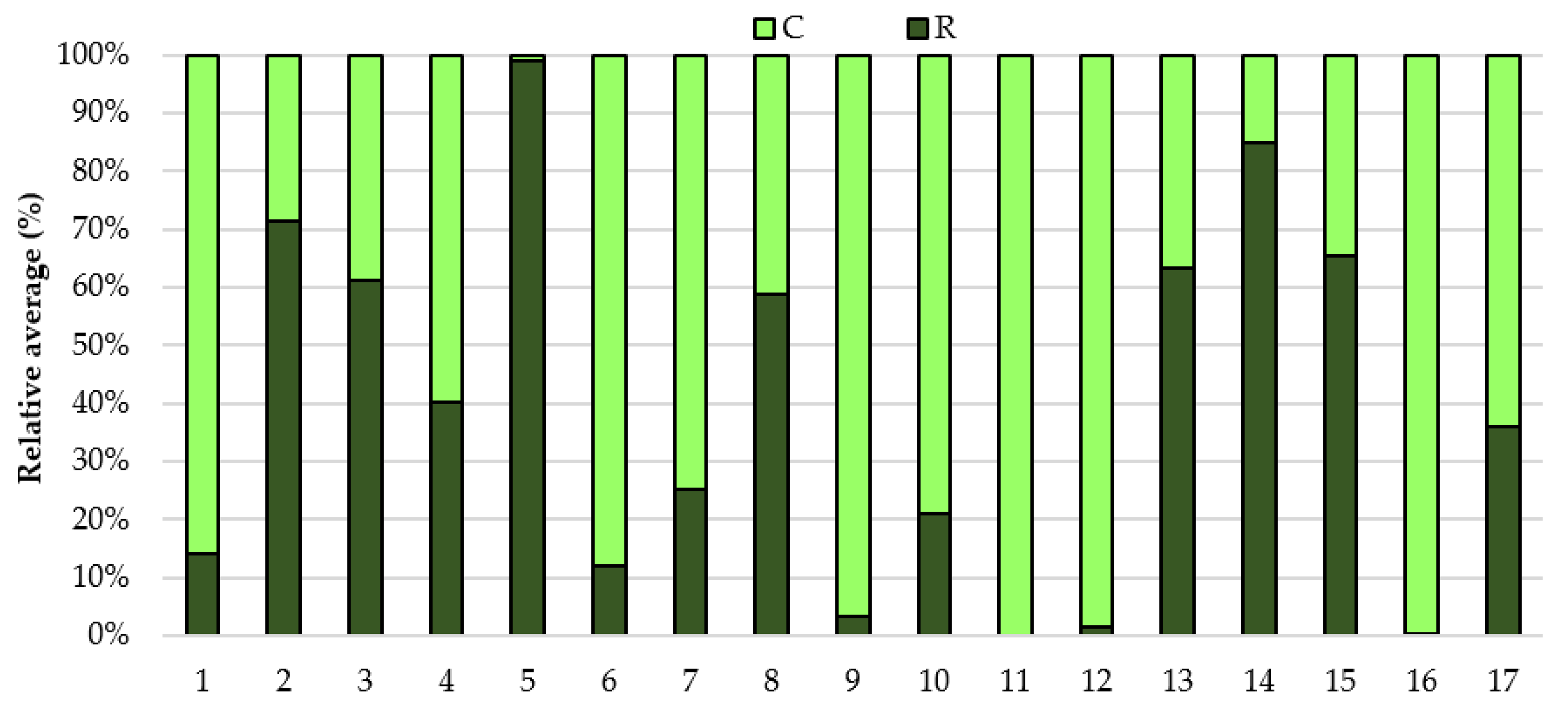
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.-M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lübke-Becker, A.; Günther, S.; Wieler, L.H.; et al. The gut microbiome of horses: Current research on equine enteral microbiota and future perspectives. Anim. Microbiome 2019, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.C.; Weese, J.S. Understanding the intestinal microbiome in health and disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef]
- Cholewińska, P.; Czyż, K.; Nowakowski, P.; Wyrostek, A. The microbiome of the digestive system of ruminants—A review. Anim. Health Res. Rev. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.L.; Song, Y.; Guan, L.L. The Role of the Gut Microbiome in Cattle Production and Health: Driver or Passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stampfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3–V5 region of the 16S rRNA gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, M.; Hoskin, S.O.; Rogers, C.W.; Grinberg, A. Fecal pH and microbial populations in thoroughbred horses during transition from pasture to concentrate feeding. J. Equine Vet. Sci. 2013, 33, 215–222. [Google Scholar] [CrossRef]
- Warzecha, C.A.; Coverdale, J.A.; Janecka, J.E.; Leatherwood, J.L.; Pinchak, W.E.; Wickersham, T.A.; McCann, J.C. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 2017, 95, 5077–5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatter, M.; Borewicz, K.; van den Bogert, B.; Wensch-Dorendorf, M.; Bochnia, M.; Greef, J.M.; Bachmann, M.; Smidt, H.; Breves, G.; Zeyner, A. Modification of the equine gastrointestinal microbiota by Jerusalem artichoke meal supplementation. PLoS ONE 2019, 14, e0220553. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.C.; Stämpfli, H.R.; Arroyo, L.G.; Allen-Vercoe, E.; Gomes, R.G.; Weese, J.S. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 2015, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Gronvold, A.M.R.; Trine, M.L.; Sorum, H.; Skancke, E.; Yannarell, A.C.; Mackie, R.I. Changes in fecal microbiota of healthy dogs administered amoxicillin. FEMS Microbiol. Ecol. 2010, 71, 313–326. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H.; Lehnhard, R.A. Physiology of acid base balance and fluid shifts with exercise. In The Athletic Horse, Principles and Practice of Equine Sports Medicine, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.T., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 69–87. [Google Scholar]
- Rowell, L.B. Cardiovascular adjustments to thermal stress. In Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow; Shepherd, J.T., Abboud, F.M., Geiger, S.R., Eds.; American Physiology Society: Bethesda, MD, USA, 1983; Volume 11, pp. 967–1023. [Google Scholar]
- Janabi, A.H.D.; Biddle, A.S.; Klein, D.J.; McKeever, K.H. The effects of acute strenuous exercise on the faecal microbiota in Standardbred racehorses. Comp. Exerc. Physiol. 2017, 13, 13–24. [Google Scholar] [CrossRef]
- Smith-Slatas, C.L.; Bourque, M.; Salazar, J.C. Clostridium septicum infections in children: A case report and review of the literature. Pediatrics 2006, 117, e796–e805. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; de la Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Newbold, C.J. Identification of a Core bacterial community within the large intestine of the horse. PLoS ONE 2013, 8, e77660. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the FM in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Potera, C. Running interference? Exercise and PCB-induced changes in the fecal microbiome. Environ. Health Perspect. 2013, 121, A199. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. FM composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Santacruz, A.; Marcos, A.; Wärnberg, J.; Martí, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009, 17, 1906–1915. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.M.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.E.; Myslicki, J.P.; Bomhof, M.R.; Belke, D.D.; Shearer, J.; Reimer, R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 2015, 40, 1–4. [Google Scholar] [CrossRef]
- Mach, N.; Ruet, A.; Clark, A.; Bars-Cortina, D.; Ramayo-Caldas, Y.; Crisci, E.; Pennarun, S.; Dhorne-Pollet, S.; Foury, A.; Moisan, M.-P.; et al. Priming for welfare: Gut microbiota is associated with equitation conditions and behaviour in horse athletes. Sci. Rep. 2020, 10, 8311. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2018, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J.; Hunter, J.O.; Darby, A.C.; Escalona, E.E.; Batty, C.; Turner, C. Characterisation of the faecal metabolome and microbiome of Thoroughbred racehorses. Equine Vet. J. 2015, 47, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, M.L.M.; Júnior, F.W.H.; Carvalho, J.R.G.; Rodrigues, I.M.; Jordão, L.R.; Fonseca, M.G.; de Rezende, A.S.C.; Queiroz Neto, A.; Weese, J.S.; Costa, M.C.; et al. Intense exercise and aerobic conditioning associated with chromium or L-carnitine supplementation modified the fecal microbiota of fillies. PLoS ONE 2016, 11, e0167108. [Google Scholar] [CrossRef]
- Janabi, A.H.D.; Biddle, A.S.; Klein, D.; McKeever, K.H. Exercise training-induced changes in the gut microbiota of Standardbred racehorses. Comp. Exerc. Physiol. 2016, 12, 119–129. [Google Scholar] [CrossRef]
- Mach, N.; Foury, A.; Kittelmann, S.; Reigner, F.; Moroldo, M.; Ballester, M.; Esquerré, D.; Rivière, J.; Sallé, G.; Gérard, P.; et al. The effects of weaning methods on gut microbiota composition and horse physiology. Front. Physiol. 2017, 8, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gregoris, T.B.; Aldred, N.; Clare, A.S.; Burgess, J.G. Improvement of phylum-and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Meth. 2011, 86, 351–356. [Google Scholar] [CrossRef]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C. Does exercise alter immune function and respiratory infections? Pres. Counc. Phys. Fit. Sports Res. Dig. 2001, 3, 1–8. [Google Scholar]
- Davis, J.M.; Murphy, E.A.; Brown, A.S.; Carmichael, M.D.; Ghaffar, A.; Mayer, E.P. Effects of moderate exercise and oat â-glucan on innate immune function and susceptibility to respiratory infection. Am. J. Physiol. Regul Integr. Comp. Physiol. 2004, 286, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Escribano, B.M.; Castejón, F.M.; Vivo, R.; Santisteban, R.; Agûera, E.I.; Rubio, M.D. Effects of training on phagocytic and oxidative metabolism of peripheral neutrophils in horses exercised in the aerobic-anaerobic transition area. Vet. Res. Commun. 2005, 29, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Raidal, S.L.; Rose, R.J.; Love, D.N. Effects of training on resting peripheral blood and BAL-derived leucocyte function in horses. Equine Vet. J. 2001, 33, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Plancade, S.; Clark, A.; Philippe, C.; Helbling, J.C.; Moisan, M.P.; Esquerré, D.; Le Moyec, L.; Robert, C.; Barrey, E.; Mach, N. Unraveling the effects of the gut microbiota composition and function on horse endurance physiology. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise is more effective at altering fecal microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Auffret, M.D.; Stewart, R.D.; Dewhurst, R.J.; Duthie, C.-A.; Snelling, T.J.; Walker, A.W.; Freeman, T.C.; Watson, M.; Roehe, R. Identification of rumen microbial genes involved in pathways linked to appetite, growth and feed conversion efficiency in cattle. Front. Genet. 2019, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen microbiome from steers differing in feed efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Guan, L.L. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microb. 2017, 83, 1–16. [Google Scholar] [CrossRef] [Green Version]

| Ingredient | Content | Ingredient | Content |
|---|---|---|---|
| Digestible energy | 13.50 MJ/kg | P | 0.50% |
| Protein | 13.00% | K | 0.90% |
| Oil | 5.50% | Mg | 0.40% |
| Fibre | 9.00% | S | 0.30% |
| Ash | 6.00% | Mn | 75.00 mg/kg |
| Starch | 26.00% | Fe | 225.00 mg/kg |
| Sugar | 4.00% | Cu (sulphate) | 40 mg/kg |
| Ca | 0.90% | Zn | 130.00 mg/kg |
| Component | Volume per 10 μL Reaction |
|---|---|
| SsoAdvanced™ Universal SYBR® Green Supermix | 5 μL |
| Forward and reverse primers | 1 μL (0.8 μM) |
| DNA template | 2 μL (0.04–0.015 × 10−4) |
| Nuclease-free water | 2 μL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górniak, W.; Cholewińska, P.; Szeligowska, N.; Wołoszyńska, M.; Soroko, M.; Czyż, K. Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses. Animals 2021, 11, 290. https://doi.org/10.3390/ani11020290
Górniak W, Cholewińska P, Szeligowska N, Wołoszyńska M, Soroko M, Czyż K. Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses. Animals. 2021; 11(2):290. https://doi.org/10.3390/ani11020290
Chicago/Turabian StyleGórniak, Wanda, Paulina Cholewińska, Natalia Szeligowska, Magdalena Wołoszyńska, Maria Soroko, and Katarzyna Czyż. 2021. "Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses" Animals 11, no. 2: 290. https://doi.org/10.3390/ani11020290
APA StyleGórniak, W., Cholewińska, P., Szeligowska, N., Wołoszyńska, M., Soroko, M., & Czyż, K. (2021). Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses. Animals, 11(2), 290. https://doi.org/10.3390/ani11020290






