1. Introduction
The lack of availability of protein raw materials for feeding organic animals, especially pigs and poultry, is one of the main limitations encountered by this sector. The productive development, health status and welfare of the animals depend directly on the adequate supply of protein in the diet [
1]. Therefore, the search for protein alternatives that are sufficient both in quality and quantity is a priority for these producers.
Insects are a part of the natural diet of free-range fish and monogastric animals throughout the world [
2,
3]. Insects are one of the options which are being considered as an alternative to traditional protein sources (e.g., soybean meal), due to their high quality and quantity of their protein [
2,
3]. Insects contain between 30% and 70% of protein on a dry matter basis [
4] and have a high nutritive value being also rich in fats, minerals and vitamins [
3] In addition, the low competitiveness with human food [
5,
6] and the reduction in the environmental impact in terms of energy cost and use of arable area [
7] make insects a promising food source in a green production environment. However, currently the use of insects in bird feeding is currently not allowed in the European Community. Given the potential ecological advantages associated with a good acceptance between producers and consumers [
8], it seems likely that political legal frameworks may change in the near future, making it possible to use insect proteins in bird feeding; being a valuable potential also for organic farming [
9].
The initial period (30 days) is considered the most important one in broiler production, as growth and development occur at a very strong rate in this phase. In fact, in this first phase, the weight of the chicken increases the initial weight up to four times. This fact determines its subsequent growth [
5,
10]. That is why the contribution of nutrients of high biological and nutritional quality, such as insects, are key to the correct productive development of the chickens. Therefore, the inclusion of insects as a protein source for the feeding of chickens, is presented as an opportunity for organic poultry. However, further research is required to determine the benefits of using insects in chicken feeding.
Currently, there are significant knowledge gaps in the field of insect production, particularly in Europe, where insects are not considered a traditional food [
4,
11,
12]. Although from the point of view of the nutrient composition of the insects, they could be suitable for the nutrition of the birds [
4,
6,
7,
9], other aspects must be worked on, including reducing the costs of insect production [
7]. On the other hand, it is well known that, in monogastric animals, the composition and characteristics of the meat are strongly influenced by the food consumed. Therefore, the quality of the meat of animals that have consumed insects in the diet should be studied [
13].
During the past years, many consumers have been interested in the non-compositional aspects of meat related to quality, as well as the intrinsic characteristics of the animals (species, breeds), the geographical origin, the food received, the productive management or the post-mortem strategies [
14]. Thus, the demand for proper analytical methods for authenticity assessment of food products is currently increasing dramatically, representing one of the major concerns for different stakeholders. Strict controls both throughout the production and during the marketing process are required. In this sense, Near Infrared Spectroscopy (NIRS) is presented as a powerful tool. The basic idea of the application of NIR technology to solve authentication problems is based on the generation of the spectral footprint of food [
15].
Based on the foregoing, the present work aims to evaluate the effect of the inclusion of Calliphora sp. (CLM) on the productive performance of medium-slow-growing chickens raised in organic production systems during their first month of life, indeed physico-chemical quality of the meat of chickens that received different percentages of a supplement of CLM. Likewise, the ability of the NIRS to properly classify the carcass samples was performed, based on the percentage of inclusion of CLM was evaluated.
4. Discussion
This study provides new insights into the inclusion of insects in the diet of medium-slow growing chickens reared in free range conditions. The present work is the first carried out using
Calliphora sp. as part of the feeding in medium-slow growing chickens in organic systems. There is only one research on meat quality to evaluate the inclusion of insects as a meal for chickens raised under organic systems and fed with
Tenebrio molitor (TM) as an insect [
28]. The rest of the studies shown below have been carried out in industrial production (broiler).
The best results in the final weight (W5) were in the animals that had consumed a major amount of CLM, which may be related to the greater amount of protein and energy consumed by these animals. This is observed in
Table 4, where the consumption per animal and study period of these nutrients is shown. These results are in line with the finding of Teguia et al. [
29] who shows a lower weight from control group, while the chickens fed with the maggot meal diet obtained the highest weight. Although the studies are not completely comparable due to the different insects used, this study suggests that the use of insects could improve chickens’ weights at 30 days, to those that received diets containing traditional feeding ingredients.
The inclusion of CLM in chicken diets improve the chicken growth performance in terms of ADG. These results are in agreement with the literature [
24]. These authors found a significant improvement in ADG in chickens that had received insect meal compared to the control during the first period of life (1–30 days). In contrast, other authors [
30,
31,
32] did not found variations on ADG when insect meal was included in the diet of the animals, in terms of control. When the level of flour inclusion was considered, no significant differences were found between the total ADG of groups T3 and T4. These findings suggest that there is no proportionality between the degree of inclusion of CLM and the final weight or the ADG during the first month of life.
An increase in the FI of chickens that had included CLM in the feeding was shown. This agrees with the findings of Lachicha et al. [
33], who considers that when insects are offered as part of the bird’s diet, a very high intake occurs in the first weeks of life; but it stabilizes from the fourth week onwards. This indicates that when birds are in their natural habitat, they incorporate insects into their diet. Ballitoc and Sun [
34] justified an increase in the consumption of the chickens that received TM attributing it to the increase in palatability of these mixtures and relating it to the natural behavior of these animals when they are free. However, other authors [
35,
36] consider that the inclusion of more than 25% of fly larvae in the diet of chickens can cause a decrease in consumption as a consequence of darkening. It has been suggested that the optimal inclusion rate to avoid rejections depends on the age of the animals. Awoniyi et al. [
37] found better results when partially replacing fishmeal (25%) with worm meal, than when total replacement occurred in old broilers; while young broilers did not reduce their consumption when offered feed that had a total replacement of the protein source.
The FCR was improved in the chickens that had eaten a higher amount of CLM (T4). This can be related to the better protein quality of the CLM larva included in the feed (
Table 1). The chicks that received treatment T3 and T4 consumed a higher amount of protein compared to the control, which is responsible for the decrease of FCR and the increase in weight (
Table 4). Similar results were found by Ballitoc and Sun [
34], who showed a decreasing trend in FCR values of broiler fed on TM from 0% to 10% of inclusion in the diet. Different authors [
7,
33,
38,
39] have described improvements over FCR in those animals that received insects as a source of protein in their diet. These authors attributed the best value for the FCR to the better protein quality of the larvae compared to other raw materials. On the other hand, Dabbou et al. [
40] observed an improvement FCR during the first period of life of the animals, but the growth period to slaughter (10–35 days) had a worse FCR value during the first period.
This study showed a high degree of homogeneity for the slaughter weight of chickens (W5) measured by CV, and the values obtained are shown in the results section. Group uniformity can be expressed as the coefficient of variation in alive weight, increased CV values are synonymous with decreased of uniformity. The level of uniformity basically dictates the final result; high CV values indicate growth retardation, rejections, and poor FCR [
41]. The high degree of homogeneity observed in the group is justified by the high-quality diet received (in terms of the amount of protein ingested). This agrees with what has been published by Hughes et al. [
41], who has described diet as one of the most important factors that influence group uniformity.
The animals used for this study were 30-days old chickens from medium-growth lines, which after the first month did not show a complete productive development. This is the reason for some differences observed when the results of this study were compared with those reported in previous studies that usually use fast-growing strains. This is due to the degree of maturity of the chicks depending on the breed studied [
42].
Although the slaughter weight (W5) increased, not differences were found in the weight of the front quarters and the breast. These results are consistent with the majority of studies on broilers and quail, where despite having increased the final weight of the animal, the weight of the breast, and the composition of the different parts of the carcass in general (wings, thighs, and breast mainly), were not affected by the inclusion of
Musca domestica (MD) or
Hermetia illucens (HI) in the chicken diets [
28,
30,
43,
44,
45]. On other hand, Hwangbo et al. [
38] and Pretorius [
46] found significantly higher breast weights in those animals that received insect meal compared to the control. Hwangbo et al. [
38] attributes the differences between the treatment and control groups with respect to breast weight to a higher rate of protein accumulation with the inclusion of MD, due to the optimal profile of essential amino acids of the latter (particularly lysine) and a high digestibility of protein, although the lack of concordance between the consulted studies can be attributed to the different nutritional composition of the insect species used in the studies and with the variation on the composition [
46]. The results clearly indicate that the inclusion of insects in the diet of chickens has the potential to be food sources, which produces carcasses and their portions (breasts, wings and thighs) of similar size compared to those in which chickens received diets containing traditional feeding ingredients [
46].
No differences were found in nutritional breast composition. According to our results, other authors [
13,
28,
30,
43,
47] indicated the absence of effects of the inclusion of insect meal in the diet on the chemical composition of the breast. In contrast Schiavone et al. [
48] published that the increase in HI in the chicken diet caused a drop in humidity and increased protein. On the other hand, Ballitoc and Sun [
34] showed an increase in moisture and protein in the breast of the group that received 2% TM. However, the explanation for this situation is not entirely clear and further investigation must be carried out. The results also showed that an increasing replacement of the feeding with CLM did not affect the oxidative state of the meat since the chicken breasts meat showed similar TBARS values in the four groups. These results correlate with those obtained by Cullere et al. [
6] who did not found variations in oxidative state when insect larval meal was included in the quail diet.
Then, the observed results suggest that the composition of the chicken breast meat (in terms of moisture, ash and fat) and the oxidative state of chickens fed with insects, is comparable with birds which were fed conventional diets. This is a key aspect from the nutritional point of view to favor the commercialization of this type of products. Probably, the apparent differences in the diet (in terms of protein), had an impact on the productive development of the animals, but without modifying the chemical composition of the carcass. This promising result reinforces the potential of this innovative ingredient as a poultry feeding.
Regarding meat fatty acid composition, the incorporation of CLM, caused a gradual increase in the content of palmitoleic acid that was directly correlated with the percentage of CLM inclusion in the chicken diet. This result is in agreement with the composition of the feed (
Table 3) and it is related with the individual composition of CLM fatty acids (
Table 1), where palmitoleic acid was the third acid with the highest concentration. Likewise, despite the differences were not significant, an increase in the concentration of oleic acid and a drop in the concentration of stearic acid (C18:0) was observed in chicken breast meat of groups that received higher inclusion of CLM in the diet (T3 and T4) with respect to the control (T1) and T2 group. These results are in agreement with what is observed in the fatty acid composition of dietary treatments. It was observed that the main fatty acid in the CLM composition was oleic acid (
Table 1), in this way as the concentration of CLM in the feed increased, the amount of this acid in the different treatments increased (T2, T3 and T4). Likewise, the lower content of stearic acid observed in the feeds that contained a higher concentration of CLM (
Table 3) is reflected in a lower concentration of the acid in the chicken breast meat.
Indeed, it was observed that chickens fed with CLM in diet (T2, T3 and T4), had a significant lower content of α-Linolenic acid (ALA C18: 3 n-3) and also a lower but not significant content of ɣ-Linolenic acid (GLA C18: 3 n-6) in the chicken breast meat (
Table 6). These results are in agreement with the observed in the individual composition in fatty acids of the experimental feeds (
Table 3). Likewise, a not significant increase of myristic acid level (C14:0) was observed, as the replacement of the control feed with CLM does, which is related to the content of this acid in the experimental diets (
Table 3). These results are in agreement with that described by by Loponte et al. [
49], who found similar results, relating this fact to a higher concentration of myristic acid in insects and that could subsequently affect the composition of the chicken breast meat. Similar, Cullere et al. [
13] described that the dietary inclusion of HI greatly changed the proportions of fatty acids in quail breast meat (increasing the concentrations of C10:0, C12:0, C14:0, C16:0 and C20:0). Other authors [
46,
48] described an increase in the content of lauric, myristic and palmitic acid (C12:0, C14:0, C16:0) in chicken breast meat, when insect meal was included in the diet. Dabbou et al. [
28] showed that the group that included TM showed higher percentages of oleic and ALA, a trend towards higher MUFA rates and lower palmitic and saturated fatty acid (SFA) rates at the same time. Dabbou et al. [
28] related this fact to the fatty acid composition of the TM species, oleic acid being the predominant one in the larval form. Dabbou et al. [
28] described that chicken breast meat lipids are mainly composed of triacylglycerol and phospholipids, the latter being rich in very long chain n-3, mainly eicosapentaenoic acid (EPA; C20:5 n-3) and acid docosahexaenoic (DHA; C22:6 n-3), which are well known for their high biological efficacy in the body and their beneficial effects on human health. While our results did not show variations in the content of DHA and EPA [
6,
28] found a significant reduction in DHA content in poultry breast meat fed with increasing levels of HI.
A significant increase in content of SFA was observed for T2 group when it was compared with T3 group. This fact would require new studies to verify this result. On the other hand, our results for the content of SFA, n-3, n-6 were slightly higher than those obtained by Loponte et al. [
49] and lower for content in MUFA and lower for content in MUFA. These authors did not found variations in the content of the main groups of fatty acids (SFA, MUFA and PUFA) in chicken breast meat of the different dietary treatments that included TM versus the control as observed in this study. These authors considered that, despite the great differences found in terms of the fatty acid profile of the TM composition compared to soybean meal, (particularly in the content of SFA and n-6), no variations in the composition were observed. Otherwise, study published by Cullere et al. [
13] showed an increase in content of SFA and MUFA, which increased from the control treatment to the different percentages of inclusion of HI larval meal. In contrast, these authors show PUFA level decreased significantly from the control treatment to those that included larval meal in the diet, with the n-3 fraction showing the greatest decrease. Ref. [
47] observed that supplementation of HI oil in the diet of broilers increased SFA and decreased PUFA in breast muscle, but did not affect MUFA content. Schiavone et al. [
48] published that the MUFA increase due to the high oleic content observed as a result of the inclusion of HI flour levels in the chicken diet.
Atherogenic (AI) and thrombogenic (TI) index correlate the different amounts of some specific SFA, MUFA and PUFA of the n-3 and n-6 series. They have been proposed to indicate the contribution of these fatty acids to the prevention or promotion of pathological phenomena in humans, such as atheromas and/or the formation of thrombi [
26]. The results showed an increase (
p < 0.05) for the TI value in group T2 with respect to T1; while the AI was not affected. It should be noted that, in both groups, AI and IT values were low, and could be considered healthy for consumers [
50,
51], since recommended AI values are below 0.5. These results are in agreement with those reported by Loponte et al. [
49]; who did not observe any difference between the breast meat of broilers fed with larva TM meal and those fed with soy in terms of quality (n-6/n-3 ratio, AI and TI). On the other hand, Dabbou et al. [
40] showed that the TM group had significantly lower AI and TI in chicken breast meat compared to the control group.
The lack of agreement between the different studies on the composition of the meat when insects are included in the diet, could be related not only with the typical lipid profile of the species of insect used but also determined by the insects breeding substrate. In fact, as already mentioned above, it has been shown that the ether extract content of the larvae can vary greatly depending on the substrate, as well as the fatty acid profile [
7].
The L* values obtained in this study were similar to those shown by previous authors when they studied medium-slow growing strains [
52,
53]. The values obtained for a* and b* parameters are slightly higher than those obtained for other breasts of the same lineage (RedBro) slaughtered after 120 days when they have reached their full development [
40]. It can be due to the precocity and lack of maturity of the chickens selected for this study (30 days old). No significant differences were observed for L* parameter depending on the diet received. This coincides with what is described by other authors [
9,
28,
43] who did not found variations for L* when larval meal was included in the diet. Likewise, Bovera et al. [
30] showed that this parameter was not modified in the breast of the animals that had been fed with TM, neither on the raw breast, nor cooked. These authors affirm that the absence of differences would allow this meat to be perfectly accepted by consumers. Similarly, for the parameters a* and b* no significant differences were observed between the different treatment groups. These results are in agreement with those described by previous authors [
13,
28,
38,
43] between the different groups. Although there were no significant differences, the a* value showed a numerical reduction when CLM was included in the diet, especially in the T4 group. Similar results were those shown by Pretorius [
46] who found significantly lower values for a* and b*, while Pieterse [
43] only found a reduction in the a* value in animals that had received insects in their diet. In contrast, Schiavone et al. [
48] showed a significant increase in the redness of the meat (a*) as the concentration of HI in the feed increased related to a possible accumulation of pigments from the insect meal in the intramuscular fat. These authors also reported a linear decrease in the b* value when the HI concentration was increased, attributing it to a progressive decrease in the content of corn in the diet, more than to the effect of the inclusion of insects in the diet.
No differences for the pH value were observed between the different treatments. Previous studies [
6,
46,
48] did not find differences for the pH value as a function of the inclusion of insect flours in the feed. However, Cullere et al. [
13] showed lower pH values in the chicken breast meat when larval meal was included in the chicken diet, while Bovera et al. [
30] found a significant increase in pH in the meat of birds that had incorporated TM in the diet. These differences found between the different studies could be conditioned by the glycogen content in the muscle at the time of slaughter, which is directly related to the stress suffered prior to slaughter. Other authors, [
40] attributed these differences to the rearing system and the genotype used in the different studies.
Cooking losses were higher (
p < 0.001) in the control group (T1) than in the groups that included CLM in the diet (T2, T3, T4). It should be noted that the control group (T1) presented slightly higher humidity values, although without differences with the rest of the treatments, and a pH slightly lower than T1 and T2. This would suggest that the meat from animals that have received CLM in the diet would have a better aptitude for the conservation and processing of the meat as the moisture content and cooking losses are reduced. On the other hand, Schiavone et al. [
48] did not found differences between the treatments while the studies carried out by Bovera et al. [
30], and Cullere et al. [
44] showed higher values of cooking losses in those groups that had included a higher concentration of insect in the bird’s diet. This was justified based on the fact that meat with a pH close to the protein isoelectric value (5.2 to 5.5) resulted in a lower water holding capacity, which produces a more intense loss of cooking.
It was observed that the pH did not affect the water holding capacity (WHC), measured through the percent of expressible juice which did not showed significant differences between the treatments. This is in agreement with [
46] that also found no differences between the different groups for drip losses. An increase of Warner Braztle Shear Force value was observed as the inclusion of CLM in the feeding increased; however, the differences were not statistically significant. This is in agreement with Bovera et al. [
30] that did not find significant differences between the groups that included TM in the diet, although as in the present study, WBSF was higher in the chicken breast meat of the chickens fed with TM. In this way, it could be argued that the increase in resistance, together with the decrease in cooking losses, could be related to some structural change in the disposition of the proteins as a consequence of the inclusion of these alternative sources. However, more in-depth studies are required to assess whether or not there are modifications to the conformation and protein composition derived from the inclusion of CLM. On the other hand, Cullere et al. [
13] described softer meats when the broilers quails had consumed insect meal in their diet.
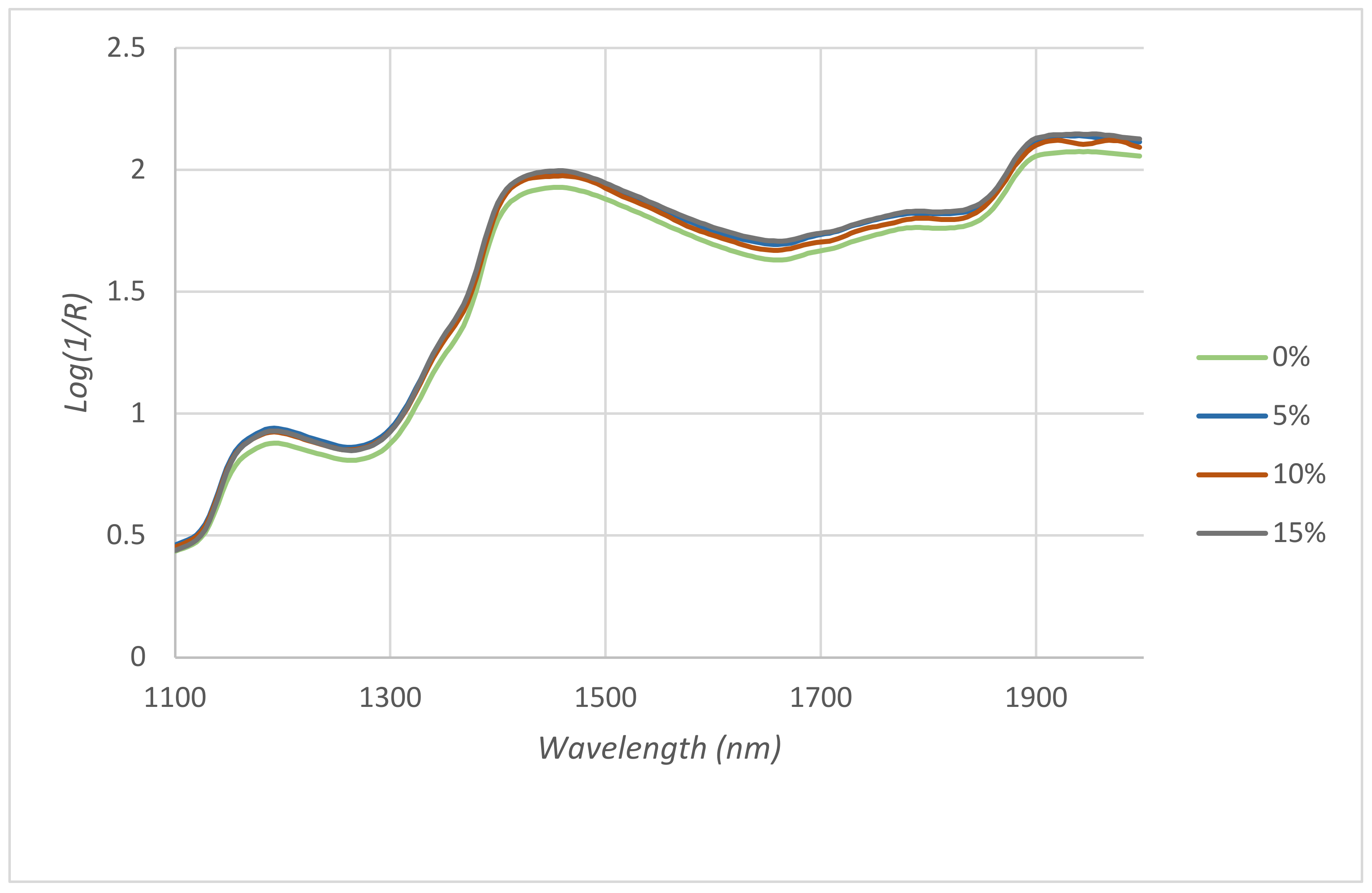
The results show the ability of the method to discriminate and to successfully differentiate samples from the control group (T1). This fact is reflected in the
Figure 1 where the average spectrum of control group appears separated from the rest of the spectra. Regarding the different treated groups, the ability of the method to correctly discriminate increased from group T2 to group T4. It was obtained that the average success rate of the method was 92.1%. These findings are in agreement with those found by Zamora-Rojas et al. [
54] who described that the NIR system was able to classify more than 90% of pig carcasses correctly based on the feed received. Ripoll et al. [
55] described that NIR spectroscopy was able to successfully classify lamb meat from three different feeding systems. Similar results were found by Berzaghi et al. [
56] who showed that the performance of the discriminant models had a correct classification of 100% between the chickens that had received the control diet and those that had received the enriched diets. The results derived from this study suggest that, due to its speed of analysis and low operating costs, the NIRS system can be used as a helpful tool to discriminate poultry meat from different dietary treatments.