Role of JAK-STAT Pathway in Broiler Chicks Fed with Chestnut Tannins
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Housing, and Treatments
2.2. Sample Collection and Processing
2.3. Bacterial Detection
2.4. Chicken-Specific Kinome (Peptide) Array
2.5. Real-Time Quantitative RT-PCR Assay
2.6. Data Analysis: Kinome Array
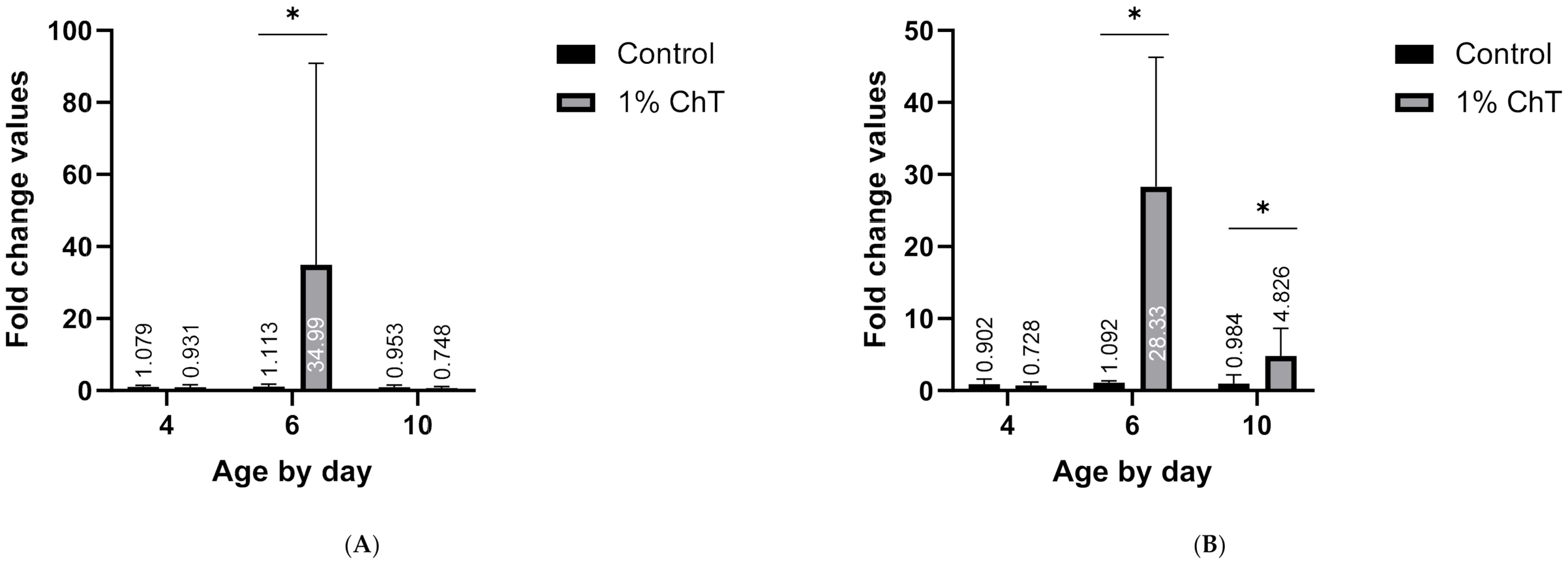
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 1. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.-M.; Boroojeni, F.G.; Zentek, J. Synergistic effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef] [Green Version]
- Diaz Carrasco, J.M.; Redondo, L.M.; Redondo, E.A.; Dominguez, J.E.; Chacana, A.P.; Fernandez Miyakawa, M.E. Use of plant extracts as an effective manner to control Clostridium perfringens induced necrotic enteritis in poultry. BioMed. Res. Int. 2016, 2016, 3278359. [Google Scholar] [CrossRef] [Green Version]
- Díaz Carrasco, J.M.; Redondo, E.A.; Pin Viso, N.D.; Redondo, L.M.; Farber, M.D.; Fernández Miyakawa, M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. BioMed. Res. Int. 2018, 2018, 1879168. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Brus, M.; Gradišnik, L.; Trapečar, M.; Škorjanc, D.; Frangež, R. Beneficial effects of water-soluble chestnut (Castanea sativa mill.) tannin extract on chicken small intestinal epithelial cell culture. Poult. Sci. 2018, 97, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural tannin wood extracts as a potential food ingredient in the food industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, K.; Zhao, J.S.; Deng, W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 717–726. [Google Scholar] [CrossRef]
- Song, P.; Zhang, R.; Wang, X.; He, P.; Tan, L.; Ma, X. Dietary grape-seed procyanidins decreased postweaning diarrhea by modulating intestinal permeability and suppressing oxidative stress in rats. J. Agric. Food Chem. 2011, 59, 6227–6232. [Google Scholar] [CrossRef]
- Williams, A.R.; Andersen-Civil, A.I.S.; Zhu, L.; Blanchard, A. Dietary phytonutrients and animal health: Regulation of immune function during gastrointestinal infections. J. Anim. Sci. 2020, 98, 1–11. [Google Scholar] [CrossRef]
- Manning, G. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenault, R.; Griebel, P.; Napper, S. Peptide arrays for kinome analysis: New opportunities and remaining challenges. Proteomics 2011, 11, 4595–4609. [Google Scholar] [CrossRef] [PubMed]
- Jalal, S.; Arsenault, R.; Potter, A.A.; Babiuk, L.A.; Griebel, P.J.; Napper, S. Genome to kinome: Species-specific peptide arrays for kinome analysis. Sci. Signal. 2009, 2, pl1. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Cardoso Dal Pont, G.; Farnell, M.B.; Jarvis, S.; Battaglia, M.; Arsenault, R.J.; Kogut, M.H. Supplementing chestnut tannins in the broiler diet mediates a metabolic phenotype of the ceca. Poult. Sci. 2020, 100, 47–54. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Lee, J.T.; Latham, R.; Carter, B.; Kogut, M.H. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 2017, 96, 4307–4316. [Google Scholar] [CrossRef]
- Eldaghayes, I.; Rothwell, L.; Williams, A.; Withers, D.; Balu, S.; Davison, F.; Kaiser, P. Infectious bursal disease virus: Strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006, 19, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon Cytokine Res. 2003, 23, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P.; Wigley, P.; Burnside, J.; Barrow, P.A.; Galyov, E.E.; Rothwell, L. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 2000, 146, 3217–3226. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.; Kindrachuk, J.; Määttänen, P.; Napper, S.; Kusalik, A. PIIKA 2: An expanded, web-based platform for analysis of kinome microarray data. PLoS ONE 2013, 8, e80837. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Arsenault, R.J.; Trost, B.; Slind, J.; Griebel, P.J.; Napper, S.; Kusalik, A. A systematic approach for analysis of peptide array kinome data. Sci. Signal. 2012, 5, pl2. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Yin, X.; Yuan, J.; Broom, L. Gut health in poultry. CAB Rev. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019, 98, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Tech. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Bartunek, P.; Koritschoner, N.P.; Brett, D.; Zenke, M. Molecular cloning, expression and evolutionary analysis of the avian tyrosine kinase JAK1. Gene 1999, 230, 129–136. [Google Scholar] [CrossRef]
- Truong, A.D.; Rengaraj, D.; Hong, Y.; Hoang, C.T.; Hong, Y.H.; Lillehoj, H.S. Analysis of JAK-STAT signaling pathway genes and their microRNAs in the intestinal mucosa of genetically disparate chicken lines induced with necrotic enteritis. Vet. Immunol. Immunopathol. 2017, 187, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wange, R.L. T cell receptor signaling: Beyond complex complexes. J. Biol. Chem. 2004, 279, 28827–28830. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar]
- Zhang, Y.; Zhang, L.; Zuo, Q.; Wang, Y.; Zhang, Y.; Xu, Q.; Li, B.; Chen, G. JAK-STAT signaling regulation of chicken embryonic stem cell differentiation into male germ cells. In Vitro Cell. Dev. Biol. 2017, 53, 728–743. [Google Scholar] [CrossRef]
- Cousins, E.; Gao, Y.; Sandford, G.; Nicholas, J. Human herpesvirus 8 viral interleukin-6 signaling through gp130 promotes virus replication in primary effusion lymphoma and endothelial cells. J. Virol. 2014, 88, 12167–12172. [Google Scholar]
- Stross, C.; Radtke, S.; Clahsen, T.; Gerlach, C.; Volkmer-Engert, R.; Schaper, F.; Heinrich, P.C.; Hermanns, H.M. Oncostatin M receptor-mediated signal transduction is negatively regulated by SOCS3 through a receptor tyrosine-independent mechanism. J. Biol. Chem. 2006, 281, 8458–8468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, J.S. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
| Immune Pathways | ||||||
|---|---|---|---|---|---|---|
| Day 4 | Day 6 | Day 10 | ||||
| Identified Pathways | Number of Peptides | p-Value | Number of Peptides | p-Value | Number of Peptides | p-Value |
| Chemokine signaling pathway | 23 | 9.19 × 10−18 | 26 | 9.19 × 10−19 | 18 | 7.10 × 10−13 |
| T cell receptor signaling pathway | 14 | 7.55 × 10−12 | 25 | 5.85 × 10−23 | 12 | 7.50 × 10−10 |
| Jak-STAT signaling pathway | 13 | 1.38 × 10−8 | 19 | 7.82 × 10−13 | 15 | 1.31 × 10−10 |
| B cell receptor signaling pathway | 13 | 2.94 × 10−12 | 19 | 3.27 × 10−18 | 12 | 3.13 × 10−11 |
| Fc gamma R-mediated phagocytosis | 13 | 2.81 × 10−11 | 13 | 4.06 × 10−10 | - | - |
| TNF signaling pathway | 13 | 2.21 × 10−10 | 21 | 1.17 × 10−17 | 10 | 1.72 × 10−7 |
| Wnt signaling pathway | 13 | 4.24 × 10−9 | 11 | 3.30 × 10−6 | - | - |
| Apoptosis | 12 | 2.09 × 10−8 | 19 | 5.79 × 10−14 | - | - |
| Toll-like receptor signaling pathway | 11 | 1.46 × 10−8 | 20 | 6.03 × 10−17 | 13 | 9.43 × 10−11 |
| Natural killer cell mediated cytotoxicity | 11 | 8.57 × 10−8 | 16 | 1.76 × 10−11 | - | - |
| NOD-like receptor signaling pathway | 10 | 8.08 × 10−6 | 18 | 1.22 × 10−11 | 12 | 1.11 × 10−7 |
| Th17 cell differentiation | 10 | 1.43 × 10−7 | 22 | 3.36 × 10−19 | 10 | 1.11 × 10−7 |
| IL-17 signaling pathway | - | - | 16 | 3.62 × 10−13 | - | - |
| Inflammatory mediator regulation of TRP channels | - | - | 10 | 6.08 × 10−7 | - | - |
| JAK-STAT Signaling Pathway | ||||||||
|---|---|---|---|---|---|---|---|---|
| Days of Necropsy | ||||||||
| 4 | 6 | 10 | ||||||
| Proteins | UniProt ID | p-Site | Fold Change | p-Value | Fold Change | p-Value | Fold Change | p-Value |
| AKT3 | Q9Y243 | S476/T305 | −1.046 | <0.01 | −1.041 | 0.026 | −1.023 | 0.034 |
| CCND1 | P24385 | T286 | - | - | 1.071 | 0.015 | - | - |
| EGFR | P00533 | Y1069 | - | - | 1.032 | 0.041 | - | - |
| EP300 | Q09472 | S89 | - | - | - | - | 1.052 | 0.031 |
| GRB2 | P62993 | Y209 | - | - | −1.063 | 0.02 | 1.044 | 0.017 |
| IL12B | P29460 | Y314 | - | - | −1.048 | 0.03 | - | - |
| IL6ST | P40189 | Y915/Y759 | 1.058 | 0.026 | −1.039 | 0.03 | 1.041 | 0.039 |
| JAK1 | P23458 | Y1034 | - | - | 1.098 | <0.01 | - | - |
| JAK2 | O60674 | Y1007 | −1.059 | 0.027 | −1.042 | <0.01 | - | - |
| MTOR | P42345 | S2481 | 1.061 | 0.042 | −1.058 | 0.01 | −1.041 | 0.036 |
| PDGFRA | P16234 | Y720 | - | - | 1.127 | <0.01 | −1.064 | <0.01 |
| PDGFRB | P09619 | Y579 | −1.115 | <0.01 | −1.061 | 0.019 | −1.061 | 0.046 |
| PIK3CB | P42338 | Y425/S1070 | 1.086 | <0.01 | - | - | −1.049 | 0.022 |
| PIK3R1 | P27986 | S608/Y467 | 1.058 | 0.028 | −1.025 | 0.045 | −1.076 | <0.01 |
| PIK3R2 | O00459 | Y365 | 1.052 | 0.038 | −1.039 | 0.04 | - | - |
| PIM1 | P11309 | S189 | - | - | −1.046 | <0.01 | −1.043 | 0.036 |
| RAF1 | P04049 | S259 | 1.053 | 0.03 | −1.048 | 0.048 | - | - |
| SOCS3 | O14543 | Y204 | −1.069 | <0.01 | −1.052 | <0.01 | - | - |
| SOS1 | Q07889 | S1193 | - | - | - | - | 1.036 | 0.029 |
| STAM2 | O75886 | Y371 | - | - | - | - | −1.057 | 0.017 |
| STAT1 | P42224 | Y701 | 1.057 | 0.048 | 1.060 | 0.033 | 1.034 | 0.034 |
| STAT3 | P40763 | Y705/S727 | 1.067 | 0.015 | 1.078 | 0.031 | 1.046 | 0.044 |
| STAT5B | P51692 | Y699 | −1.071 | <0.01 | 1.061 | <0.01 | 1.087 | <0.01 |
| Chemokine Signaling Pathway | |||
|---|---|---|---|
| Proteins | Day 4 | Day 6 | Day 10 |
| AKT3 | ↓ | ↓ | ↓ |
| ARRB1 | ↓ | ↓ | - |
| ARRB2 | - | ↓ | - |
| CHUK | - | ↑ | - |
| CRK | - | - | ↑ |
| CRKL | ↓ | - | - |
| CRR2 | ↑ | - | - |
| GRB2 | - | ↓ | ↑ |
| GRK5 | ↓ | ↓ | - |
| GSK3A | ↓ | ↑ | ↓ |
| GSK3B | ↓ | ↑ | ↑ |
| JAK2 | ↓ | ↓ | - |
| LYN | - | ↑ | - |
| MAP2K1 | ↑ | - | - |
| NFKB1 | - | ↑ | - |
| NFKBIA | - | ↑ | ↓ |
| PAK1 | ↓ | - | - |
| PIK3R1 | ↑ | ↓ | ↓ |
| PIK3R2 | ↑ | ↓ | - |
| PRKCD | ↓ | ↓ | - |
| PTK2 | ↑ | ↑ | ↓ |
| PTK2B | ↑ | ↑ | ↑ |
| PXN | ↓ | ↑ | ↓ |
| RAF1 | ↑ | ↓ | - |
| SHC1 | - | ↑ | - |
| SHC3 | - | ↑ | - |
| SRC | - | ↓ | ↑ |
| STAT1 | ↑ | ↑ | ↑ |
| STAT3 | ↑ | ↑ | ↑ |
| STAT5B | ↓ | ↑ | ↑ |
| T Cell Receptor Signaling Pathway | |||
|---|---|---|---|
| Proteins | Day 4 | Day 6 | Day 10 |
| AKT3 | ↓ | ↓ | ↓ |
| CHUK | - | ↓ | - |
| FOS | - | ↑ | - |
| FYN | ↓ | ↓ | - |
| GRB2 | - | ↓ | ↑ |
| GSK3B | ↓ | ↑ | ↑ |
| JUN | ↓ | ↓ | - |
| LCK | - | ↑ | - |
| MAP2K1 | ↑ | - | - |
| MAP2K2 | - | ↓ | ↑ |
| MAP3K7 | ↓ | ↑ | ↑ |
| NFATC1 | ↑ | - | - |
| NFATC2 | - | ↓ | - |
| NFATC3 | ↑ | ↓ | ↑ |
| NFKB1 | - | ↑ | - |
| NFKBIA | - | ↑ | ↓ |
| PAK1/2 | ↓ | ↓ | - |
| PDPK1 | ↓ | ↓ | - |
| PIK3R1 | ↑ | ↓ | ↓ |
| PIK3R2 | ↑ | ↓ | ↓ |
| PTPRC | - | ↓ | - |
| RAF1 | ↑ | ↓ | - |
| ZAP70 | - | ↓ | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Cardoso Dal Pont, G.; Battaglia, M.; Arsenault, R.J.; Kogut, M.H. Role of JAK-STAT Pathway in Broiler Chicks Fed with Chestnut Tannins. Animals 2021, 11, 337. https://doi.org/10.3390/ani11020337
Lee A, Cardoso Dal Pont G, Battaglia M, Arsenault RJ, Kogut MH. Role of JAK-STAT Pathway in Broiler Chicks Fed with Chestnut Tannins. Animals. 2021; 11(2):337. https://doi.org/10.3390/ani11020337
Chicago/Turabian StyleLee, Annah, Gabriela Cardoso Dal Pont, Michele Battaglia, Ryan J. Arsenault, and Michael H. Kogut. 2021. "Role of JAK-STAT Pathway in Broiler Chicks Fed with Chestnut Tannins" Animals 11, no. 2: 337. https://doi.org/10.3390/ani11020337






