Fermentation Characteristics and In Vitro Digestibility of Fibers and Fiber-Rich Byproducts Used for the Feeding of Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Enzymatic Pre-Digestion of Substrates
2.3. Management and Feeding of Donor Animals
2.4. Sampling of Feces and Sample Preparation
2.5. Batch-Culture Incubation
2.6. Analysis of Gas Production
2.7. Analysis of CH4 Production
2.8. In Vitro Digestibility of Dry Matter and Organic Matter
2.9. Additional Analyses and Calculations
2.10. Statistical Analysis
3. Results
3.1. Chemical Composition and Physicochemical Properties of Test Substrates
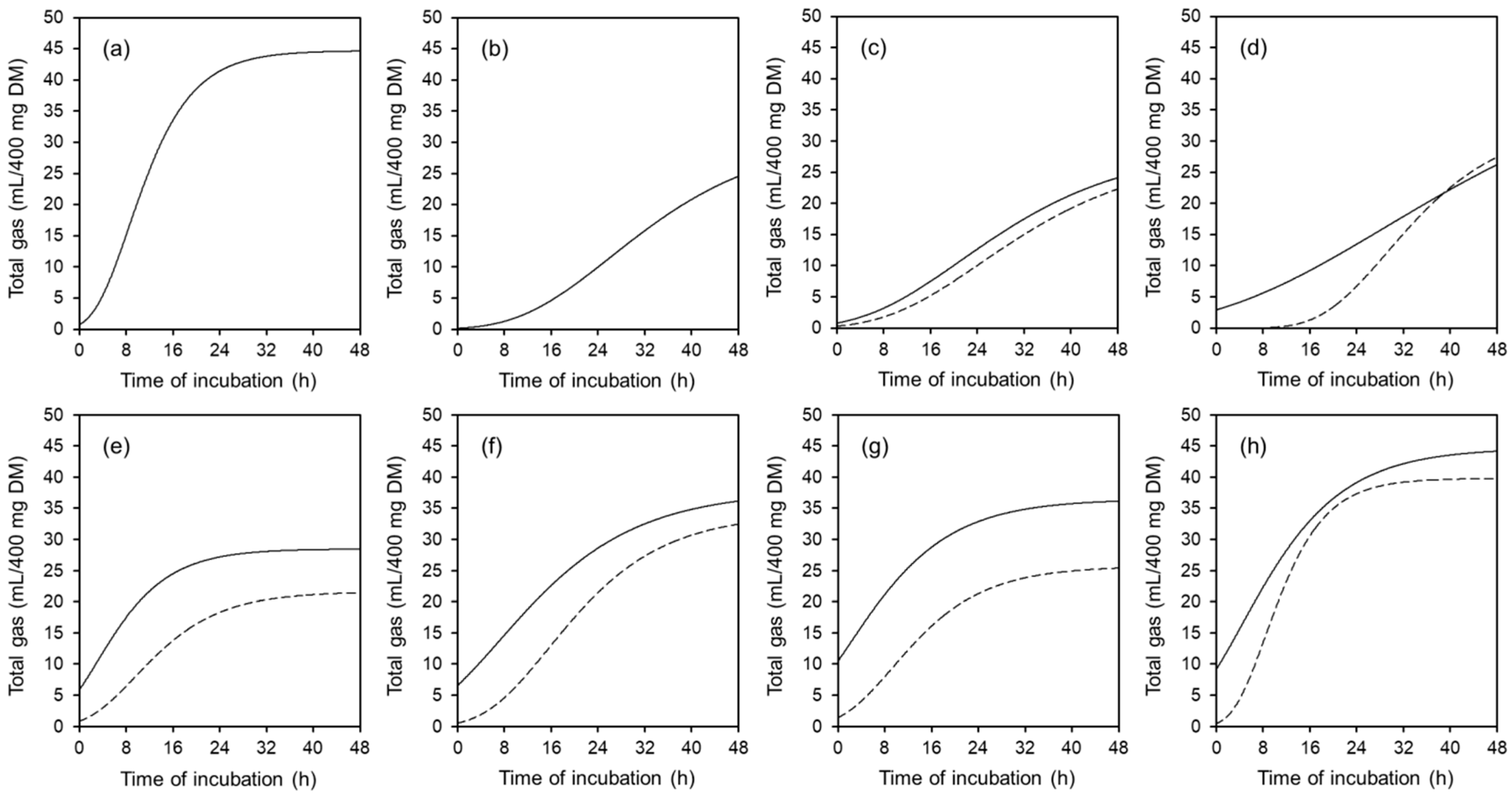
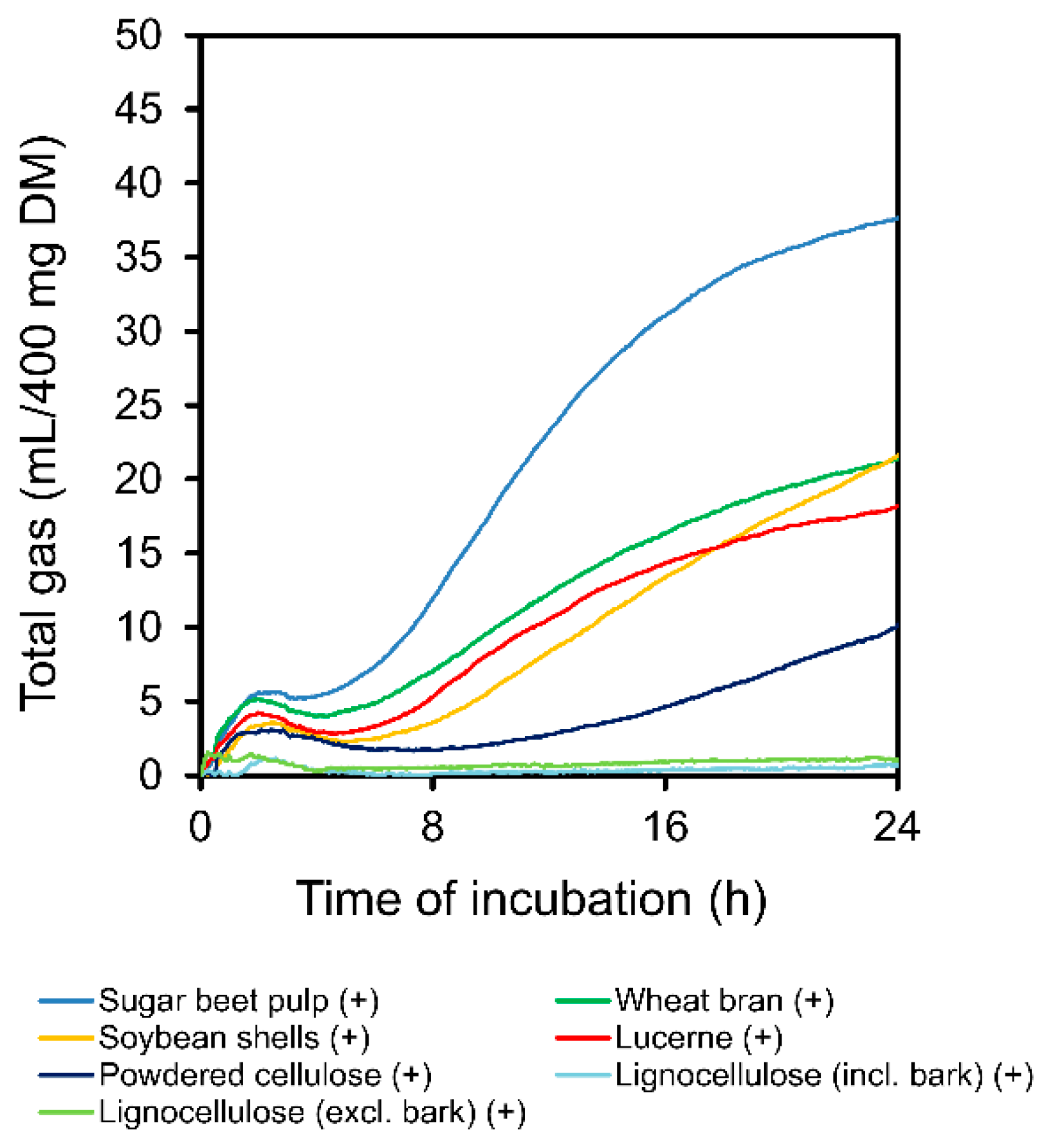
3.2. Total Gas and CH4 Production
3.3. Post-Incubation pH, NH3, and Short Chain Fatty Acids
3.4. In Vitro Digestibility of Dry Matter and Organic Matter
3.5. Correlations
4. Discussion
4.1. Chemical Composition and Physicochemical Properties
4.2. Fermentation Characteristics
4.3. In Vitro Digestibility of Dry Matter and Organic Matter
4.4. Correlations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenk, C. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 2001, 90, 21–33. [Google Scholar] [CrossRef]
- Wilfart, A.; Montagne, L.; Simmins, H.; Noblet, J.; van Milgen, J. Effect of fibre content in the diet on the mean retention time in different segments of the digestive tract in growing pigs. Livest. Sci. 2007, 109, 27–29. [Google Scholar] [CrossRef]
- Bindelle, J.; Leterme, P.; Buldgen, A. Nutritional and environmental consequences of dietary fibre in pig nutrition: A review. Biotechnol. Agron. Soc. Environ. 2008, 12, 69–80. [Google Scholar]
- von Heimendahl, E.; Breves, G.; Abel, H. Fiber-related digestive processes in three different breeds of pigs. J. Anim. Sci. 2010, 88, 972–981. [Google Scholar] [CrossRef] [Green Version]
- Tretola, M.; Luciano, A.; Ottoboni, M.; Baldi, A.; Pinotti, L. Influence of traditional vs alternative dietary carbohydrates sources on the large intestinal microbiota in post-weaning piglets. Animals 2019, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Drochner, W.; Coenen, M. Pflanzliche Strukturstoffe in der Schweineernährung. Übers. Tierernährg. 1986, 14, 1–50. [Google Scholar]
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fibre on intestinal growth, cell proliferation, and morphology in growing pigs. J. Anim. Sci. 1994, 72, 2270–2278. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Verstegen, M.W.A. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci. 2007, 109, 194–203. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.A.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 2005, 123–124, 445–462. [Google Scholar] [CrossRef]
- Bender, A.; Breves, G.; Stein, J.; Leonhard-Marek, S.; Schröder, B.; Winckler, C. Colonic fermentation as affected by antibiotics and acidic pH: Application of an in vitro model. Z. Gastroenterol. 2001, 39, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Noblet, J.; Jaguelin-Peyraud, Y. Prediction of digestibility of organic matter and energy in the growing pig from an in vitro method. Anim. Feed Sci. Technol. 2007, 134, 211–222. [Google Scholar] [CrossRef]
- VDLUFA. Die Chemische Untersuchung von Futtermitteln, Methodenbuch, 3rd ed.; VDLUFA: Darmstadt, Germany, 2012. [Google Scholar]
- GfE. Prediction of metabolisable energy of compound feeds for pigs. Proc. Soc. Nutr. Physiol. 2008, 17, 199–204. [Google Scholar]
- Fontaine, J.; Bech-Andersen, S.; Butikofer, U.; de Froidmont-Görtz, I. Determination of tryptophan in feed by HPLC—development of an optimal hydrolysis and extraction procedure by the EU commission DG XII in three international collaborative studies. Agribiol. Res. 1998, 51, 97–108. [Google Scholar] [CrossRef]
- Conway, E.J.; Byrne, A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem. J. 1933, 27, 419–429. [Google Scholar]
- Kyriazakis, I.; Emmans, G.C. The voluntary feed intake of pigs given feeds based on wheat bran, dried citrus pulp and grass meal, in relation to measurements of feed bulk. Br. J. Nutr. 1995, 73, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Slama, J.; Schedle, K.; Wurzer, G.K.; Gierus, M. Physicochemical properties to support fibre characterization in monogastric animal nutrition. J. Sci. Food Agr. 2019, 99, 3895–3902. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agyekum, A.K.; Nyachoti, C.M. Nutritional and metabolic consequences of feeding high-fiber diets to swine: A review. Engineering 2017, 3, 716–725. [Google Scholar]
- Johnston, I.R. The composition of the cell wall of Aspergillus niger. Biochem. J. 1965, 96, 651–658. [Google Scholar] [PubMed]
- Giger-Reverdin, S. Characterisation of feedstuffs for ruminants using some physical parameters. Anim. Feed Sci. Technol. 2000, 86, 53–69. [Google Scholar]
- Nigam, P.; Singh, D. Solid-state (substrate) fermentation systems and their applications in biotechnology. J. Basic Microbiol. 1994, 34, 405–423. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. The nutritional significance of “dietary fibre” analysis. Anim. Feed Sci. Technol. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Ehle, F.R.; Jeraci, J.L.; Robertson, J.B.; Van Soest, P.J. The influence of dietary fiber on digestibility, rate of passage and gastrointestinal fermentation in pigs. J. Anim. Sci. 1982, 55, 1071–1081. [Google Scholar] [CrossRef]
- Chen, C.N.; Lee, T.T.; Yu, B. Comparison of the dietary fiber digestibility and fermentability of feedstuffs determined by conventional methods and in vitro gas production technique in pigs. Acta Agric. Scand. A Anim. Sci. 2013, 63, 201–207. [Google Scholar]
- Bindelle, J.; Buldgen, A.; Boudry, C.; Leterme, P. Effect of inoculum and pepsin-pancreatin hydrolysis on fibre fermentation measured by the gas production technique in pigs. Anim. Feed Sci. Technol. 2007, 132, 111–122. [Google Scholar] [CrossRef]
- Jha, R.; Bindelle, J.; Van Kessel, A.; Leterme, P. In vitro fibre fermentation of feed ingredients with varying fermentable carbohydrate and protein levels and protein synthesis by colonic bacteria isolated from pigs. Anim. Feed Sci. Technol. 2011, 165, 191–200. [Google Scholar] [CrossRef]
- Uerlings, J.; Bindelle, J.; Schroyen, M.; Richel, A.; Bruggeman, G.; Willems, E.; Everaert, N. Fermentation capacities of fructan- and pectin-rich by-products and purified fractions via an in vitro piglet faecal model. J. Sci. Food Agric. 2019, 99, 5720–5733. [Google Scholar] [CrossRef]
- Coles, L.T.; Moughan, P.J.; Darragh, A.J. In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple-stomached animals. Anim. Feed Sci. Technol. 2005, 123–124, 421–444. [Google Scholar] [CrossRef]
- Anderson, W.F.; Akin, D.E. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J. Ind. Microbiol. Biotechnol. 2008, 35, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Polo, C.C.; Pereira, L.; Mazzafera, P.; Flores-Borges, D.N.A.; Mayer, J.L.S.; Guizar-Sicairos, M.; Holler, M.; Barsi-Andreeta, M.; Westfahl Jr., H.; Meneau, F. Correlations between lignin content and structural robustness in plants revealed by X-ray ptychography. Sci. Rep. 2020, 10, 6023. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.E.; Borneman, W.S.; Rigsby, L.L.; Martin, S.A. p-Coumaroyl and feruloyl arabinoxylans from plant cell walls as substrates for ruminal bacteria. Appl. Environ. Microbiol. 1993, 59, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Wilfart, A.; Jaguelin-Peyraud, Y.; Simmins, H.; Noblet, J.; van Milgen, J.; Montagne, L. A step-wise in vitro method to estimate kinetics of hydrolysis of feeds. Livest. Sci. 2007, 109, 179–181. [Google Scholar] [CrossRef]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Hegarty, R.; Gerdes, R. Hydrogen production and transfer in the rumen. Rec. Adv. Anim. Nutr. 1998, 12, 37–44. [Google Scholar]
- De Graeve, K.G.; Grivet, J.P.; Durand, M.; Beaumatin, P.; Cordelet, C.; Hannequart, G.; Demeyer, D. Competition between reductive acetogenesis and methanogenesis in the pig large-intestinal flora. J. Appl. Bacteriol. 1994, 76, 55–61. [Google Scholar] [CrossRef]
- Canh, T.T.; Sutton, A.L.; Aarnink, A.J.; Verstegen, M.W.; Schrama, J.W.; Bakker, G.C. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J. Anim. Sci. 1998, 76, 1887–1895. [Google Scholar]
- Mroz, Z.; Moeser, A.J.; Vreman, K.; van Diepen, J.T.; van Kempen, T.; Canh, T.T.; Jongbloed, A.W. Effects of dietary carbohydrates and buffering capacity on nutrient digestibility and manure characteristics in finishing pigs. J. Anim. Sci. 2000, 78, 3096–3106. [Google Scholar]
- Zhao, J.; Bai, Y.; Zhang, G.; Liu, L.; Lai, C. Relationship between dietary fiber fermentation and volatile fatty acids’ concentration in growing pigs. Animals 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagonias, G.; Pearce, G.R. The digestion of fibre by pigs. 2. Volatile fatty acid concentrations in large intestine digesta. Br. J. Nutr. 1985, 53, 531–536. [Google Scholar]
- Carneiro, M.S.C.; Lordelo, M.M.; Cunha, L.F.; Freire, J.P.B. Effects of dietary fibre source and enzyme supplementation on faecal apparent digestibility, short chain fatty acid production and activity of bacterial enzymes in the gut of piglets. Anim. Feed Sci. Technol. 2008, 146, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Roediger, W.E.W. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 2, 712–715. [Google Scholar] [CrossRef]
- Smith, J.G.; Yokoyama, W.H.; German, J.B. Butyric acid from the diet: Action at the level of gene expression. Crit. Rev. Food Sci. 1998, 38, 259–297. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Serena, A.; Bjørnbak Kjær, A.K.; Jørgensen, H.; Engberg, R. Rye bread enhances the production and plasma concentration of butyrate but not the plasma concentrations of glucose and insulin in pigs. J. Nutr. 2005, 135, 1696–1704. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar]
- Huang, Z.; Urriola, P.E.; Shurson, G.C. Use of in vitro dry matter digestibility and gas production to predict apparent total tract digestibility of total dietary fiber for growing pigs. J. Anim. Sci. 2017, 95, 5474–5484. [Google Scholar] [CrossRef] [Green Version]
| Item | Pre-Starter | Starter |
|---|---|---|
| Dry matter | 911 | 907 |
| Crude ash | 53 | 46 |
| Crude protein | 241 | 228 |
| Lys | 15.9 | 13.9 |
| Met | 5.8 | 5.2 |
| Cys | 3.9 | 3.6 |
| Thr | 11.5 | 10.3 |
| Trp | 3.7 | 3.3 |
| Val | 12.7 | 11.3 |
| AEE | 48 | 48 |
| Crude fiber | 38 | 39 |
| aNDFom | 217 | 220 |
| ADFom | 54 | 48 |
| ADL | 9 | 11 |
| Sugar | 58 | 38 |
| Starch | 411 | 438 |
| Gross energy | 19.2 | 19.1 |
| MES | 15.7 | 15.7 |
| Substrate | DM | CA | CP | AEE | CF | aNDFom | ADFom | ADL | Sugar | Starch | GE | WBC | WHC | SC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lignocellulose (including bark) | 933 | 13 | 11 | 1 | 679 | 897 | 779 | 315 | 4 | n.a. | 20.4 | 6.7 | 5.4 | 80 |
| Lignocellulose (excluding bark) | 938 | 2 | 5 | 2 | 701 | 918 | 763 | 246 | 15 | n.a. | 20.1 | 8.2 | 6.2 | 44 |
| Powdered cellulose | 939 | 2 | 7 | 4 | 773 | 999 | 966 | 1 | 0 | n.a. | 17.4 | 14.3 | 9.7 | 9 |
| A. niger mycelium 2 | 904 | 3 | 144 | 30 | 373 | 857 | 602 | 221 | 3 | n.a. | 19.6 | 4.4 | 4.1 | 367 |
| Lucerne chaff | 936 | 73 | 151 | 18 | 313 | 474 | 374 | 82 | 29 | 21 | 18.8 | 7.7 | 8.0 | 110 |
| Soybean shells | 912 | 51 | 112 | 15 | 414 | 707 | 508 | 18 | 16 | 3 | 17.7 | 7.5 | 6.8 | 240 |
| Wheat bran | 919 | 58 | 163 | 47 | 125 | 508 | 156 | 43 | 59 | 134 | 19.5 | 6.4 | 6.4 | 59 |
| Sugar beet pulp | 921 | 68 | 105 | 6 | 188 | 500 | 235 | 25 | 61 | 6 | 17.3 | 10.4 | 10.6 | 411 |
| Substrate | GP36 | GP48 | GP36 | GP48 | ||
|---|---|---|---|---|---|---|
| − | + | − | + | |||
| Starch | 44.6 a | 45.9 a | ||||
| Purified cellulose | 18.7 cde | 23.9 cd | ||||
| Lignocellulose (including bark) | 5.3 ef | 6.4 ef | 5.2 | 1.5 | 6.6 | 1.7 |
| Lignocellulose (excluding bark) | 1.9 f | 2.7 f | 2.5 | 1.8 | 2.5 | 2.0 |
| Powdered cellulose | 17.8 de | 22.2 de | 18.0 | 17.5 | 22.3 | 22.4 |
| A. niger mycelium | 20.4 cde | 23.1 cd | 20.5 | 19.4 | 24.2 | 27.9 |
| Lucerne chaff | 28.4 bcd | 28.9 bcd | 28.0 A | 20.7 B | 28.9 A | 21.6 B |
| Soybean shells | 34.0 bc | 35.1 b | 33.0 A | 28.9 B | 35.3 A | 32.7 B |
| Wheat bran | 34.2 b | 36.6 b | 34.4 A | 24.5 B | 35.4 A | 25.3 B |
| Sugar beet pulp | 43.5 ab | 43.5 ab | 42.8 | 39.4 | 42.6 | 39.3 |
| Substrate | Pre-Digestion | a | b | b + c | R2 |
|---|---|---|---|---|---|
| Starch | − | 44.7 | 8.5 | 14.5 | 0.993 |
| Purified cellulose | − | 31.4 | 26.1 | 41.7 | 0.996 |
| Powdered cellulose | − | 29.3 | 21.1 | 37.6 | 0.991 |
| + | 28.2 | 24.5 | 40.8 | 0.990 | |
| A. niger mycelium | − | 44.0 | 28.9 | 57.9 | 0.922 |
| + | 33.1 | 29.3 | 40.5 | 0.998 | |
| Lucerne chaff | − | 28.5 | 3.1 | 9.9 | 0.984 |
| + | 21.6 | 9.5 | 17.7 | 0.991 | |
| Soybean shells | − | 37.8 | 7.3 | 20.4 | 0.993 |
| + | 34.2 | 15.6 | 26.7 | 0.997 | |
| Wheat bran | − | 36.4 | 2.1 | 11.7 | 0.971 |
| + | 25.7 | 9.3 | 18.1 | 0.993 | |
| Sugar beet pulp | − | 44.6 | 4.5 | 14.1 | 0.984 |
| + | 39.8 | 8.5 | 14.1 | 0.995 |
| Substrate | 2–4 h | 22–24 h | 46–48 h | 2–4 h | 22–24 h | 46–48 h | |||
|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | ||||
| Starch | 70 e | 1257 b | 1338 c | ||||||
| Purified cellulose | 43 f | 846 c | 2387 a | ||||||
| Lignocellulose (including bark) | 15 fg | −11 e | 743 de | 12 | 7 | −28 | 3 | 681 | 671 |
| Lignocellulose (excluding bark) | 4 g | −26 e | 324 e | 6 A | −11 B | 10 | −100 | 459 | 1023 |
| Powdered cellulose | 33 f | 881 c | 2251 a | 33 | 27 | 903 | 755 | 2225 | 2182 |
| A. niger mycelium | 59 e | 417 d | 1105 cd | 57 A | −2 B | 411 A | 244 B | 1164 | 1336 |
| Lucerne chaff | 206 d | 1542 a | 2240 a | 240 A | 65 B | 1539 A | 873 B | 2206 A | 1530 B |
| Soybean shells | 239 c | 1366 ab | 2236 a | 239 A | 61 B | 1359 A | 995 B | 2220 | 2136 |
| Wheat bran | 388 a | 1264 b | 1899 b | 380 A | 70 B | 1257 A | 713 B | 1882 | 1531 |
| Sugar beet pulp | 316 b | 1140 b | 1761 bc | 315 A | 140 B | 1141 B | 1576 A | 1755 | 1945 |
| Substrate 2 | pH |
|---|---|
| Blank (only fecal inoculum) | 6.44 a |
| Starch | 4.75 f |
| Purified cellulose | 5.67 bcd |
| Lignocellulose (including bark) | 6.39 a |
| Lignocellulose (excluding bark) | 6.48 a |
| Powdered cellulose | 5.80 bc |
| A. niger mycelium | 5.43 d |
| Lucerne chaff | 5.84 b |
| Soybean shells | 5.52 d |
| Wheat bran | 5.67 c |
| Sugar beet pulp | 5.12 e |
| Substrate | NH3 | NH3 | |
|---|---|---|---|
| − | + | ||
| Starch | −4.96 f | ||
| Purified cellulose | 0.54 d | ||
| Lignocellulose (including bark) | −0.14 d | −0.14 | 0.38 |
| Lignocellulose (excluding bark) | −0.17 d | −0.17 | −0.19 |
| Powdered cellulose | 0.59 d | 0.59 | 0.45 |
| A. niger mycelium | 1.38 d | 1.38 A | 0.38 B |
| Lucerne chaff | 3.57 b | 3.57 A | 1.43 B |
| Soybean shells | 2.40 c | 2.40 | 1.63 |
| Wheat bran | 6.75 a | 6.75 A | 2.55 B |
| Sugar beet pulp | −1.79 e | −1.79 B | 0.22 A |
| Substrate | Acetic Acid | Propionic Acid | n-Butyric Acid | n-Valeric Acid | Acetic Acid | Propionic Acid | n-Butyric Acid | n-Valeric Acid | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | − | + | |||||
| Starch | 9.78 bc | 9.71 a | 3.14 a | 1.01 a | ||||||||
| Purified cellulose | 7.70 cd | 2.21 d | 0.50 c | 0.03 e | ||||||||
| Lignocellulose (including bark) | 1.45 e | 0.02 e | 0.24 cd | −0.02 ef | 1.45 | 0.10 | 0.04 | −0.26 | 0.24 | −0.22 | −0.02 | −0.07 |
| Lignocellulose (excluding bark) | 0.85 e | −0.11 e | −0.19 d | −0.08 f | 0.85 | −0.06 | −0.11 | −0.40 | −0.19 | −0.27 | −0.08 | −0.08 |
| Powdered cellulose | 6.57 d | 1.82 d | 0.27 c | −0.04 ef | 6.57 | 6.41 | 1.82 | 1.68 | 0.27 | 0.35 | −0.04 | −0.04 |
| A. niger mycelium | 6.83 d | 3.75 cd | 0.94 bc | 0.13 de | 6.83 | 6.58 | 3.75 | 3.53 | 0.94 | 1.31 | 0.13 | 0.22 |
| Lucerne chaff | 10.18 abc | 2.83 d | 1.18 b | 0.31 cd | 10.18 | 7.54 | 2.83 | 1.80 | 1.18 A | 0.37 B | 0.31 A | 0.04 B |
| Soybean shells | 13.21 a | 4.15 c | 1.44 b | 0.23 d | 13.21 | 10.84 | 4.15 | 3.36 | 1.44 | 0.97 | 0.23 | 0.12 |
| Wheat bran | 9.59 c | 4.54 c | 2.86 a | 0.62 b | 9.59 | 6.72 | 4.54 | 2.86 | 2.86 | 1.88 | 0.63 A | 0.13 B |
| Sugar beet pulp | 13.27 ab | 6.16 b | 1.25 b | 0.45 c | 13.27 | 12.70 | 6.16 | 5.75 | 1.25 | 2.14 | 0.45 | 0.35 |
| Substrate | Pre-Digestion | Fermentation | Total |
|---|---|---|---|
| Lignocellulose (including bark) | 0.02 DE | 0.15 B | 0.07 E |
| Lignocellulose (excluding bark) | 0.03 D | 0.12 B | 0.09 DE |
| Powdered cellulose | <0.01 E | 0.10 B | 0.14 CD |
| A. niger mycelium | 0.06 C | 0.40 A | 0.28 B |
| Lucerne chaff | 0.19 B | 0.37 aA | 0.22 bBCD |
| Soybean shells | 0.04 CDE | 0.40 A | 0.35 B |
| Wheat bran | 0.37 A | 0.44 aA | 0.25 bBC |
| Sugar beet pulp | 0.22 B | 0.40 bA | 0.55 aA |
| Substrate | Pre-Digestion | Fermentation | Total |
|---|---|---|---|
| Lignocellulose (including bark) | <0.01 E | 0.15 B | 0.07 E |
| Lignocellulose (excluding bark) | 0.01 DE | 0.14 B | 0.11 DE |
| Powdered cellulose | 0.01 CDE | 0.14 B | 0.21 BCD |
| A. niger mycelium | 0.01 B | 0.40 A | 0.31 BC |
| Lucerne chaff | 0.02 ABCDE | 0.38 aA | 0.21 bBC |
| Soybean shells | 0.03 ABCD | 0.42 A | 0.34 B |
| Wheat bran | 0 | 0.46 aA | 0.21 bC |
| Sugar beet pulp | 0.04 A | 0.45 bA | 0.60 aA |
| WBC | WHC | SC | pH | GP36 | GP48 | CH4 | NH3 | Acetic Acid | Propionic Acid | n-Butyric Acid | IVDMD | IVOMD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 1 | 0.85 | −0.27 | −0.11 | 0.09 | 0.12 | 0.28 | −0.34 | 0.08 | −0.04 | −0.27 | −0.35 | −0.29 |
| WHC | 1 | 0.05 | −0.43 | 0.48 | 0.49 | 0.39 | −0.25 | 0.42 | 0.33 | 0.02 | −0.03 | 0.05 | |
| SC | 1 | −0.68 | 0.52 | 0.50 | −0.08 | −0.30 | 0.47 | 0.64 | 0.16 | 0.45 | 0.48 | ||
| pH | 1 | −0.85 | −0.88 | −0.28 | −0.06 | −0.78 | −0.82 | −0.51 | −0.47 | −0.54 | |||
| GP36 | 1 | 0.99 | 0.30 | 0.15 | 0.78 | 0.77 | 0.57 | 0.56 | 0.67 | ||||
| GP48 | 1 | 0.35 | 0.22 | 0.77 | 0.76 | 0.58 | 0.56 | 0.67 | |||||
| CH4 | 1 | 0.38 | 0.58 | 0.38 | 0.36 | 0.11 | 0.08 | ||||||
| NH3 | 1 | 0.26 | 0.20 | 0.68 | 0.33 | 0.29 | |||||||
| Acetic acid | 1 | 0.88 | 0.69 | 0.52 | 0.58 | ||||||||
| Propionic acid | 1 | 0.71 | 0.54 | 0.61 | |||||||||
| n-Butyric acid | 1 | 0.57 | 0.58 | ||||||||||
| IVDMD | 1 | 0.96 | |||||||||||
| IVOMD | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, M.; Michel, S.; Greef, J.M.; Zeyner, A. Fermentation Characteristics and In Vitro Digestibility of Fibers and Fiber-Rich Byproducts Used for the Feeding of Pigs. Animals 2021, 11, 341. https://doi.org/10.3390/ani11020341
Bachmann M, Michel S, Greef JM, Zeyner A. Fermentation Characteristics and In Vitro Digestibility of Fibers and Fiber-Rich Byproducts Used for the Feeding of Pigs. Animals. 2021; 11(2):341. https://doi.org/10.3390/ani11020341
Chicago/Turabian StyleBachmann, Martin, Sebastian Michel, Jörg Michael Greef, and Annette Zeyner. 2021. "Fermentation Characteristics and In Vitro Digestibility of Fibers and Fiber-Rich Byproducts Used for the Feeding of Pigs" Animals 11, no. 2: 341. https://doi.org/10.3390/ani11020341






