Observational Study on Variation of Longitudinal Platelet Counts in Calves over the First 14 Days of Life and Reference Intervals from Cross-Sectional Platelet and Leukocyte Counts in Dairy Calves up to Two Months of Age
Abstract
:Simple Summary
Abstract
1. Introduction
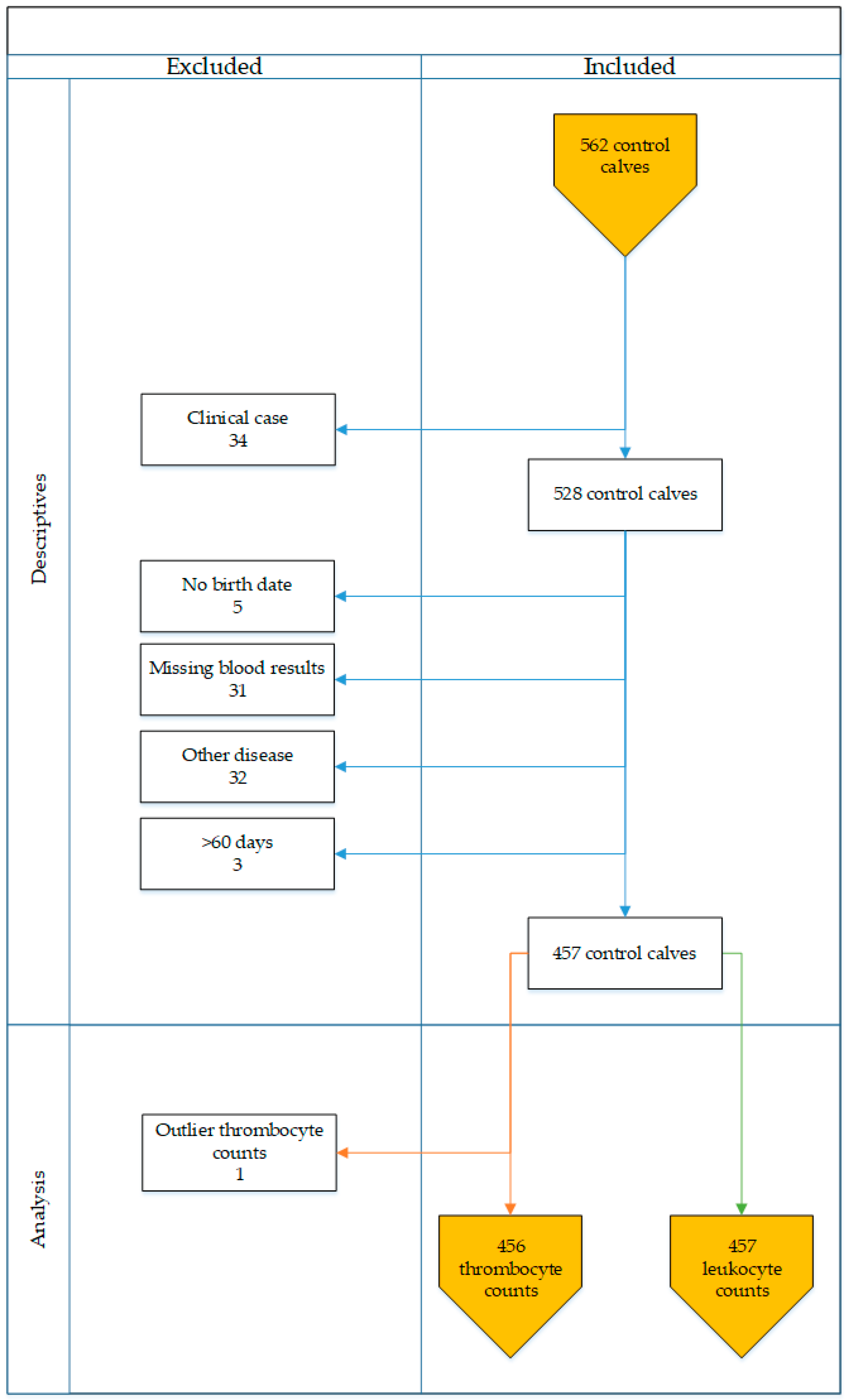
2. Materials and Methods
2.1. Ethic Statements
2.2. Longitudinal Study
2.2.1. Animal Recruitment
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Blood Sample Collection and Handling
2.2.4. Housing and Feeding
2.3. Field Study
2.3.1. Setting
2.3.2. Animal Recruitment
2.3.3. Inclusion and Exclusion Criteria
2.3.4. Blood Sample Collection and Handling
2.4. Statistical Analyses
2.4.1. Longitudinal Study
2.4.2. Field Study
3. Results
3.1. Longitudinal Study
3.2. Field Study
3.2.1. Platelet Counts
3.2.2. Leukocyte Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witt, K.; Weber, C.N.; Meyer, J.; Buchheit-Renko, S.; Müller, K.E. Haematological analysis of calves with bovine neonatal pancytopenia. Vet. Rec. 2011, 169, 228. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.J.; Harvey, J.W. Interpretation and Diagnosis. In Veterinary Laboratory Medicine; Saunders: St. Louis, MO, USA, 2004; p. 5. [Google Scholar]
- Moritz, A. Der Einsatz Lasergestützter Multiparameter-Hämatologiesysteme in der Veterinärmedizin; Justus-Liebig Universität Giessen: Giessen, Germany, 2002. (In German) [Google Scholar]
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of bovine hematology reference intervals from 1957 to 2006. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Brun-Hansen, H.C.; Kampen, A.H.; Lund, A. Hematologic values in calves during the first 6 months of life. Vet. Clin. Pathol. 2006, 35, 182–187. [Google Scholar]
- Panousis, N.; Siachos, N.; Kitkas, G.; Kalaitzakis, E.; Kritsepi-Konstantinou, M.; Valergakis, G.E. Hematology reference intervals for neonatal Holstein calves. Res. Vet. Sci. 2018, 118, 1–10. [Google Scholar] [CrossRef]
- Tennant, B.; Harrold, D.; Reina-Guerra, M.; Kendrick, J.W.; Laben, R.C. Hematology of the neonatal calf: Erythrocyte and leukocyte values of normal calves. Cornell Vet. 1974, 64, 516–532. [Google Scholar]
- Bastian, M.; Holsteg, M.; Hanke-Robinson, H.; Duchow, K.; Cussler, K. Bovine Neonatal Pancytopenia: Is this alloimmune syndrome caused by vaccine-induced alloreactive antibodies? Vaccine 2011, 29, 5267–5275. [Google Scholar] [CrossRef]
- Pardon, B.; Steukers, L.; Dierick, J.; Ducatelle, R.; Saey, V.; Maes, S.; Vercauteren, G.; De Clercq, K.; Callens, J.; De Bleecker, K.; et al. Haemorrhagic diathesis in neonatal calves: An emerging syndrome in Europe. Transbound. Emerg. Dis. 2010, 57, 135–146. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef]
- Jones, B.A.; Sauter-Louis, C.; Henning, J.; Stoll, A.; Nielen, M.; Van Schaik, G.; Smolenaars, A.; Schouten, M.; Den Uijl, I.; Fourichon, C.; et al. Calf-level factors associated with bovine neonatal pancytopenia—A multi-country case-control study. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, F.; Hinzmann, R.; Panteghini, M. Reference intervals: The way forward. Ann. Clin. Biochem. 2009, 46, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Processing Data for Outliers. Biometrics 1953, 9, 74. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Horn, P.S.; Pesce, A.J. Reference intervals: An update. Clin. Chim. Acta 2003, 334, 5–23. [Google Scholar] [CrossRef]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef]
- Ježek, J.; Nemec, M.; Starič, J.; Klinkon, M. Age Related Changes and Reference Intervals of Haematological Variables in Dairy Calves. Bull. Vet. Inst. Pulawy 2011, 55, 471–478. [Google Scholar]
- Egli, C.P.; Blum, J.W. Clinical, Haematological, Metabolic and Endocrine Traits During the First Three Months of Life of Suckling Simmentaler Calves Held in a Cow-Calf Operation. J. Vet. Med. Ser. A 2010, 45, 99–118. [Google Scholar] [CrossRef]
- Sola-Visner, M. Platelets in the neonatal period: Developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematol. Am. Soc. Hematol. Educ. Progr. 2012, 2012, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Zanker, I.A.; Hammon, H.M.; Blum, J.W. Delayed feeding of first colostrum: Are there prolonged effects on haematological, metabolic and endocrine parameters and on growth performance in calves? J. Anim. Physiol. Anim. Nutr. 2001, 85, 53–66. [Google Scholar] [CrossRef]
- Walka, M.M.; Sonntag, J.; Dudenhausen, J.W.; Obladen, M. Thrombopoietin concentration in umbilical cord blood of healthy term newborns is higher than in adult controls. Biol. Neonate 1999, 75, 54–58. [Google Scholar] [CrossRef]
- Wiedmeier, S.E.; Henry, E.; Sola-Visner, M.C.; Christensen, R.D. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J. Perinatol. 2009, 29, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Radostits, O.M.; Blood, D.C.; Gay, C.C. Veterinary Medicine: A Text Book of the Disease of Cattle, Sheep, Pigs, Goats and Horses, 10th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; ISBN 978-0-7020-2777-2. [Google Scholar]
- Latimer, K.S.; Mahaffey, E.D.; Prasse, K.W. Duncan’s and Prasse’s Veterinary Labortory Medicine: Clinical Pathology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Henry, E.; Christensen, R.D. Reference Intervals in Neonatal Hematology. Clin. Perinatol. 2015, 42, 483–497. [Google Scholar] [CrossRef]
- Biino, G.; Santimone, I.; Minelli, C.; Sorice, R.; Frongia, B.; Traglia, M.; Ulivi, S.; Di Castelnuovo, A.; Gögele, M.; Nutile, T.; et al. Age- And Sex-Related Variations in Platelet Count in Italy: A Proposal of Reference Ranges Based on 40987 Subjects’ Data. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Andres, O.; Schulze, H.; Speer, C.P. Platelets in neonates: Central mediators in haemostasis, antimicrobial defence and inflammation. Thromb. Haemost. 2015, 113, 3–12. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Italiano, J.; Ferrer-Marin, F.; Gutti, R.; Bailey, M.; Poterjoy, B.; Rimsza, L.; Sola-Visner, M. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood 2011, 117, 4106–4117. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, A.; Nakahata, T.; Matsubara, K.; Hayashi, Y.; Kato, T.; Suzuki, Y.; Shimbo, T. Age-Related changes in thrombopoietin in children: Reference interval for serum thrombopoietin levels. Br. J. Haematol. 1999, 106, 884–888. [Google Scholar] [CrossRef]
- Ma, D.C.; Sun, Y.H.; Chang, K.Z.; Zuo, W. Developmental change of megakaryocyte maturation and DNA ploidy in human fetus. Eur. J. Haematol. 2009, 57, 121–127. [Google Scholar] [CrossRef]
- Mattia, G.; Vulcano, F.; Milazzo, L.; Barca, A.; Macioce, G.; Giampaolo, A.; Hassan, H.J. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood 2002, 99, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.J.; Hoffmeister, K.M.; Hu, Z.; Mager, D.E.; Ait-Oudhia, S.; Debrincat, M.A.; Pleines, I.; Josefsson, E.C.; Kile, B.T.; Italiano, J.; et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood 2014, 123, 3381–3389. [Google Scholar] [CrossRef] [Green Version]
- England, J.M.; Rowan, R.M.; Van Assendelft, O.W.; Bull, B.S.; Coulter, W.; Fujimoto, K.; Groner, W.; Richardson-Jones, A.; Klee, G.; Koepke, J.A.; et al. Recommendations of the international council for standardization in haematology for ethylenediaminetetraacetic acid anticoagulation of blood for blood cell counting and sizing. Am. J. Clin. Pathol. 1993, 100, 371–372. [Google Scholar] [CrossRef]
- Lombarts, A.J.P.F.; De Kieviet, W. Recognition and prevention of pseudothrombocytopenia and concomitant pseudoleukocytosis. Am. J. Clin. Pathol. 1988, 89, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Vicari, A.; Banfi, G.; Bonini, P.A. EDTA-Dependent pseudothrombocytopaenia: A 12-month epidemiological study. Scand. J. Clin. Lab. Investig. 1988, 48, 537–542. [Google Scholar] [CrossRef]
- George, J.N. Platelets. Lancelet 2000, 355, 1531–1539. [Google Scholar] [CrossRef]
- Prins, M.; van Leeuwen, M.W.; Teske, E. Stability and reproducibility of ADVIA 120-measured red blood cell and platelet parameters in dogs, cats, and horses, and the use of reticulocyte haemoglobin content (CH(R)) in the diagnosis of iron deficiency. Tijdschr. Diergeneeskd. 2009, 134, 272–278. [Google Scholar]


| Week Number (Age in Days) | Total Amount | Male | Female | Sex Unrecorded |
|---|---|---|---|---|
| 1 (0–7) | 39 | 15 | 19 | 5 |
| 2 (8–14) | 142 | 60 | 81 | 1 |
| 3 (15–21) | 105 | 17 | 84 | 4 |
| 4 (22–28) | 99 | 12 | 87 | 0 |
| 5 (29–35) | 34 | }1 | }69 | }2 |
| 6 (36–42) | 21 | |||
| 7 (43–49) | 12 | |||
| 8 + 9 (50–60) | 5 |
| Age at Blood Sampling (Hours) | N | M | F | Mean | Mean Male | Mean Female | Median | SD | Min. | Max. | Range | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 7 | 8 | 435 | 471 | 403 | 394 | 175 | 235 | 822 | 587 | 338–532 |
| 1 | 14 | 7 | 7 | 439 | 485 | 394 | 400 | 172 | 242 | 873 | 631 | 340–539 |
| 2 | 15 | 7 | 8 | 453 | 493 | 419 | 437 | 143 | 260 | 816 | 556 | 374–532 |
| 3 | 15 | 7 | 8 | 440 | 473 | 411 | 396 | 158 | 234 | 850 | 616 | 352–528 |
| 6 | 14 | 6 | 8 | 429 | 470 | 398 | 412 | 147 | 229 | 769 | 540 | 344–531 |
| 12 | 15 | 7 | 8 | 403 | 444 | 368 | 382 | 147 | 220 | 796 | 576 | 322–485 |
| 24 | 14 | 6 | 8 | 381 | 441 | 335 | 345 | 138 | 207 | 729 | 522 | 301–461 |
| 48 | 15 | 7 | 8 | 395 | 439 | 356 | 395 | 123 | 232 | 669 | 437 | 326–463 |
| 72 | 15 | 7 | 8 | 443 | 494 | 398 | 427 | 184 | 215 | 969 | 754 | 341–545 |
| 96 | 16 | 8 | 8 | 498 | 519 | 477 | 475 | 194 | 258 | 981 | 723 | 395–602 |
| 120 | 13 | 6 | 7 | 642 | 685 | 605 | 581 | 265 | 299 | 1277 | 978 | 482–803 |
| 144 | 12 | 5 | 7 | 743 | 819 | 689 | 751 | 291 | 312 | 1355 | 1043 | 558–928 |
| 168 | 13 | 6 | 7 | 807 | 864 | 759 | 769 | 268 | 446 | 1402 | 956 | 645–969 |
| 192 | 12 | 5 | 7 | 822 | 899 | 767 | 815 | 235 | 479 | 1297 | 818 | 673–972 |
| 240 | 13 | 5 | 8 | 773 | 833 | 736 | 753 | 173 | 528 | 1211 | 683 | 669–878 |
| 288 | 16 | 7 | 9 | 787 | 907 | 694 | 765 | 171 | 516 | 1262 | 746 | 696–879 |
| 336 | 16 | 7 | 9 | 773 | 877 | 691 | 751 | 161 | 516 | 1199 | 683 | 687–859 |
| RIs and Descriptives | Platelet Count (×109/L) | Leukocyte Count (×109/L) |
|---|---|---|
| n = 435 | n = 457 | |
| RI calves 0–60 days | 4.0–18.9 | |
| RI calves 6–60 days | 287–1372 | |
| Descriptive results | n = 456 | n = 457 |
| Mean | 787 | 9.3 |
| Median | 773 | 9.3 |
| Standard Deviation | 269 | 1.5 |
| Minimum | 132 | 2.7 |
| Maximum | 1663 | 37.0 |
| Range | 1531 | 34.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strous, E.; Vanhoudt, A.; Smolenaars, A.; van Schaik, G.; Schouten, M.; de Pater, H.; Roelofs, B.; Nielen, M. Observational Study on Variation of Longitudinal Platelet Counts in Calves over the First 14 Days of Life and Reference Intervals from Cross-Sectional Platelet and Leukocyte Counts in Dairy Calves up to Two Months of Age. Animals 2021, 11, 347. https://doi.org/10.3390/ani11020347
Strous E, Vanhoudt A, Smolenaars A, van Schaik G, Schouten M, de Pater H, Roelofs B, Nielen M. Observational Study on Variation of Longitudinal Platelet Counts in Calves over the First 14 Days of Life and Reference Intervals from Cross-Sectional Platelet and Leukocyte Counts in Dairy Calves up to Two Months of Age. Animals. 2021; 11(2):347. https://doi.org/10.3390/ani11020347
Chicago/Turabian StyleStrous, Emma, Arne Vanhoudt, Anja Smolenaars, Gerdien van Schaik, Matthijs Schouten, Henrik de Pater, Bjorn Roelofs, and Mirjam Nielen. 2021. "Observational Study on Variation of Longitudinal Platelet Counts in Calves over the First 14 Days of Life and Reference Intervals from Cross-Sectional Platelet and Leukocyte Counts in Dairy Calves up to Two Months of Age" Animals 11, no. 2: 347. https://doi.org/10.3390/ani11020347






