Bushmeat Species Identification: Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow (LF) Strip for Identification of Formosan Reeves’ Muntjac (Muntiacus reevesi micrurus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Cooked Meat
2.3. Preparation of Purified Genomic DNA
2.4. Preparation of Crude Genomic DNA
2.5. Primer Design
2.6. Probe Design
2.7. Optimization of frmRPA-LF
2.8. Lateral Flow Analysis
2.9. Cross-Reactivity Analysis
2.10. Detection Limit
3. Results
3.1. Primer and Probe Selection
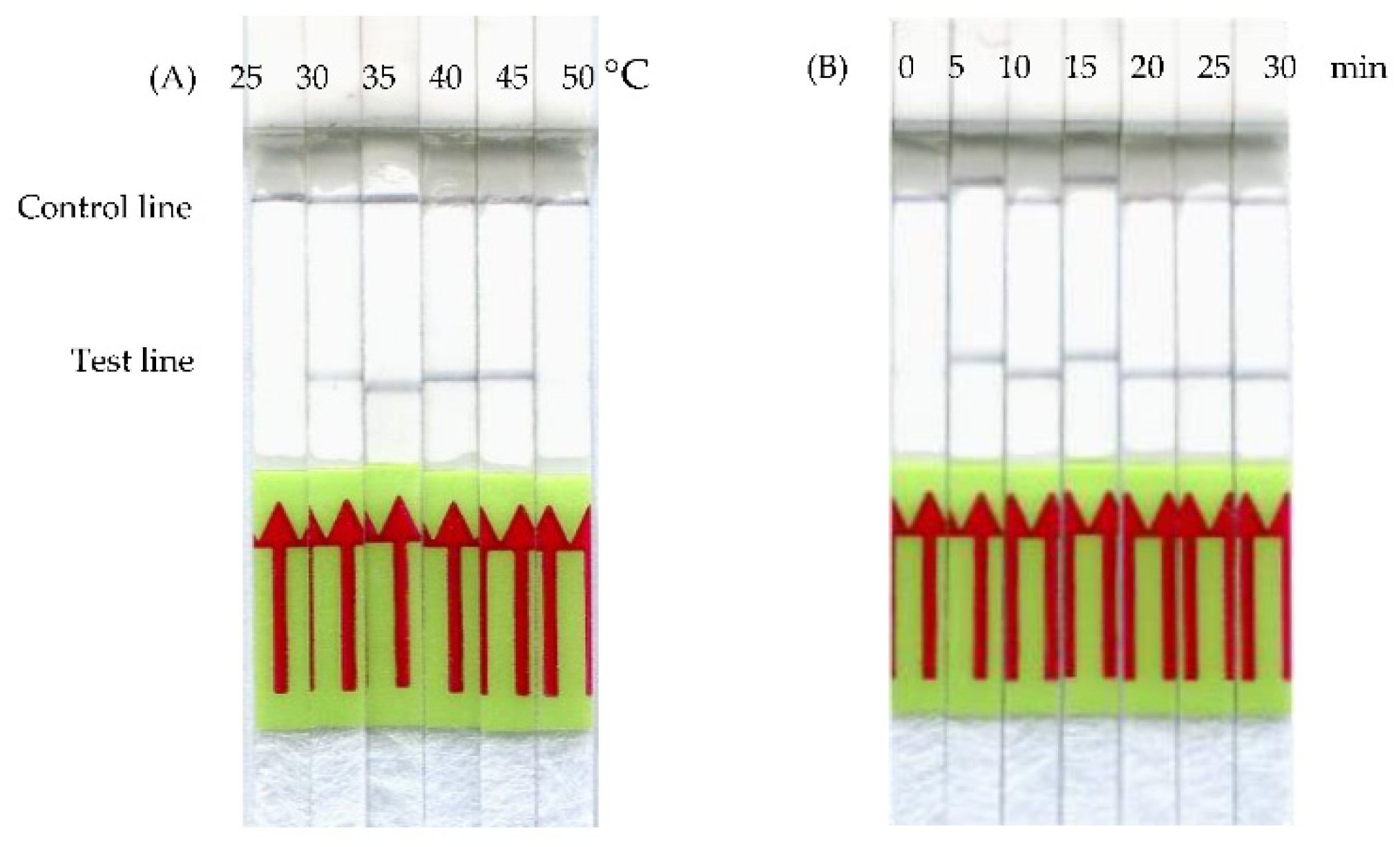
3.2. Optimization of frmRPA-LF Reaction
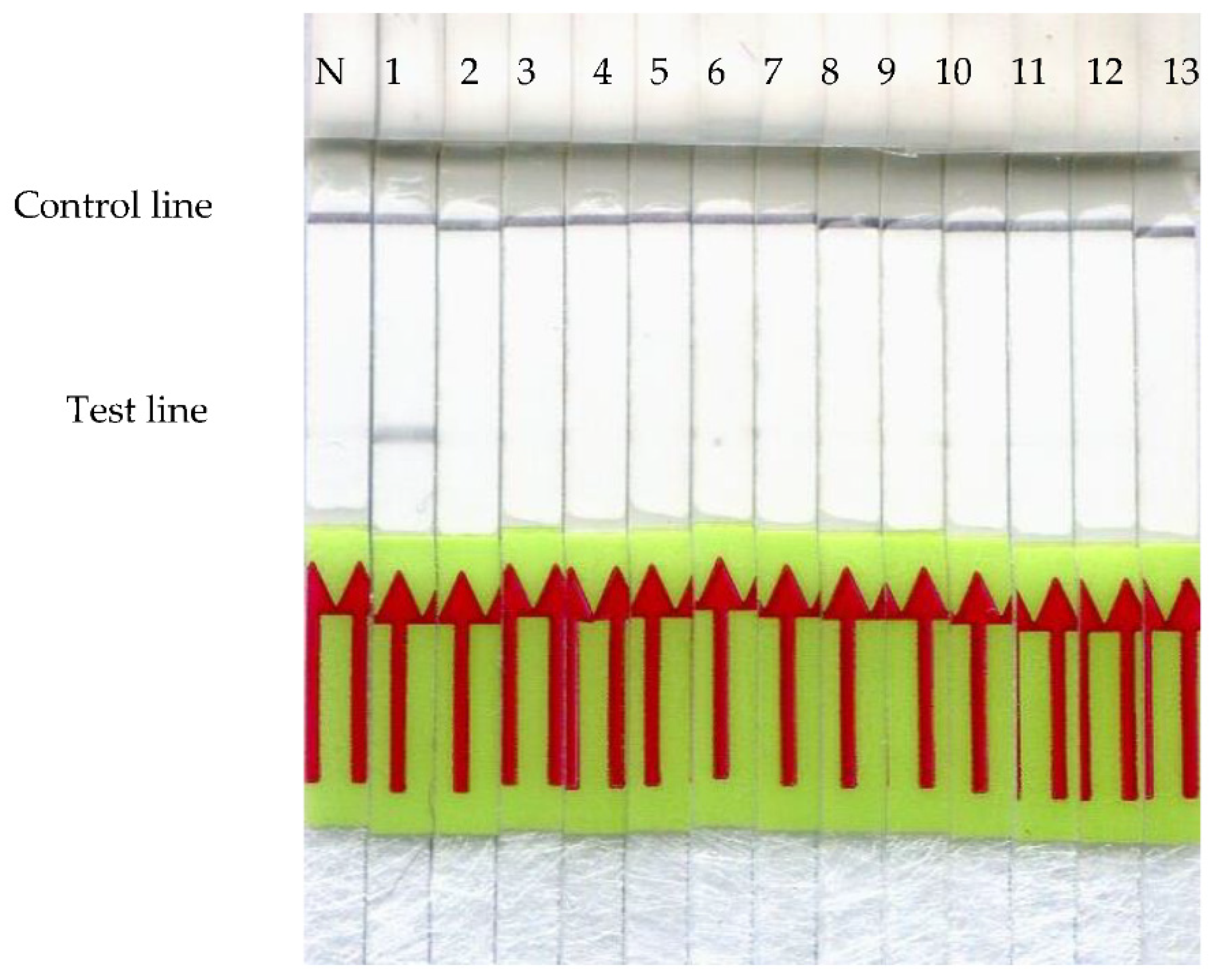
3.3. Cross-Reactivity Analysis
3.4. Detection Limit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsieh, Y.H.P.; Chen, Y.T.; Gajewski, K. Monoclonal antibody-based sandwich ELISA for reliable identification of imported Pangasius catfish. J. Food Sci. 2009, 74, C602–C607. [Google Scholar] [CrossRef]
- Kotoura, S.; Murakami-Yamaguchi, Y.; Nakamura, M.; Miake, K.; Sugiyama, M.; Tanabe, S.; Narita, H. A Sandwich ELISA for the determination of beef meat content in processed foods. Food Sci. Technol. Res. 2009, 15, 613–618. [Google Scholar] [CrossRef]
- Liu, L.; Chen, F.C.; Dorsey, J.L.; Hsieh, Y.H.P. Sensitive monoclonal antibody-based sandwich ELISA for the detection of porcine skeletal muscle in meat and feed products. J. Food Sci. 2006, 71, M1–M6. [Google Scholar] [CrossRef]
- Macedo-Silva, A.; Barbosa, S.F.; Alkmin, M.G.A.; Vaz, A.J.; Shimokomaki, M.; Tenuta-Filho, A. Hamburger meat identification by dot-ELISA. Meat Sci. 2000, 56, 189–192. [Google Scholar] [CrossRef]
- Chan, K.W.; Lo, C.; Chu, C.H.; Chin, L.T.; Wang, Y.T.; Yang, W.C. Development of a colloidal gold-based immunochromatographic test strip for detection of cetacean myoglobin. J. Vis. Exp. 2016, 113, 53433. [Google Scholar] [CrossRef] [PubMed]
- Boyacı, İ.H.; Temiz, H.T.; Uysal, R.S.; Velioğlu, H.M.; Yadegari, R.J.; Rishkan, M.M. A Novel method for discrimination of beef and horsemeat using raman spectroscopy. Food Chem. 2014, 148, 37–41. [Google Scholar] [CrossRef]
- Zajac, A.; Hanuza, J.; Dyminska, L. Raman spectroscopy in determination of horse meat content in the mixture with other meats. Food Chem. 2014, 156, 333–338. [Google Scholar] [CrossRef]
- Castello, A.; Francino, O.; Cabrera, B.; Polo, J.; Sanchez, A. Identification of bovine material in porcine spray-dried blood derivatives using the polymerase chain reaction technique. Biotechnol. Agron. Soc. Environ. 2004, 8, 267–273. [Google Scholar]
- Colgan, S.; O’Brien, L.; Maher, M.; Shilton, N.; McDonnell, K.; Ward, S. Development of a DNA based assay for species identification in meat and bone meal. Food Res. Int. 2001, 34, 409–414. [Google Scholar] [CrossRef]
- Calvo, J.H.; Zaragoza, P.; Osta, R. A quick and more sensitive method to identify pork in processed and unprocessed food by PCR amplification of a new specific DNA fragment. J. Anim. Sci. 2001, 79, 2108–2112. [Google Scholar] [CrossRef] [Green Version]
- Calvo, J.H.; Rodellar, C.; Zaragoza, P.; Osta, R. Beef-And bovine-derived material identification in processed and unprocessed food and feed by PCR amplification. J. Agric. Food Chem. 2002, 50, 5262–5264. [Google Scholar] [CrossRef]
- Herman, B.L. Determination of the animal origin of raw food by species-specific PCR. J. Dairy Res. 2001, 68, 429–436. [Google Scholar] [CrossRef]
- Veerkaar, E.L.C.; Nijman, I.J.; Boutaga, K.; Lenstra, J. Differentiation of cattle species in beef by PCR-RFLP of mitochondrial and satellite DNA. Meat Sci. 2002, 60, 365–369. [Google Scholar] [CrossRef]
- Girish, P.S.; Anjaneyulu, A.S.R.; Viswas, K.N.; Shivakumar, B.M.; Anand, M.; Patel, M.; Sharma, B. Meat species identification by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) of mitochondrial 12S rRNA gene. Meat Sci. 2005, 70, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, F.; Comincini, S.; Ferretti, L.; Valfre, F.; Moretti, V.M. Application of quantitative real-time PCR in the detection of prion-protein gene species-specific DNA sequences in animal meals in animal meals and feedstuffs. J. Food Prot. 2006, 69, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Andreo, M.; Lugo, L.; Garrido-Pertierra, A.; Prieto, M.I.; Puyet, A. Identification and quantification of species in complex DNA mixtures by real-time polymerase chain reaction. Anal. Biochem. 2005, 339, 73–82. [Google Scholar] [CrossRef]
- Laube, I.; Zagon, J.; Broll, H. Quantitative determination of commercially relevant species in foods by real-time PCR. Int. J. Food Sci. Technol. 2007, 42, 336–341. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase polymerase amplification for diagnostic applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends. Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Li, Z.; Torres, J.E.P.; Goossens, J.; Stijlemans, B.; Sterckx, Y.G.J.; Magez, S. Development of a recombinase polymerase amplification lateral flow assay for the detection of active Trypanosoma evansi infections. PLoS Negl. Trop. Dis. 2020, 14, e0008044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Yao, J.; Yuan, S.; Liu, H.; Wei, N.; Zhang, J.; Shan, W. Development of a lateral flow recombinase polymerase amplification assay for rapid and visual detection of Cryptococcus neoformans/C. Gattii in cerebral spinal fluid. BMC Infect. Dis. 2019, 19, 108. [Google Scholar] [CrossRef]
- Silva, G.; Oyekanmi, J.; Nkere, C.K.; Bömer, M.; Kumar, P.L.; Seal, E.S. Rapid detection of potyviruses from crude plant extracts. Anal. Biochem. 2018, 546, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Zhou, D.H.; Zhang, L.X.; Zheng, W.B.; Ma, J.G.; Wang, M.; Zhu, X.Q.; Xu, M.J. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for equipment-free detection of Cryptosporidium spp. oocysts in dairy cattle feces. Parasitol. Res. 2016, 115, 3551–3555. [Google Scholar] [CrossRef]
- Yang, M.; Ke, Y.; Wang, X.; Ren, H.; Liu, W.; Lu, H.; Zhang, W.; Liu, S.; Chang, G.; Tian, S.; et al. Development and evaluation of a rapid and sensitive EBOV-RPA test for rapid diagnosis of Ebola virus disease. Sci. Rep. 2016, 6, 26943. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; van der Linden, H.; Hartskeerl, R.A. Development of a recombinase polymerase amplification assay for the detection of pathogenic Leptospira. Int. J. Environ. Res. Public Health 2014, 11, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.S.; McNerney, R.; Low, H.T.; Leader, B.T.; Perez-Osorio, A.C.; Meyer, J.C.; O’Sullivan, D.M.; Brooks, D.G.; Piepenburg, O.; Forrest, M.S. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification. PLoS ONE 2014, 9, e103091. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Trabasso, P.; Moretti, M.L.; Mikami, Y.; Kamei, K.; Gonoi, T. Identification of fungal pathogens by visible microarray system in combination with isothermal gene amplification. Mycopathologia 2014, 178, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Temmam, S.; Davoust, B.; Chaber, A.L.; Lignereux, Y.; Michelle, C.; Monteil-Bouchard, S.; Raoult, D.; Desnues, C. Screening for viral pathogens in African simian bushmeat seized at a French airport. Transbound. Emerg. Dis. 2017, 64, 1159–1167. [Google Scholar] [CrossRef]
- Ortea, I.; Cañas, B.; Calo-Mata, P.; Barros-Velázquez, J.; Gallardo, J.M. Argininekinase peptide mass fingerprinting as a proteomic approach for species identification and taxonomic analysis of commercially relevant shrimp species. J. Agric. Food Chem. 2009, 57, 5665–5672. [Google Scholar] [CrossRef]
- Giovannacci, I.; Guizard, C.; Carlier, M.; Duval, V.; Martin, J.L.; Demeulemester, C. Species identification of meat products by ELISA. Int. J. Food Sci. Technol. 2004, 39, 863–867. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Sheu, S.C.; Bridgman, R.C. Development of a monoclonalantibody specific to cooked mammalian meats. J. Food Prot. 1998, 61, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Lockley, A.K.; Bardsley, R.G. DNA-Based methods for food authentication. Trends Food Sci. Technol. 2000, 11, 67–77. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.R.; Sharma, B.D.; Gokulakrishnan, P.; Mediratta, S.K.; Sharma, D. Identification of species origin of meat and meat products on the DNA basis: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Lillis, L.; Lehman, D.; Singhal, M.C.; Cantera, J.; Singleton, J.; Labarre, P.; Toyama, A.; Piepenburg, O.; Parke, M.; Wood, R.; et al. Non-Instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS ONE 2014, 9, e108189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-Dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lei, T.; Liu, Z.; Kuang, Y.; Lyu, J.; Wang, Q. A novel technique to detect EGFR mutations in lung cancer. Int. J. Mol. Sci. 2016, 17, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Liu, H.; Ye, F.; Xiang, G.; Shan, W.; Xing, W. Rapid and visual detection of Mycobacterium tuberculosis complex using recombinase polymerase amplification combined with lateral flow strips. Mol. Cell. Probes 2017, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yu, Y.; Weidmann, M.; Pan, Y.; Yan, S.; Wang, Y. Rapid detection of shrimp white spot syndrome virus by real time, isothermal recombinase polymerase amplification assay. PLoS ONE 2014, 9, e104667. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Jin, L.; Long, K.; Tang, Q.; Ma, J.; Wang, X.; Zhu, L.; Jiang, A.; Tang, G.; Jiang, Y.; et al. Analysis of mitochondrial DNA sequence and copy number variation across five high-altitude species and their low-altitude relatives. Mitochondrial DNA Part B 2018, 3, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Hopwood, A.J.; Fairbrother, K.S.; Lockley, A.K.; Bardsley, R.G. An actin gene-related polymerase chain reaction (PCR) test for identification of chicken in meat mixtures. Meat Sci. 1999, 53, 227–231. [Google Scholar] [CrossRef]
- Matsunaga, T.; Chikuni, K.; Tanabe, R.; Muroya, S.; Shibata, K.; Yamada, J.; Shinmura, Y. A quick and simple method for the identification of meat species and meat products by PCR assay. Meat Sci. 1999, 51, 143–148. [Google Scholar] [CrossRef]
- Meyer, R.; Candrian, U.; Lüthy, J. Detection of pork in heated meat products by the polymerase chain reaction. J. AOAC Int. 1994, 77, 617–622. [Google Scholar] [CrossRef]
- Arslan, A.; Ilhak, O.I.; Calicioglu, M. Effect of method of cooking on identification of heat processed beef using polymerase chain reaction (PCR) technique. Meat Sci. 2006, 72, 326–330. [Google Scholar] [CrossRef] [PubMed]


| Meat Type | DNeasy® Blood & Tissue Kit | UniversAll Tissue Extraction Buffer |
|---|---|---|
| Raw | 27.7 | 4.84 |
| Stir-fried in soy sauce | 46.4 | 3.02 |
| Boiled | 80.2 | 5.66 |
| Pan-fried | 40.4 | 4.06 |
| Roasted | 44.4 | 4.64 |
| Stir-fried in sesame oil | 46.4 | 5.84 |
| Stir-fried in barbecue sauce | 58.6 | 2.42 |
| Stewed | 45.2 | 1.56 |
| Primer | Sequence (5′-3′) | Gene Location (EF035447) |
|---|---|---|
| frm-forward | 5′-ACAGGATCCAACAATCCAACAGGAATCCCATC-3′ | 607–638 |
| frm-reverse | 5′-Biotin-GGGGAGGTGTATTGAGTGGATTTGCTGGGGTATAG-3′ | 765–799 |
| frm-probe | 5′-FAM-TACCATCAAAGATATTCTGGGTGCCTTACTTCT (THF) AACTCTCTCTCTAA C3 spacer-3′ | 672–718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Yang, W.-C.; Chan, K.-W. Bushmeat Species Identification: Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow (LF) Strip for Identification of Formosan Reeves’ Muntjac (Muntiacus reevesi micrurus). Animals 2021, 11, 426. https://doi.org/10.3390/ani11020426
Hsu Y-H, Yang W-C, Chan K-W. Bushmeat Species Identification: Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow (LF) Strip for Identification of Formosan Reeves’ Muntjac (Muntiacus reevesi micrurus). Animals. 2021; 11(2):426. https://doi.org/10.3390/ani11020426
Chicago/Turabian StyleHsu, Yun-Hsiu, Wei-Cheng Yang, and Kun-Wei Chan. 2021. "Bushmeat Species Identification: Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow (LF) Strip for Identification of Formosan Reeves’ Muntjac (Muntiacus reevesi micrurus)" Animals 11, no. 2: 426. https://doi.org/10.3390/ani11020426





