Differences in Reproductive Success in Young and Old Females of a Long-Lived Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Study Site
2.2. Mediterranean Spur-Thighed Hatchlings
2.3. Hatchling Body Mass and Size Analysis
2.4. Modelling Hatchling Survival
3. Results
3.1. Description of Hatchling Biometry from Emergence and Environmental Differences
3.2. Hatchling Body Mass and Size
3.3. Hatchling Survival
4. Discussion
4.1. Maternal Characteristics and Offspring Fitness
4.2. Influence of Interannual Variation on Recruitment Success
4.3. Increasing Descriptive Knowledge of Tortoise Hatchling Biometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stearns, S.C. The evolution of life history traits: A critique of the theory and a review of the data. Ann. Rev. Ecol. Syst. 1977, 8, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Michod, R.E. Evolution of life histories in response to age specific mortality factors. Am. Nat. 1979, 113, 531–550. [Google Scholar] [CrossRef]
- Lindstedt, S.; Calder, W. Body size, physiological time, and longevity of homeothermic animals. Q. Rev. Biol. 1981, 56, 1–16. [Google Scholar] [CrossRef]
- Iverson, J.B. Correlates of reproductive output in turtles (order Testudines). Herpetol. Monogr. 1992, 6, 25. [Google Scholar] [CrossRef]
- Promislow, D.E.L. On size and survival: Progress and pitfalls in the allometry of life span. J. Gerontol. 1993, 48, B115–B123. [Google Scholar] [CrossRef]
- Epperson, D.M.; Colleen, D.H. Nestling and hatchling ecology of Gopher tortoises (Gopherus polyphemus) in southern Mississippi. J. Herpetol. 2003, 37, 315–324. [Google Scholar] [CrossRef]
- Heppell, S.S. Application of life-history theory and population model analysis to turtle conservation. Copeia 1998, 1998, 367. [Google Scholar] [CrossRef] [Green Version]
- Congdon, J.D.; Dunham, A.E. Delayed sexual maturity and demographics of Blanding´s turtles Emyoidea blandingii: Implications for conservation and management of long-lived organisms. Conservat. Biol. 2003, 7, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Fay, R.; Barbraud, C.; Delord, K.; Weimerskirch, H. Variation in the age of first reproduction: Different strategies or individual quality? Ecology 2017, 97, 1842–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamel, S.; Côté, S.D.; Gaillard, J.M.; Festa-Bianchet, M. Individual variation in reproductive costs of reproduction: High-quality females always do better. J. Anim. Ecol. 2009, 78, 143–151. [Google Scholar] [CrossRef] [PubMed]
- McGraw, J.B.; Caswell, H. Estimation of individual fitness from life-history data. Am. Nat. 1996, 147, 47–64. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Viallefont, A.; Cooke, F.; Lebreton, J.D. Age-specific costs of first-time breeding. Auk 1995, 112, 67–76. [Google Scholar] [CrossRef]
- Kim, S.Y.; Velando, A.; Torres, R.; Drummond, H. Effects of recruiting age on senescence, lifespan and lifetime reproductive success in a long-lived seabird. Oecologia 2011, 166, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Congdon, J.D.; Nagle, R.D.; Kinney, O.M.; Sels, R.C.V. Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp. Gerontol. 2003, 38, 765–772. [Google Scholar] [CrossRef]
- Lansing, A.I. A transmissible, cumulative and reversible factor in aging. J. Gerontol. 1947, 2, 228–239. [Google Scholar] [CrossRef]
- García-Palomares, S.; Navarro, S.; Pertusa, J.F.; Hermeregildo, C.; García-Perez, M.A.; Rausell, F.; Cano, A.; Tarín, J.J. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. Biol. Reprod. 2009, 80, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Rollinson, N.; Hutchings, J.A. Environmental quality predicts optimal egg size in the wild. Am. Nat. 2013, 182, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, A. Movement patterns, habitat use and growth hatchling tortoises. Gopherus polyphemus Copeia 2006, 1, 68–76. [Google Scholar] [CrossRef]
- Jenouvrier, S.; Tavecchia, G.; Thibault, J.C.; Choquet, R.; Bretagnolle, V. Recruitment processes in long-lived species with delayed maturity: Estimating key demographic parameters. Oikos 2008, 117, 620–628. [Google Scholar] [CrossRef]
- Segura, A.; Jimenez, J.; Acevedo, P. Predation of young tortoises by ravens: The effect of habitat structure on tortoise detectability and abundance. Sci. Rep. 2020, 10, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Heydrich, C.; Jackson, K.; Wendland, L.D.; Brown, M.B. Gopher Tortoise hatchling survival: Field study and meta-analysis. Herpetologica 2012, 68, 334–344. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intergovernmental Panel on Climate Change. Special Report on Global Warming of 1.5 °C; IPCC: Malmo, Sweden, 2017. [Google Scholar]
- Frankenberg, E.; Werner, Y.L. Egg, clutch and maternal sizes in lizards: Intra- and interspecific relations in Near-Eastern Agamidae and Lacertidae. Herpetol. J. 1992, 2, 7–18. [Google Scholar]
- Hailey, A.; Loumbourdis, N.S. Egg size and shape, clutch dynamics, and reproductive effort in European tortoises. Can. J. Zool. 1988, 66, 1527–1536. [Google Scholar] [CrossRef]
- Warne, R.W.; Charnow, E.L. Reproductive allometry and the size-number trade-off for lizards. Am. Nat. 2008, 172, E80–E98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Paniagua, C.; Keller, C.; Andreu, A.C. Long-term demographic fluctuations of the spur-thighed tortoise, Testudo graeca in SW Spain. Ecography 2001, 24, 707–721. [Google Scholar] [CrossRef]
- Dunham, A.E.; Miles, D.M.; Reznick, D. Life history patterns in squamate reptiles. In Biology of the Reptilian; Gans, C., Huey, R., Eds.; Alan R. Liss: New York, NY, USA, 1988; pp. 443–511. [Google Scholar]
- Keller, C.; Díaz-Paniagua, C.; Andreu, A.C. Survival rates and mortality causes of Testudo graeca hatchlings in southwestern Spain. J. Herpet. 1998, 32, 238–243. [Google Scholar] [CrossRef]
- Rodríguez-Caro, R.C.; Gracia, E.; Morais, R.; Anadón, J.D.; Gimenez, A. One scute ring per year in Testudo graeca? A novel method to identify ring deposition patterns in tortoises. Acta Herpetol. 2015, 10, 77–84. [Google Scholar]
- Anadón, J.D.; Giménez, A.; Graciá, E.; Pérez, I.; Fernandez, M.; Fahd, S.; El Mouden, E.H.; Kalboussi, M.; Jdeidi, T.; Larbes, S.; et al. Distribution of Testudo graeca in the western Mediterranean according to climatic factors. Amphib. Reptil. 2012, 33, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Segura, A.; Acevedo, P. The importance of protected and unprotected areas for the Mediterranean spur-thighed tortoise demography in northwest Morocco. Amphib. Reptil. 2019, 40, 369–371. [Google Scholar] [CrossRef]
- Ben Kaddour, K.; El Mouden, E.H.; Slimani, T.; Lagarde, F. Dimorphisme sexual et cinetique de croissance et de maturation chez Testudo g. graeca, dans le Jbilets centrales, Maroc. Rev. Ecol. 2005, 60, 256–278. [Google Scholar]
- El Mouden, E.H.; Slimani, T.; Ben Kaddour, K. Croissance et dimorphisme sexual chez la tortue maresque (Testudo graeca graeca L. 1758). Chelonii 2002, 3, 325–330. [Google Scholar]
- Jímenez-Franco, M.V.; Gímenez, A.; Rodríguez-Caro, R.; Sánz-Aguilar, A.; Botella, F.; Anadón, J.D.; Wiegan, T.; Graciá, E. Sperm storage reduces the strength of the mate-finding Allee effect. Ecol. Evol. 2020, 10, 1938–1948. [Google Scholar] [CrossRef]
- Rodríguez-Caro, R.; Wiegand, T.; White, E.; Sanz-Aguilar, A.; Giménez, A.; Graciá, E.; van Benthem, K.J.; Anadón, J.D. A low cost approach to estimate demographicrates using inverse modelling. Biol. Conserv. 2019, 237, 365. [Google Scholar] [CrossRef]
- Lebreton, J.D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef] [Green Version]
- Lebreton, J.D.; Pradel, R. Multistate recapture models: Modelling incomplete in- dividual histories. J. Appl. Stat. 2002, 29, 353–369. [Google Scholar] [CrossRef]
- Thomson, D.L.; Cooch, E.G.; Conroy, M.J. Modeling Demographic Processes in Marked Populations; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Sánz-Aguilar, A.; Igual, J.M.; Oro, D.; Genovart, M.; Tavecchia, G. Estimating recruitment and survival in partially monitored populations. J. Appl. Ecol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 25 February 2020).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Cooch, E.; White, G. Program MARK: A Gentle Introduction, 6th ed.; Cornell University: Ithaca, NY, USA, 2008; Available online: http://www.phidot.org/software/mark/docs/book/ (accessed on 25 February 2020).
- Pradel, R.; Wintrebert, C.M.A.; Gimenez, O. A Proposal for a foodness of fit test to the Arnason-Schwarz multisite capture-recapture model. Biometrics 2003, 59, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Choquet, R.; Lebreton, J.D.; Gimenez, O.; Reboulet, A.M.; Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating Capture-Recapture data. Ecography 2009, 32, 1071–1074. [Google Scholar] [CrossRef]
- Paterson, J.E.; Steinberg, B.D.; Litzgus, J.D. Effects of body size, habitat selection and exposure on hatchling turtle survival. J. Zool. 2014, 294, 278–285. [Google Scholar] [CrossRef]
- O’Donoghue, M. Early survival of juvenile snowshoe hares. Ecology 1994, 75, 1582–1592. [Google Scholar] [CrossRef]
- Janzen, F.J.; Tucker, J.K.; Paukstis, G.L. Experimental analysis of an early life-history stage: Direct or indirect selection on body size of hatchling turtles? Funct. Ecol. 2007, 21, 162–170. [Google Scholar] [CrossRef]
- Tucker, J.K. Body size and migration of hatchling turtles: Inter- and intraspecific comparisons. J. Herpetol. 2000, 34, 541–546. [Google Scholar] [CrossRef]
- Golubovik, A. Ontogenetic shift of antipredator behavior in Hermans tortoise. Behav. Ecol. Sociobiol. 2015, 69, 1201–1208. [Google Scholar] [CrossRef]
- Acker, P.; Robert, A.R.; Bourget, R.; Colas, B. Heterogeneity of reproductive age increases the viability of semelparous populations. Funct. Ecol. 2014, 28, 458–468. [Google Scholar] [CrossRef]
- Ferrer, M.; Bisson, I. Age and territory quality effects on fecundity in Spanish Imperial Eagle (Aquila adalberti). Auk 2003, 120, 180–186. [Google Scholar] [CrossRef]
- Gaillard, J.M.; Festa-Bianchet, M.; Yoccoz, N.G.; Loison, A.; Toigo, C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 367–393. [Google Scholar] [CrossRef]
- McMahon, C.R.; Burton, H.R.; Bester, M.N. A demographic comparison of two southern elephant seal populations. J. Anim. Ecol. 2003, 72, 61–74. [Google Scholar] [CrossRef]
- Henen, B.T. Seasonal and annual energy budgets of female Desert Tortoises (Gopherus agassizii). Ecology 1997, 78, 283–296. [Google Scholar] [CrossRef]
- Loehr, V.J.; Henen, B.T.; Hofmeyr, M.D. Reproductive responses to rainfall in the Namaqualand speckled tortoise. Copeia 2011, 2, 278–284. [Google Scholar] [CrossRef]
- Leuteritz, T.E.J.; Ravolanaivo, R. Reproductive ecology and egg production of the Radiated Tortoise (Geochelone radiate) in southern Madagascar. Afr. Zool. 2005, 40, 233–242. [Google Scholar] [CrossRef]
- Lovich, J.E.; Medica, P.; Avery, H.; Meyer., K.; Bowser., G.; Brown., A. Studies of reproductive output of the desert tortoise at Joshua Tree National Park, the Mojave National Preserve, and comparative sites. Park Sci. 1999, 19, 22–24. [Google Scholar]
- Lewis-Winokur, V.; Winokur, R.M. Incubation temperature affects sexual differentiation, incubation time, and posthatching survival in desert tortoises (Gopherus agassizi). Can. J. Zool. 1995, 73, 2091–2097. [Google Scholar] [CrossRef]
- Hofmeyr, M.D.; Henen, B.T.; Loehr, V.J.T. Overcoming environmental and morphological constraints: Egg size and pelvic kinesis in the smallest tortoise. Homopus SignatusCan. J. Zool. 2005, 83, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Anadón, J.D.; Giménez, A.; Martínez, M.; Martínez, J.; Pérez, I.; Esteve, M.A. Factors determining the distribution of the spur thighed tortoise Testudo graeca in south esat Spain: A hierarchical approach. Ecography 2006, 29, 339–346. [Google Scholar] [CrossRef]
- Regnier, T.; Bolliet, V.; Gaudin, P.; Labonne, J. Bigger is not always better: Egg size influences survival throughout incubation in brown trout (Salmo trutta). Ecol. Freshw. Fish 2013, 22, 169–177. [Google Scholar] [CrossRef]
- Lovich, J.E.; Agha, M.; Meyer, K.; Ennen, J.; Loughran, C.; Madrak, S.; Bjurlin, C. Climatic variation affects clutch phenology in Agassiz’s desert tortoise. Gopherus Agassizii Endanger. Species Res. 2012, 19, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.; Gallardo, C.; Gaertner, M.A.; Arribas, A.; Castro, M. Future climate extreme events in the Mediterranean simulated by a regional climate model: A first approach. Glob. Planet Change 2004, 44, 163–180. [Google Scholar] [CrossRef]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional climate projections. Climate change 2007, The physical science basis. In Climate Change 2007, The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Fernández-Chacón, A.; Bertolero, A.; Amengual, A.D.; Tavecchia, G.; Homar, V.; Oro, D. Spatial heterogeneity in the effects of climate change on the population dynamics of a Mediterranean tortoise. Glob. Change Biol. 2011, 17, 3075–3088. [Google Scholar] [CrossRef]
- Hagerman, S.; Dowlatabadi, H.; Satterfield, T.; McDaniels, T. Expert views on biodiversity conservation in an era of climate change. Glob. Environ. Change 2010, 20, 192–207. [Google Scholar] [CrossRef]
- Hannah, L.; Midgley, G.F.; Millar, D. Climate change-integrated conservation strategies. Glob. Ecol. Biogeogr. 2002, 11, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.A.; Bowman, R.D.; Hull, T.W.; Sowell, S. Movements and home range of hatchling and yearling gopher tortoises. Gopherus Polyphemus Chelon. Conserv. Biol. 1995, 1, 173–180. [Google Scholar]
- Keller, C.; Díaz-Paniagua, C.; Andreu, A.C. Post-emergent activity and growth rates of hachling spur-thighed tortoise. Testudo Graeca Can. J. Zool. 1997, 75, 1089–1098. [Google Scholar] [CrossRef]
- Pike, D.; Seigel, R.A. Variation in hatchling tortoise survivorship at three geographic localities. Herpetologica 2006, 62, 125–131. [Google Scholar] [CrossRef]
- Morafka, D.J. Neonates: Missing links in the life histories of North American tortoises. In Biology of North American Tortoises; Bury, R.B., Germano, D.J., Eds.; National Biological Survey: Washington, DC, USA, 1994; pp. 161–173. [Google Scholar]
- Avery, H.W.; Spotila, J.R.; Congdon, J.D.; Fischer, R.U.; Standora, E.A.; Avery, S.B. Roles of diet protein and temperature in growth and nutritional energetics of juvenile slider turtles. Trachemys Scripta Physiol. Zool. 1993, 66, 902–925. [Google Scholar] [CrossRef]
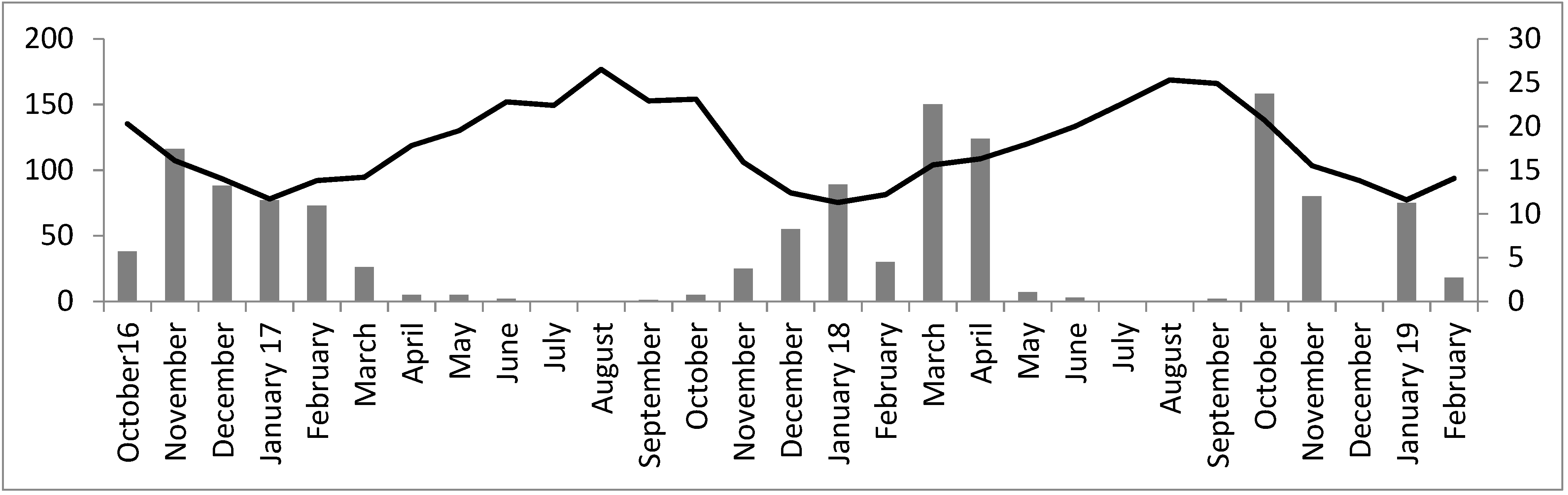


| Items | No Hatchlings/Tortoise | Hatchling Body Size | Hatchling Body Mass | |
|---|---|---|---|---|
| 2017 | Young (n = 14) | 1.4 | 38 (36–39) | 11.5 (11–13) |
| Old (n = 37) | 3.7 | 34 (29–38) | 9.8 (8–13) | |
| 2018 | Young (n = 3) | 0.3 | 37 (36–37) | 12.7 (12–13) |
| Old (n = 18) | 1.8 | 36 (33–39) | 14.6 (12–17) |
| Model | Variable | Estimate | Std. Error |
|---|---|---|---|
| Size | Intercept | 29.53 | 0.48 |
| Month | 0.51 | 0.03 | |
| Female age (young) | 5.51 | 0.89 | |
| Period (2018/2019) | 1.51 | 0.58 | |
| Month * female age (young) | −0.30 | 0.06 | |
| Body mass | Intercept | −6.42 | 0.90 |
| Month | 1.72 | 0.07 | |
| Female age (young) | 4.86 | 1.65 | |
| Period (2018/2019) | 9.56 | 1.65 | |
| Month * female age (young) | −0.31 | 0.13 | |
| Month * period (2018/2019) | −0.55 | 0.14 | |
| Period (2018/2019) * female age (young) | −3.19 | 1.50 |
| Model Specification | AICc | ΔAICc | ω | nPars | Dev. |
|---|---|---|---|---|---|
| φ(e)ρ(e + t) | 322.50 | 0 | 0.48 | 11 | 145.29 |
| φ(e)ρ(t) | 323.20 | 0.70 | 0.33 | 10 | 148.48 |
| φ(.)ρ(t) | 326.25 | 3.76 | 0.07 | 9 | 153.97 |
| φ(e)ρ(e * t) | 326.39 | 3.89 | 0.07 | 16 | 135.91 |
| φ(.)ρ(e + t) | 327.34 | 4.84 | 0.04 | 10 | 152.62 |
| φ(.)ρ(e * t) | 331.10 | 8.60 | 0.00 | 15 | 143.39 |
| φ(e)ρ(.) | 359.50 | 37.00 | 0.00 | 3 | 200.84 |
| φ(.)ρ(.) | 363.50 | 41.00 | 0.00 | 2 | 206.95 |
| Model Specification | AICc | ΔAICc | ω | nPars | Dev. |
|---|---|---|---|---|---|
| φ(pe)ρ(pe * t) | 236.80 | 0.00 | 1.00 | 14 | 112.30 |
| φ(pe)ρ(.) | 252.57 | 15.77 | 0.00 | 3 | 155.86 |
| φ(pe)ρ(t) | 252.77 | 15.96 | 0.00 | 11 | 136.69 |
| φ(.)ρ(pe) | 253.06 | 16.26 | 0.00 | 3 | 156.35 |
| φ(pe)ρ(pe) | 253.73 | 16.92 | 0.00 | 4 | 154.81 |
| φ(.)ρ(t) | 253.89 | 17.08 | 0.00 | 10 | 140.46 |
| φ(.)ρ(.) | 255.39 | 18.59 | 0.00 | 2 | 160.83 |
| φ(pe)ρ(pe + t) | 255.48 | 18.68 | 0.00 | 12 | 136.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura, A.; Rodriguez-Caro, R.C.; Graciá, E.; Acevedo, P. Differences in Reproductive Success in Young and Old Females of a Long-Lived Species. Animals 2021, 11, 467. https://doi.org/10.3390/ani11020467
Segura A, Rodriguez-Caro RC, Graciá E, Acevedo P. Differences in Reproductive Success in Young and Old Females of a Long-Lived Species. Animals. 2021; 11(2):467. https://doi.org/10.3390/ani11020467
Chicago/Turabian StyleSegura, Amalia, Roberto C. Rodriguez-Caro, Eva Graciá, and Pelayo Acevedo. 2021. "Differences in Reproductive Success in Young and Old Females of a Long-Lived Species" Animals 11, no. 2: 467. https://doi.org/10.3390/ani11020467
APA StyleSegura, A., Rodriguez-Caro, R. C., Graciá, E., & Acevedo, P. (2021). Differences in Reproductive Success in Young and Old Females of a Long-Lived Species. Animals, 11(2), 467. https://doi.org/10.3390/ani11020467






