Anandamide Influences Interleukin-1? Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Experimental Procedures
2.3. IL-1β Concentration Assesment
2.4. Relative Gene Expression Determination
2.5. Statistical Analysis
3. Results
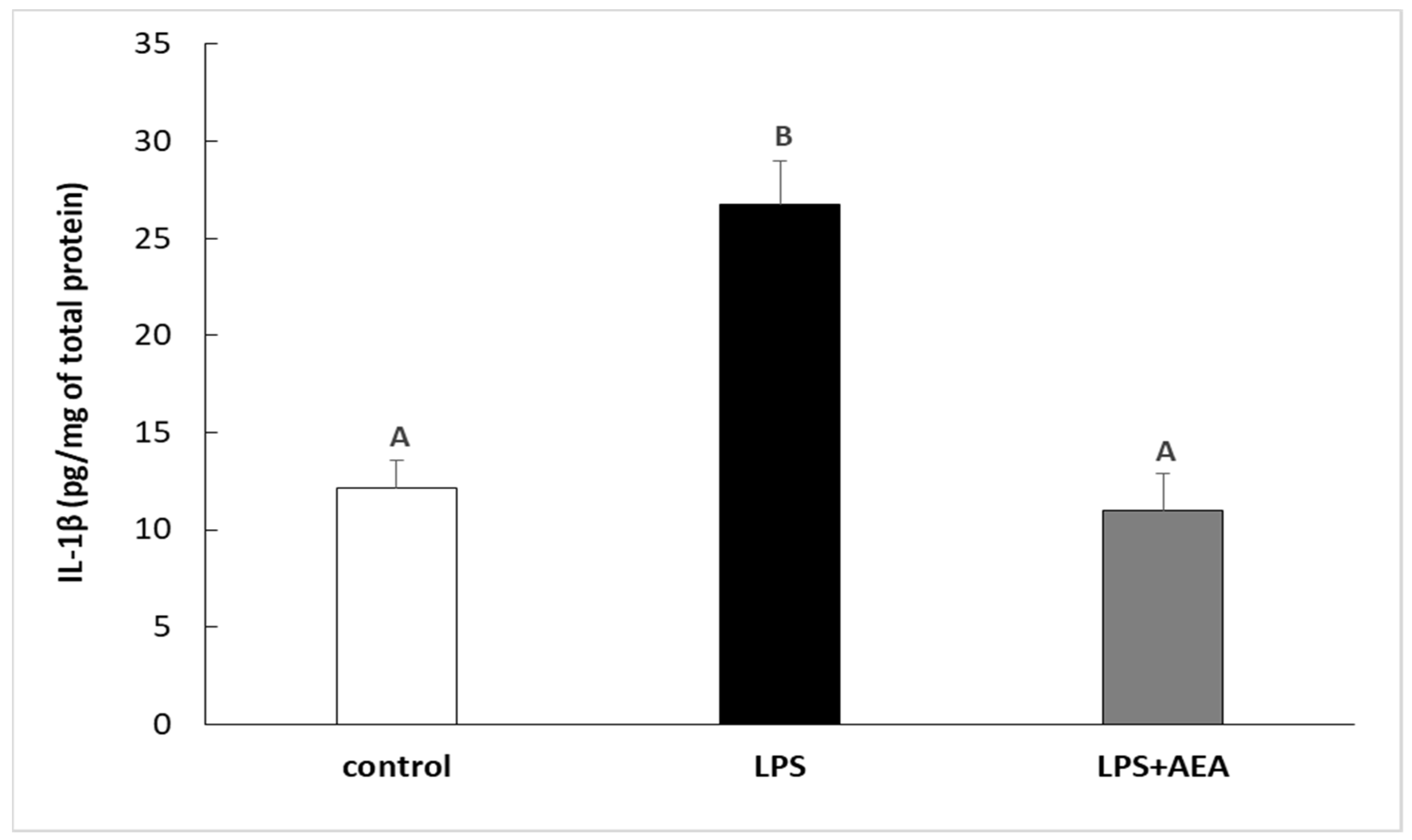
3.1. Effect of AEA Injection on LPS-Induced Synthesis of IL-1β in the Hypothalamus of Endotoxin-Treated Ewes
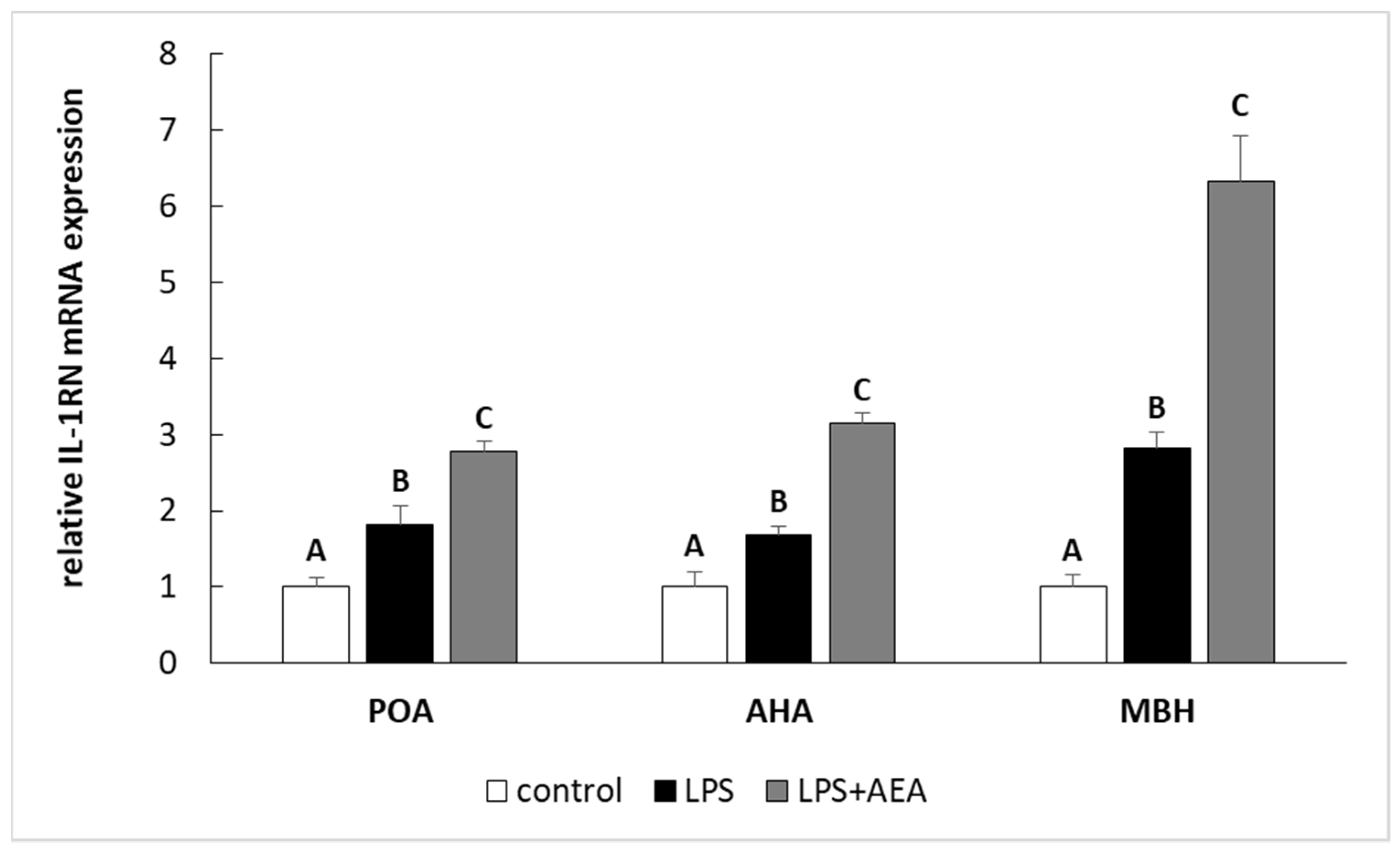
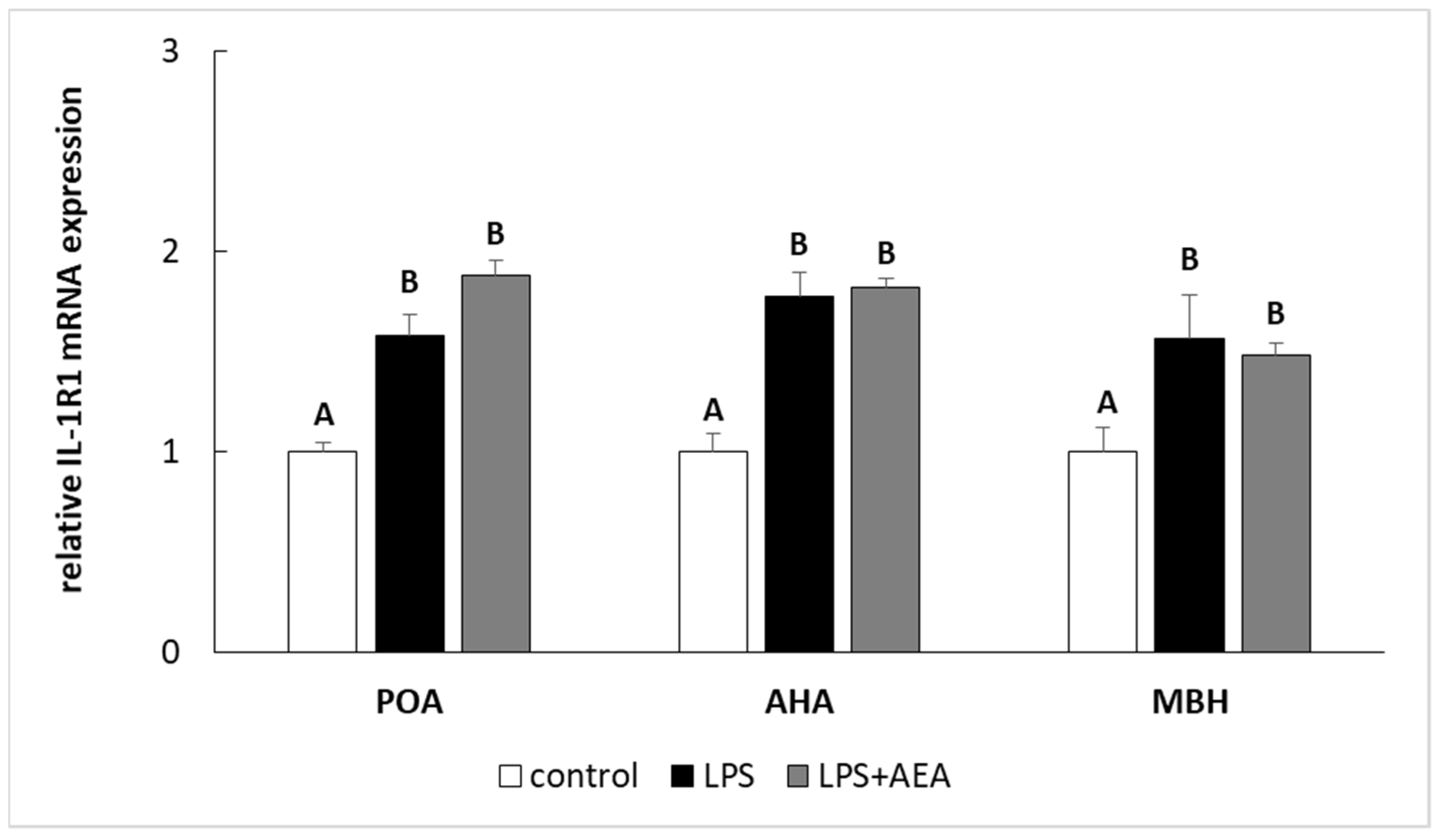
3.2. Effect of AEA Injection on IL-1 Gene Expression System in the Hypothalamus of Endotoxin-Treated Ewes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tomaszewska-Zaremba, D.; Herman, A.; Haziak, K. How does bacterial endotoxin influence gonadoliberin/gonadotropins secretion and action? J. Anim. Feed Sci. 2016, 25, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Peripheral injection of SB203580 inhibits the inflammatory-dependent synthesis of proinflammatory cytokines in the hypothalamus. BioMed Res. Int. 2014, 2014, 475152. [Google Scholar] [CrossRef]
- McCusker, R.H.; Kelley, K.W. Immune–neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [Green Version]
- Herman, A.P.; Kopycińska, K.; Krawczyńska, A.; Romanowicz, K.; Tomaszewska-Zaremba, D. The effect of repeated endo-toxin injections on gonadotropin secretion in ewes. J. Anim. Feed Sci. 2014, 23, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.; Abrams, M.; DeSouza, L.; Wagner, C.; Whitlock, B.; Sartin, J. Endotoxin inhibition of luteinizing hormone in sheep. Domest. Anim. Endocrinol. 2003, 25, 13–19. [Google Scholar] [CrossRef]
- Herman, A.P.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Antushevich, H.; Herman, A.; Paczesna, K.; Romanowicz, K.; Tomaszewska-Zaremba, D. Peripheral inhibitor of AChE, neostigmine, prevents the inflammatory dependent suppression of GnRH/LH secretion during the follicular phase of the estrous cycle. BioMed Res. Int. 2017, 2017, 6823209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, A.P.; Krawczyńska, J.; Bochenek, J.; Haziak, K.; Romanowicz, K.; Misztal, T.; Antushevich, H.; Herman, A.; To-maszewska-Zaremba, D. The effect of rivastigmine on the LPS-induced suppression of GnRH/LH secretion during the follicu-lar phase of the estrous cycle in ewes. Anim. Reprod. Sci. 2013, 138, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Karsch, F.J.; Battaglia, D.F.; Breen, K.M.; Debus, N.; Harris, T.G. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress 2002, 5, 101–112. [Google Scholar] [CrossRef]
- Battaglia, D.F.; Bowen, J.M.; Krasa, H.B.; Thrun, L.A.; Viguié, C.; Karsch, F.J. Endotoxin inhibits the reproductive neuroen-docrine axis while stimulating adrenal steroids: A simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 1997, 138, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Caldani, M.; Batailler, M.; Dubois, M.P. LHRH-immunoreactive structures in the sheep brain. Histochemistry 1988, 89, 129–139. [Google Scholar] [CrossRef]
- Herman, A.P.; Misztal, T.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Injection of Exogenous IL-1β in the Control Activities of Hypothalamic-Pituitary-Gonadal Axis in Anestrous Ewes. Reprod. Domest. Anim. 2011, 47, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, S.R.; Leonhardt, S.; Jarry, H.; Wuttke, W.; Kim, K. Effect of interleukin-1beta on gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in castrated male rats. J. Neuroendocrinol. 2000, 12, 421–429. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Luheshi, G.N. Interleukin 1 in the brain: Biology, pathology and therapeutic target. Trends Neurosci. 2000, 23, 618–625. [Google Scholar] [CrossRef]
- Herman, A.P.; Tomaszewska-Zaremba, D. Effect of endotoxin on the expression of GnRH and GnRHR genes in the hypothalamus and anterior pituitary gland of anestrous ewes. Anim. Reprod. Sci. 2010, 120, 105–111. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Kowalewska, M.; Herman, A.; Szczepkowska, A.; Skipor, J. The effect of melatonin from slow-release implants on basic and TLR-4-mediated gene expression of inflammatory cytokines and their receptors in the choroid plexus in ewes. Res. Vet. Sci. 2017, 113, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, M.; Szczepkowska, A.; Herman, A.; Pellicer-Rubio, M.; Jałyński, M.; Skipor, J. Melatonin from slow-release implants did not influence the gene expression of the lipopolysaccharide receptor complex in the choroid plexus of seasonally anoestrous adult ewes subjected or not to a systemic inflammatory stimulus. Small Rumin. Res. 2017, 147, 1–7. [Google Scholar] [CrossRef]
- Skipor, J.; Kowalewska, M.; Szczepkowska, A.; Majewska, A.; Misztal, T.; Jalynski, M.; Herman, A.P.; Zabek, K. Plasma and cerebrospinal fluid interleukin-1β during lipopolysaccharide-induced systemic inflammation in ewes implanted or not with slow-release melatonin. J. Anim. Sci. Biotechnol. 2017, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Barnard, D.F.; Gabella, K.M.; Kulp, A.C.; Parker, A.D.; Dugan, P.B.; Johnson, J.D. Sex differences in the regulation of brain IL-1β in response to chronic stress. Psychoneuroendocrinology 2019, 103, 203–211. [Google Scholar] [CrossRef]
- Li, T.G.; Pu, R.; Shui, L.; Bai, S.M.; Lu, J.; Chen, Y.S.; Tu, Y. Effect of moxibustion on inflammatory cytokine levels in hippo-campus and hypothalamus in rats with fatigue. Zhen Ci Yan Jiu 2019, 44, 195–199. [Google Scholar]
- Tomaszewska-Zaremba, D.; Herman, A.P.; Misztal, T. Does central IL-1b affect GnRH secretion in the hypothalamus of an-oestrous ewes via different regulatory pathways? J. Anim. Feed Sci. 2013, 22, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Igaz, P.; Salvi, R.; Rey, J.-P.; Glauser, M.; Pralong, F.P.; Gaillard, R.C. Effects of Cytokines on Gonadotropin-Releasing Hormone (GnRH) Gene Expression in Primary Hypothalamic Neurons and in GnRH Neurons Immortalized Conditionally. Endocrinology 2006, 147, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, S.L.; Sánchez-Miranda, E.; Castillo-Arellano, J.I.; Cervantes-Villagrana, R.D.; Ibarra-Sánchez, A.; González-Espinosa, C. Anandamide inhibits FcεRI-dependent degranulation and cytokine synthesis in mast cells through CB2 and GPR55 receptor activation. Possible involvement of CB2-GPR55 heteromers. Int. Immunopharmacol. 2018, 64, 298–307. [Google Scholar] [CrossRef]
- Centonze, D.; Finazzi-Agro, A.; Bernardi, G.; Maccarrone, M. The endocannabinoid system in targeting inflammatory neuro-degenerative diseases. Trends Pharmacol. Sci. 2007, 28, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.J.; Bi, G.H.; He, Y.; Galaj, E.; Gardner, E.L.; Xi, Z.X. Cannabinoid CB1 and CB2 receptor mechanisms underlie can-nabis reward and aversion in rats. Br. J. Pharmacol. 2019, 176, 1268–1281. [Google Scholar] [CrossRef]
- Maccarrone, M.; Wenger, T. Effects of Cannabinoids on Hypothalamic and Reproductive Function. In Handbook of Experimental Pharmacology; Springer International Publishing: Berlin/Heidelberg, Germany, 2005; pp. 555–571. [Google Scholar]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noroś, R.R. Nutrient Requirements for Cattle and Sheep in the Traditional System; Instytut Zootechniki: Krakow, Poland, 1993. (In Polish) [Google Scholar]
- Herman, A.P.; Tomaszewska-Zaremba, D.; Kowalewska, M.; Szczepkowska, A.; Oleszkiewicz, M.; Krawczyńska, A.; Wójcik, M.; Antushevich, H.; Skipor, J. Neostigmine attenuates proinflammatory cytokine expression in preoptic area but not choroid plexus during lipopolysaccharide-induced systemic inflammation. Med. Inflamm. 2018, 2018, 9150207. [Google Scholar] [CrossRef] [PubMed]
- Krawczyńska, A.; Antushevich, H.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Herman, A.P.; Zięba, D.A. Photoperiodic conditions as a factor modulating leptin influence on pro-inflammatory cytokines and their receptors gene expression in ewe’s aorta. J. Anim. Feed Sci. 2019, 28, 128–137. [Google Scholar] [CrossRef]
- Justinova, Z.; Solinas, M.; Tanda, G.; Redhi, G.H.; Goldberg, S.R. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J. Neurosci. 2005, 25, 5645–5650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtulewicz, K.; Tomaszewska-Zaremba, D.; Krawczyńska, A.; Tomczyk, M.; Herman, A.P. The effect of inflammation on the synthesis of luteinizing hormone and gonadotropin-releasing hormone receptor expression in the pars tuberalis of ewe during different photoperiodic conditions. Can. J. Anim. Sci. 2018, 98, 675–687. [Google Scholar] [CrossRef]
- Rasmussen, R. Quantification on the LightCycler. In Rapid Cycle Real-Time PCR; Meuer, S., Wittwer, C., Nakagawara, K.-I., Eds.; Springer: Berlin, Germany, 2001; pp. 21–34. [Google Scholar]
- Herman, A.P.; Bochenek, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Herman, A.; Romanowicz, K.; To-maszewska-Zaremba, D. Central interleukin-1β suppresses the nocturnal secretion of melatonin. Med. Inflamm. 2016, 2016, 2589483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, F.; Santos-Junior, N.N.; Rodrigues, R.P.D.A.; Costa, L.H.A.; Catalão, C.H.R.; Rocha, M.J.A. Interleukin-1 Receptor Antagonist Decreases Hypothalamic Oxidative Stress During Experimental Sepsis. Mol. Neurobiol. 2016, 53, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Janský, L.; Vybíral, S.; Pospíšilová, D.; Roth, J.; Dornand, J.; Zeisberger, E.; Kamínková, J. Production of systemic and hypo-thalamic cytokines during the early phase of endotoxin fever. Neuroendocrinology 1995, 62, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Haziak, K.; Antushevitch, H.; Herman, A.; Tomaszewska-Zaremba, D. Inhibi-tion of acetylcholinesterase activity by rivastigmine decreases lipopolysaccharide-induced IL-1β expression in the hypothala-mus of ewes. Domest. Anim. Endocrinol. 2013, 44, 109–114. [Google Scholar] [CrossRef]
- Herman, A.; Misztal, T.; Zaremba, D.T. Expression of Interleukin (IL)-1β and IL-1 Receptors Genes in the Hypothalamus of Anoestrous Ewes after Lipopolysaccharide Treatment. Reprod. Domest. Anim. 2010, 45, e426–e433. [Google Scholar] [CrossRef] [PubMed]
- Pinteaux, E.; Rothwell, N.J.; Boutin, H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia 2006, 53, 551–556. [Google Scholar] [CrossRef]
- Jin, C.; Fu, W.-L.; Zhang, D.-D.; Xing, W.-W.; Xia, W.-R.; Wei, Z.; Zou, M.-J.; Zhu, X.-M.; Xu, D.-G. The protective role of IL-1Ra on intestinal ischemia reperfusion injury by anti-oxidative stress via Nrf2/HO-1 pathway in rat. Biomed. J. 2019, 42, 36–45. [Google Scholar] [CrossRef]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family–Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef]
- André, R.; Lerouet, D.; Kimber, I.; Pinteaux, E.; Rothwell, N.J. Regulation of expression of the novel IL-1 receptor family members in the mouse brain. J. Neurochem. 2005, 95, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bebes, A.; Kovács-Sólyom, F.; Prihoda, J.; Kui, R.; Kemény, L.; Gyulai, R. Interleukin-1 Receptors Are Differentially Expressed in Normal and Psoriatic T Cells. Mediat. Inflamm. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Boraschi, D.; Tagliabue, A. The interleukin-1 receptor family. Semin. Immunol. 2013, 25, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kownacki, P.; Konturek, P.C. Role of sensory nerves in gastroprotective effect of anandamide in rats. J. Physiol. Pharmacol. 2011, 62, 207. [Google Scholar] [PubMed]
- Mecha, M.; Feliu, A.; Inigo, P.M.; Mestre, L.; Carrillo-Salinas, F.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R(+)WIN55,212. J. Clin. Investig. 2003, 111, 1231–1240. [Google Scholar] [CrossRef] [Green Version]
- Puffenbarger, R.A.; Boothe, A.C.; Cabral, G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat micro-glial cells. Glia 2000, 29, 58–69. [Google Scholar] [CrossRef]
- McCoy, K.L. Interaction between Cannabinoid System and Toll-Like Receptors Controls Inflammation. Mediat. Inflamm. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tarn, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocan-nabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutando, L.; Busquets-Garcia, A.; Puighermanal, E.; Gomis-González, M.; Delgado-García, J.M.; Gruart, A.; Maldonado, R.; Ozaita, A. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J. Clin. Investig. 2013, 123, 2816–2831. [Google Scholar] [CrossRef]
- Zhu, W.; Newton, C.; Daaka, Y.; Friedman, H.; Klein, T.W. Delta 9-Tetrahydrocannabinol enhances the secretion of interleukin 1 from endotoxin-stimulated macrophages. J. Pharmacol. Exp. Ther. 1994, 270, 1334–1339. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H. Interplays between inflammasomes and viruses, bacteria (pathogenic and probiotic), yeasts and parasites. Immunol. Lett. 2020, 228, 1–14. [Google Scholar] [CrossRef]
- Li, G.; Xia, M.; Abais, J.M.; Boini, K.; Li, P.-L.; Ritter, J.K. Protective Action of Anandamide and Its COX-2 Metabolite against l-Homocysteine-Induced NLRP3 Inflammasome Activation and Injury in Podocytes. J. Pharmacol. Exp. Ther. 2016, 358, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory Effect of Cannabidiol on the Activation of NLRP3 Inflammasome Is Associated with Its Modulation of the P2X7 Receptor in Human Monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thøgersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of Interleukin 1β by Matrix Metalloproteinases. J. Biol. Chem. 1996, 271, 14657–14660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, K.; Oyama, T.; Sakuta, T.; Tokuda, M.; Torii, M. Anandamide Induces Matrix Metalloproteinase-2 Production through Cannabinoid-1 Receptor and Transient Receptor Potential Vanilloid-1 in Human Dental Pulp Cells in Culture. J. Endod. 2012, 38, 786–790. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Pinteaux, E.; Moore, J.D.; Molina-Holgado, E.; Guaza, C.; Gibson, R.M.; Rothwell, N.J. Endogenous Interleukin-1 Receptor Antagonist Mediates Anti-Inflammatory and Neuroprotective Actions of Cannabinoids in Neurons and Glia. J. Neurosci. 2003, 23, 6470–6474. [Google Scholar] [CrossRef]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef] [Green Version]


| Group | No. of Animals | Experimental Treatment I | Dose (ng/kg) | Experimental Treatment II | Dose (µg/kg) | |
|---|---|---|---|---|---|---|
| 1 | Control | 6 | NaCl | 0 | NaCl | 0 |
| 2 | LPS treated | 6 | LPS | 400 | NaCl | 0 |
| 3 | LPS + AEA i.v. treated | 6 | LPS | 400 | AEA i.v. | 10 |
| Total number of animals | 18 | |||||
| GenBank Acc. No. | Gene | Amplicon Size (bp) | Forward/Reverse | Sequence 5′→3′ | Reference |
|---|---|---|---|---|---|
| NM_001034034 | GAPDH (glyceraldehyde-3-phosphate dehydrogenase) | 134 | forward | AGAAGGCTGGGGCTCACT | [2] |
| reverse | GGCATTGCTGACAATCTTGA | ||||
| U39357 | ACTB (beta actin) | 168 | forward | CTTCCTTCCTGGGCATGG | [2] |
| reverse | GGGCAGTGATCTCTTTCTGC | ||||
| BC108088.1 | HDAC1 (histone deacetylase1) | 115 | forward | CTGGGGACCTACGGGATATT | [2] |
| reverse | GACATGACCGGCTTGAAAAT | ||||
| X54796.1 | IL-1B (interleukin 1 beta) | 137 | forward | CAGCCGTGCAGTCAGTAAAA | [2] |
| reverse | GAAGCTCATGCAGAACACCA | ||||
| NM_001206735.1 | IL-1R1 (interleukin 1 receptor, type I) | 124 | forward | GGGAAGGGTCCACCTGTAAC | [2] |
| reverse | ACAATGCTTTCCCCAACGTA | ||||
| NM_001046210.1 | IL-1R2 (interleukin 1 receptor, type II) | 161 | forward | CGCCAGGCATACTCAGAAA | [36] |
| reverse | GAGAACGTGGCAGCTTCTTT | ||||
| NM_001308595.1 | IL-1RN (interleukin 1 receptor antagonist) | 145 | forward | AGGATCTGGGATGTCAACCA | [36] |
| reverse | CATGGATCCCCAGGAACATA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.; Tomaszewska-Zaremba, D.; Bochenek, J.; Herman, A.; Herman, A.P. Anandamide Influences Interleukin-1? Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation. Animals 2021, 11, 484. https://doi.org/10.3390/ani11020484
Tomczyk M, Tomaszewska-Zaremba D, Bochenek J, Herman A, Herman AP. Anandamide Influences Interleukin-1? Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation. Animals. 2021; 11(2):484. https://doi.org/10.3390/ani11020484
Chicago/Turabian StyleTomczyk, Monika, Dorota Tomaszewska-Zaremba, Joanna Bochenek, Anna Herman, and Andrzej P. Herman. 2021. "Anandamide Influences Interleukin-1? Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation" Animals 11, no. 2: 484. https://doi.org/10.3390/ani11020484
APA StyleTomczyk, M., Tomaszewska-Zaremba, D., Bochenek, J., Herman, A., & Herman, A. P. (2021). Anandamide Influences Interleukin-1? Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation. Animals, 11(2), 484. https://doi.org/10.3390/ani11020484






