Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feline and Human Cell Lines
2.2. Immunocytochemistry
2.3. Cytotoxicity Assays
2.4. Immunoblotting
2.5. Flow Cytometry
3. Results
3.1. CAT-MT, FMCp, and FMCm Are Feline Luminal Mammary Carcinoma Cell Lines
3.2. HDACis and MTIs Present Antitumor Effects on Luminal Feline Cell Lines CAT-MT and FMCp
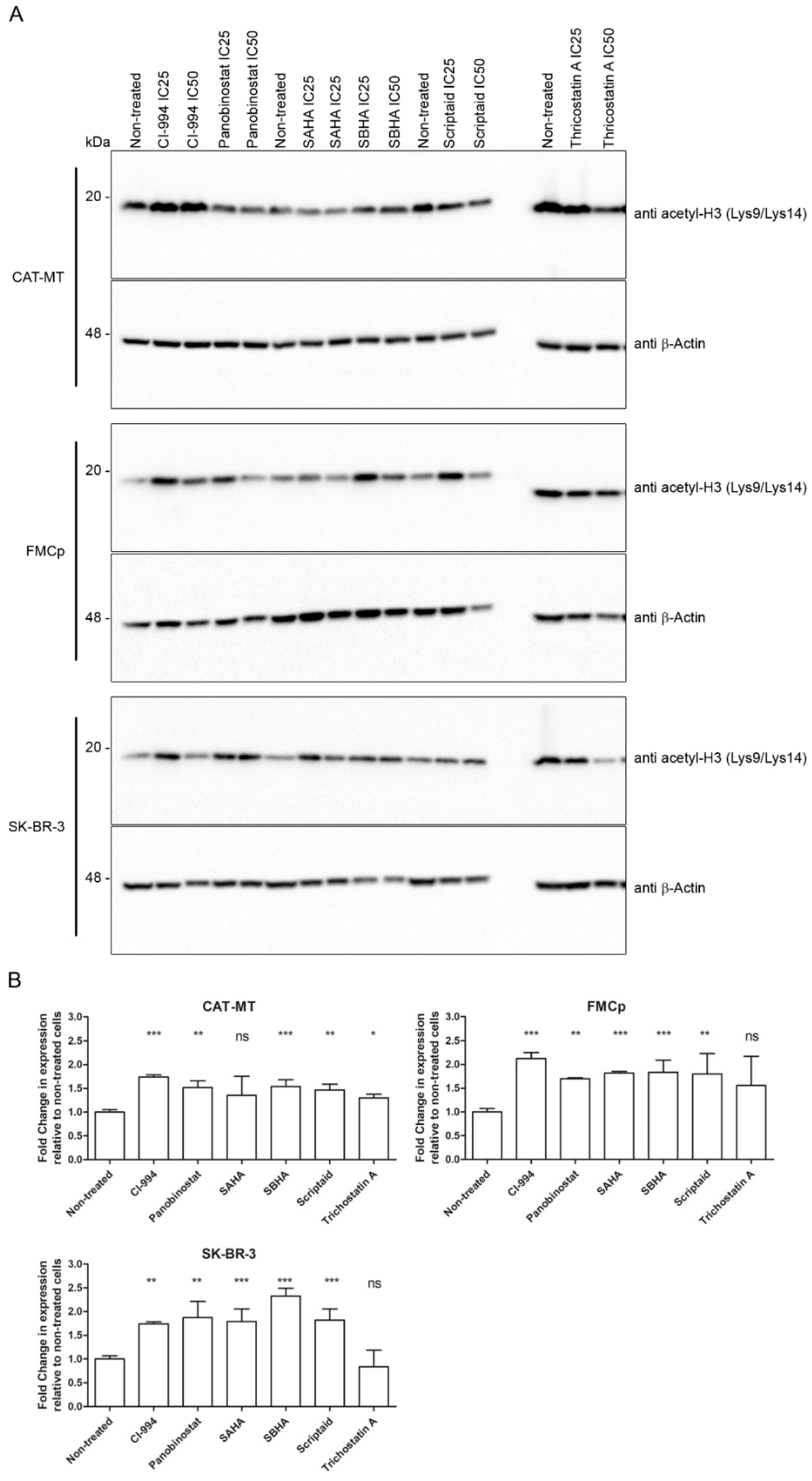
3.3. The Cytotoxic Effect of HDACis Is Related to Histone Acetylation
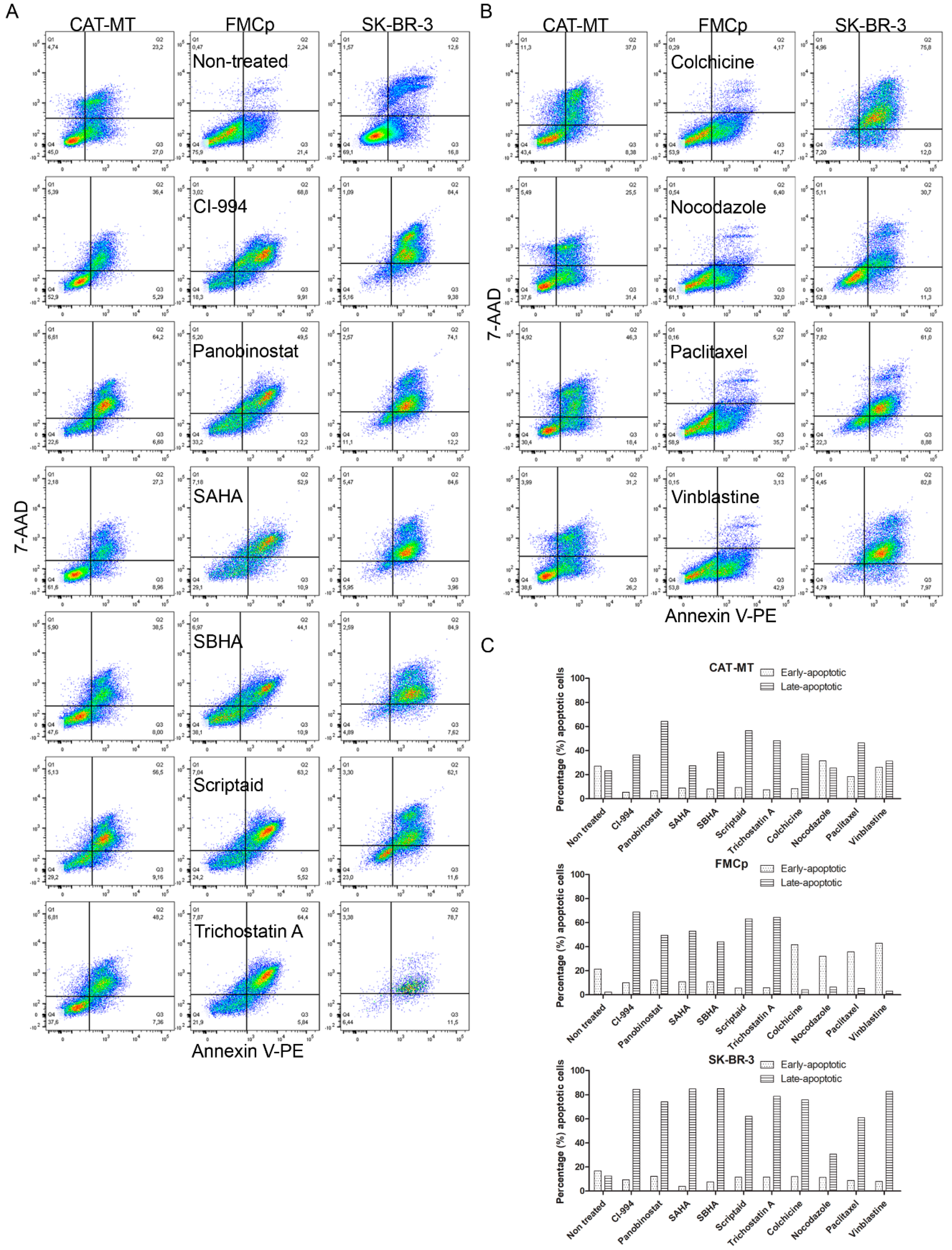
3.4. Apoptosis Is the Main Mechanism by Which HDACis and MTIs Induce Tumor Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Soares, M.; Madeira, S.; Correia, J.; Peleteiro, M.; Cardoso, F.; Ferreira, F. Molecular based subtyping of feline mammary carcinomas and clinicopathological characterization. Breast 2016, 27, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Peleteiro, M.C.; Ferreira, F. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases—disease progression and clinical implications from a 3-year follow-up study. Tumor Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; MacEwen, E.G. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000, 18, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early Detection, Agressive Therapy: Optimizing the management of the feline mammary masses. J. Feline Med. Surg. 2010, 214–224. [Google Scholar] [CrossRef]
- Wei, W.Z.; Jones, R.F.; Juhasz, C.; Gibson, H.; Veenstra, J. Evolution of animal models in cancer vaccine development. Vaccine 2015, 33, 7401–7407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorenmo, K.U.; Worley, D.R.; Goldschmidt, M.H. Tumors of the Mammary Gland, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Chun, P. Histone deacetylase inhibitors in hematological malignancies and solid tumors. Arch. Pharm. Res. 2015, 38, 933–949. [Google Scholar] [CrossRef]
- Stahl, M.; Kohrman, N.; Gore, S.D.; Kim, T.K.; Zeidan, A.M.; Prebet, T. Epigenetics in Cancer: A Hematological Perspective. PLoS Genet. 2016, 12, 1–21. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef] [Green Version]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies to Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- McDonnel, S.J.; Liepnieks, M.L.; Murphy, B.G. Treatment of chronically FIV-infected cats with suberoylanilide hydroxamic acid. Antiviral Res. 2014, 108, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnel, S.J.; Tell, L.A.; Murphy, B.G. Pharmacokinetics and pharmacodynamics of suberoylanilide hydroxamic acid in cats. J. Vet. Pharmacol. Ther. 2013, 196–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnel, S.J.; Sparger, E.E.; Luciw, P.A.; Murphy, B.G. Pharmacologic reactivation of latent feline immunodeficiency virus ex vivo in peripheral CD4+ T-lymphocytes. Virus Res. 2012, 170, 174–179. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norval, M.; Maingay, J.; Else, R.W. Characteristics of a feline mammary carcinoma cell line. Res. Vet. Sci. 1985, 39, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Uyama, R.; Hong, S.-H.; Nakagawa, T.; Yazawa, M.; Kadosawa, T.; Mochizuki, M.; Tsujimoto, H.; Nishimura, R.; Sasaki, N. Establishment and characterization of eight feline mammary adenocarcinoma cell lines. J. Vet. Med. Sci. 2005, 67, 1273–1276. [Google Scholar] [CrossRef] [Green Version]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Young, L.J.T. Mus tales: A hands-on view. J. Mammary Gland Biol. Neoplasia 2008, 13, 343–349. [Google Scholar] [CrossRef]
- Rangarajan, A.; Weinberg, R.A. Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer 2003, 3, 952–959. [Google Scholar] [CrossRef]
- Kao, J.; Salari, K.; Bocanegra, M.; Choi, Y.L.; Girard, L.; Gandhi, J.; Kwei, K.A.; Hernandez-Boussard, T.; Wang, P.; Gazdar, A.F.; et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Minke, J.M.H.M.; Schuuring, E.; van den Berghe, R.; Stolwijk, J.A.M.; Boonstra, J.; Cornelisse, C.; Hilkens, J.; Misdorp, W. Isolation of Two Distinct Epithelial Cell Lines from a Single Feline Mammary Carcinoma with Different Tumorigenic Potential in Nude Mice and Expressing Different Levels of Epidermal Growth Factor Receptors. Cancer Res. 1991, 51, 4028–4037. [Google Scholar] [PubMed]
- Muleya, J.S.; Nakaichi, M.; Sugahara, J.; Taura, Y.; Murata, T.; Nakama, S. Establishment and Characterization of a New Cell Line Derived from Feline Mammary Tumor. J. Vet. Med. Sci. 1998, 60, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Adega, F.; Chaves, R. Establishment and characterization of a new feline mammary cancer cell line, FkMTp. Cytotechnology 2016, 68, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Fojo, T.; Menefee, M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann. Oncol. 2007, 18, 3–8. [Google Scholar] [CrossRef]
- Chen, G.K.; Durán, G.E.; Mangili, A.; Beketic-Oreskovic, L.; Sikic, B.I. MDR1 activation is the predominant resistance mechanism selected by vinblastine in MES-SA cells. Br. J. Cancer 2000, 83, 892–898. [Google Scholar] [CrossRef]
- Eto, S.; Saeki, K.; Yoshitake, R.; Yoshimoto, S.; Shinada, M.; Ikeda, N.; Kamoto, S.; Tanaka, Y.; Kato, D.; Maeda, S.; et al. Anti-tumor effects of the histone deacetylase inhibitor vorinostat on canine urothelial carcinoma cells. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kisseberth, W.C.; Murahari, S.; London, C.A.; Kulp, S.K.; Chen, C.S. Evaluation of the effects of histone deacetylase inhibitors on cells from canine cancer cell lines. Am. J. Vet. Res. 2008, 69, 938–945. [Google Scholar] [CrossRef]
- Dias, J.N.R.; Aguiar, S.I.; Pereira, D.M.; André, A.S.; Gano, L.; Correia, J.D.G.; Carrapiço, B.; Rütgen, B.; Malhó, R.; Goncalves, J.; et al. The histone deacetylase inhibitor panobinostat is a potent antitumor agent in canine diffuse large B-cell lymphoma. Oncotarget 2018, 9, 28586–28598. [Google Scholar] [CrossRef]
- Murahari, S.; Jalkanen, A.L.; Kulp, S.K.; Chen, C.S.; Modiano, J.F.; London, C.A.; Kisseberth, W.C. Sensitivity of osteosarcoma cells to HDAC inhibitor AR-42 mediated apoptosis. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nève, P.; Ketelbant-Balasse, P.; Willlems, C.; Dumont, J.E. Effect of inhibitors of microtubules and microfilaments on dog thyroid slices in vitro. Exp. Cell 1972, 74. [Google Scholar] [CrossRef]
- Khanna, C.; Rosenberg, M.; Vail, D.M. A review of paclitaxel and novel formulations including those suitable for use in dogs. J. Vet. Intern. Med. 2015, 29, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules 2014, 19, 15196–15212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michishita, M.; Ohtsuka, A.; Nakahira, R.; Tajima, T.; Nakagawa, T.; Sasaki, N.; Arai, T.; Takahashi, K. Anti-tumor effect of bevacizumab on a xenograft model of feline mammary carcinoma. J. Vet. Med. Sci. 2016, 78, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Harman, R.M.; Curtis, T.M.; Argyle, D.J.; Coonrod, S.A.; Van de Walle, G.R. A Comparative Study on the In Vitro Effects of the DNA Methyltransferase Inhibitor 5-Azacytidine (5-AzaC) in Breast/Mammary Cancer of Different Mammalian Species. J. Mammary Gland Biol. Neoplasia 2016, 21, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledet, M.M.; Anderson, R.; Harman, R.; Muth, A.; Thompson, P.R.; Coonrod, S.A.; Van de Walle, G.R. BB-Cl-Amidine as a novel therapeutic for canine and feline mammary cancer via activation of the endoplasmic reticulum stress pathway. BMC Cancer 2018, 18, 1–13. [Google Scholar] [CrossRef]


| Cell Line | CAT-MT | FMCp | FMCm | SK-BR-3 | ||||
|---|---|---|---|---|---|---|---|---|
| Score | Obs. | Score | Obs. | Score | Obs. | Score | Obs. | |
| ER | 3 | 5% (Weak) | 8 | 70% (Strong) | 3 | 10% (Weak) | 0 | No signal |
| PR | 8 | 80% (Strong) | 0 | No signal | 0 | No signal | 4 | 3% (Average) |
| HER2 | 0 | Negative | 0 | Negative | 0 | Negative | +3 | Intense |
| CK 5/6 | Negative | <1% | Negative | <1% | Negative | <1% | Negative | <1% |
| Ki-67 | Strong | 50.2% | Strong | 57.4% | Strong | 68.5% | Strong | 53.6% |
| Molecular Subtype | Luminal A | Luminal B | Luminal B | HER2+ | ||||
| Drug | Cell Line | CAT-MT | FMCp | FMCm | SK-BR-3 | IC50 Units |
|---|---|---|---|---|---|---|
| HDACis | CI-994 | 16.470 (±1.904) | 9.616 (±2.150) | ND | 16.860 (±3.183) | μM |
| Panobinostat | 0.042 (±0.067) | ND | ND | 0.015 (±0.027) | ||
| SAHA | 4.416 (±0.453) | 2.571 (±0.578) | ND | 3.798 (±0.344) | ||
| SBHA | 45.230 (±4.692) | 33.830 (±6.454) | ND | 62.980 (±4.033) | ||
| Scriptaid | 3.392 (±0.403) | 3.090 (±0.691) | ND | 5.095 (±0.487) | ||
| Trichostatin A | 0.263 (±0.062) | ND | ND | 0.572 (±0.029) | ||
| MTIs | Colchicine | 1.472 (±0.484) | 5.876 (±0.968) | ND | 0.590 (±0.350) | nM |
| Nocodazole | 12.270 (±3.455) | 30.840 (±8.499) | ND | 37.120 (±8.844) | ||
| Paclitaxel | 1.939 (±1.134) | 8.646 (±2.337) | ND | ND | ||
| Vinblastine | 0.570 (±1.080) | 6.563 (±1.514) | ND | 236.200 (±91.868) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, F.; Gameiro, A.; Correia, J.; Ferreira, F. Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells. Animals 2021, 11, 502. https://doi.org/10.3390/ani11020502
Almeida F, Gameiro A, Correia J, Ferreira F. Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells. Animals. 2021; 11(2):502. https://doi.org/10.3390/ani11020502
Chicago/Turabian StyleAlmeida, Filipe, Andreia Gameiro, Jorge Correia, and Fernando Ferreira. 2021. "Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells" Animals 11, no. 2: 502. https://doi.org/10.3390/ani11020502







