Abstract
Simple Summary
Nest establishment is a critical stage of the ant life cycle because it determines the chances of colony success. Here, we study the effect of different numbers of queens (i.e., one or six) on the position of queens and workers inside and outside the artificial nests of an invasive (Linepithema humile) and Mediterranean native (Tapinoma nigerrimum) species. Our results suggest that queens in nests with six queens entered the nest faster than single queens. Similarly, during nest establishment, workers in nests with six queens entered the nest faster, with this effect being more pronounced for the native species. Once nests were established, fewer workers were engaged in outside-nest tasks in nests with six queens. This was especially true for workers of the native species engaged in patrolling. These results suggest that the number of queens can influence both queen and worker behavior, and that invasive and native species have different responses.
Abstract
As a critical stage in the life cycle of ant colonies, nest establishment depends on external and internal factors. This study investigates the effect of the number of queens on queen and worker behavior during nest establishment in invasive Argentine ants (Linepitema humile) and native Mediterranean Tapinoma nigerrimum. We set up experimental colonies with the same number of workers but with one or six queens. At different time points, we recorded the positions of queens and workers inside and outside the nest. Our results highlight the influence of the number of queens on the position of queens and workers with between-species differences. Queens of both species entered the nests more quickly when there were six queens. During nest establishment, more workers were inside nests with six queens for both species, with this effect being greater for T. nigerrimum. Once nests were established, fewer workers of both species were engaged in nest maintenance and feeding in nests with six queens; T. nigerrimum had fewer workers engaged in patrolling. These results suggest that the number of queens is a key factor driving queen and worker behavior during and after nest establishment with different species responses.
Keywords:
invasive; ant; native; establishment; queens; workers; Linepithema humile; Tapinoma nigerrimum 1. Introduction
In ants, as well as most other species, the propagule size (i.e., the number of introduced individuals), propagule number (i.e., the number of introduction events), and propagule pressure (i.e., the composite measure between propagule size and number) determine the likelihood of establishment [,,]. In theory, a larger initial propagule size and more frequent introduction events increase the chances of establishment success []. Despite the use of laboratory, field, or combined approaches [,,] to study the role of propagule size and pressure in ant establishment success, they are among the most poorly understood factors of ant ecology.
In dependent colony foundation, the success of nest establishment is also influenced by the role played by each caste. For example, in the absence of workers, queens of polygynous species are unable to produce new workers and thus rarely survive. Moreover, brood production is limited by worker provision rather than the egg-laying capacity of queens []. Consequently, the number of workers and queens has a critical influence on the successful establishment of emerging ant colonies and the subsequent population dynamics []. Regardless of the organization level (i.e., individual or colony), experimental studies often address the effect of workers on a species’ competitive ability []. However, queens can also affect the productivity and survival of workers [], as well as the behavior of the queen [] and workers []. The most efficient management strategy to avoid the establishment of invasive or potentially invasive species is prevention [,]. Thus, determining the role of factors such as propagule size and number of queens in experimental nests can shed light on nest establishment strategies, particularly for invasive ant species.
In this study, we evaluated the effect of species and the number of queens on both queen and worker behavior for nest establishment in a laboratory experimental setup. This type of setup is the best option to compare species because we can control abiotic (e.g., temperature and humidity) and biotic (e.g., number of species interacting) factors. As we were interested in comparing two species in isolation, this study was not possible to conduct in the field, as many species are simultaneously present and all species interact with each other. We focused on two ant species, one invasive (Linepithema humile) and one native (Tapinoma nigerrimum). Among invasive ant species, L. humile is a cosmopolitan species that has successfully invaded all continents apart from Antarctica, and it continues to spread []. Its success is mainly due to its trophic habits (e.g., mass recruitment and omnivorous diet), reproduction strategy (i.e., high polygyny) and colony structure (e.g., colonies with lack of boundaries due to the absence of aggression). These characteristics allow L. humile to rapidly recruit workers and accumulate food, leading to the formation of super colonies []. For instance, in the Mediterranean basin, L. humile has formed a super colony that extends from southern Spain along the Mediterranean coast to Italy []. In this area, L. humile lives in sympatry with the native T. nigerrimum, which has similar life history traits. This Mediterranean species is abundant and widespread, forms polydomous and polygynous colonies, and exhibits mass recruitment to obtain food sources. Consequently, these species have similar space and trophic requirements which likely promotes competition for those resources, as has already been shown in experimental conditions []. Therefore, understanding what characteristics may provide advantages for one species over the other could provide insight into the potential value of the native species T. nigerrimum as a biological control agent of the invasive L. humile. Studies comparing these two species have been focused on differences in worker behavior and abundance [,]. Here, we focus on the potential effect of queens on nest establishment, one of the critical phases of species invasion [].
2. Material and Methods
2.1. Ant Collection
In April 2014, we collected L. humile queens, workers, and brood from La Ciotat in southern France (43.18° N, 5.61° E). We simultaneously collected T. nigerrimum queens, workers, and brood from nearby Auriol in southern France (43.37° N, 5.64° E) where L. humile nests are also present. These field stations located near Marseille, France, have a Mediterranean climate with moderately low temperatures in winter and dry hot periods in summer. Vegetation types at both field stations are mainly composed of shrub species (Retama sp. and Thymus sp.) and forest (Pinus sp. and Quercus sp.). The site surroundings were dense residential areas and large expanses of agricultural land. We collected workers, queens, and brood at different stages of development for both species.
2.2. Experimental Design
Ant colonies were fed with a sucrose solution and a protein-based solution twice a week []. The inner surfaces of the large plastic boxes where the ants were kept were coated with fluoropolymer resin (fluon) to stop the ants from escaping. The laboratory temperature was kept at 24–26 °C throughout the experiment, which is optimal for both species [].
Each experimental colony was composed of a nest (length: 15.3 cm, width: 7.8 cm, height: 1.5 cm) and a foraging arena (length: 15 cm, width: 15 cm, height: 15 cm) connected by a small transparent tube. Nests were mainly made of plaster and kept between two plates of transparent polyvinyl chloride (PVC). Nests were covered with transparent red paper that produced darkness for ants, thus simulating natural conditions. The foraging arena was a plastic box with a plaster bottom where we placed food.
We used an experimental design with two initial numbers of queens (1 and 6 queens) and 300 workers for each species. We named the colonies according to the four treatments: 1-queen invasive, 1-queen native, 6-queen native, and 6-queen invasive. We chose these numbers of queens and workers based on the results of [], which showed that both workers and queens have a positive effect on the productivity of the other caste and that queens have a positive effect on worker survivorship. We set up the corresponding number of queens and workers for each treatment and made 20 replicates per treatment (n = 80 nests, with a total of 280 queens and 24,000 workers). These initial colonies did not contain brood.
For each experimental colony, we placed the workers and queen(s) inside the foraging arena and took photos to monitor queen and worker behavior. To maintain the humidity of the nests, we added 2 mL of water twice a week using a syringe. We also cleaned the foraging arenas by removing the mounds of dead individuals made by workers as well as any remaining food. Most workers and all queens stayed inside the nests while we performed the nest cleaning and maintenance.
To follow the queen and worker positions in the experimental setups at different times, we took photos (Panasonic LUMIX DMC-FZ45) of the experimental nest chambers and the corresponding foraging arena. We considered three main positions of workers or queens: (i) inside the nest; (ii) in the foraging arena (outside the nest around food and water supplies), and (iii) climbing the walls of the foraging arena (Figure S1).
During the first day after setup, we recorded the position of the ants at four different times after they were placed inside the experimental arena: 0.5 h, 1 h, 1.5 h, and 2 h. We also recorded the nests the following day (i.e., after 24 h) and 7 days after the start of the experimental setup. Photos were analyzed using the “counting” tool in the Adobe Photoshop software to determine the number of individuals in each position of the experimental nest (Figure S1).
According to the biology of ants, we fitted two models to consider two periods after setup: establishment of the colony (i.e., from setup to 2 h) and maintenance of the colony over a longer period (i.e., 24 h and 7 days after setup). As these models presented similar results to those obtained from the full model (i.e., six time points) (Table S1; Figure S2), we have only presented and discussed results from the first two models.
2.3. Statistical Analyses
2.3.1. Queens during Nest Establishment
We assessed the time for the first queen to enter the nest using Kaplan–Meier (K–M) survival analysis []. In our case, the outcome event is a queen situated inside the nest. We used K–M estimates with censored data, since queens from three nests had not yet entered the nest by the end of observation on the first day (i.e., the event of interest did not occur for those queens).
2.3.2. Workers Inside the Nests
We fitted a generalized linear mixed model (GLMM) during nest establishment (i.e., between 0.5 h to 2 h after setup) and another model for 24 h and 7 days after setup, aiming to analyze the relationship between the number of workers inside the nests, the number of queens, and the species for each of these periods. We used the number of queens (as a factor), species, time, and their interaction as fixed effects. We included nest identity (ID) as the random effect, because there were multiple correlated observations from the same nests (i.e., number of workers inside the nest at four different times). Using nest ID as a random effect allowed us to consider the correlation between multiple observations from the same nest while only estimating one variance. We used a Poisson distribution, as the dependent variable (i.e., number of workers) is count data (i.e., real positive numbers), and a negative binomial distribution in the case of overdispersion. We used the deviance goodness of fit test and inspected the normality of residuals, heteroscedasticity, and independence of random effects (Table S2). The goodness-of-fit test based on deviance is the log-likelihood ratio statistic for testing the fitted model against the saturated model in which there is a regression coefficient for every observation. When significant interactions were detected, pairwise comparisons were done with the package emmeans [].
2.3.3. Workers Outside the Nests
Workers can be engaged in different tasks outside the nest such as patrolling, nest maintenance, and feeding []. Once nests were established, we quantified the number of workers outside the nest engaged in one of the following tasks: patrolling (i.e., workers on walls), nest maintenance (i.e., workers in the foraging arena carrying plaster pieces and cleaning the nest), and feeding (i.e., workers located inside or around protein and/or sugar tubes). We used a generalized linear model (GLM) to analyze each of these tasks as follows: the number of workers as a response variable, and the number of queens (as a factor), species, and their interaction as fixed variables. We used the deviance goodness of fit test to select models and analysis of variance (ANOVA) to compare them (Tables S1 and S3).
Statistical analyses were conducted using R (R Core Team 2019) with an α-level of 0.05. We used the lme4 package [] to perform generalized linear mixed-effects analysis. We used the survival package [] to perform survival analysis. p-values were obtained by likelihood ratio tests of the full model, with the effect compared to the model without the effect. The plots presented here were made using the ggplot2 package [], and values are presented as mean ± 95% confidence interval (CI).
3. Results
3.1. Queens during Nest Establishment
There was a difference in the time spent by the first queen to enter the nest between the number of queens (likelihood ratio test = 21.60, df = 12, p < 0.001); queens from six-queens nests entered faster than queens from one-queen nests (Hazard Ratio: HR = 2.923, p < 0.001) (Table S3, Figure 1). Nevertheless, there was no differences in the time spent by the first queen to enter the nest between species in one-queen nests (likelihood ratio test = 2.680, df = 1, p = 0.100) (Table S4, Figure 1) nor six-queen nests (likelihood ratio test = 1.200, df = 1, p = 0.300) (Table S5, Figure 1).
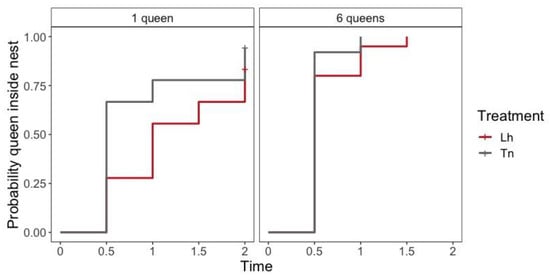
Figure 1.
Kaplan–Meier plot of time (in hours) for first queen to enter the nest shows the survivor function stratified according to the number of queens and species. Plus signs (+) indicate censored data and their corresponding x values for the time at which censoring occurred. Lh = L. humile nest and Tn = T. nigerrimum nest.
3.2. Workers Inside the Nest
We compared the negative binomial model with random intercept (model 1) with a model without random intercept (model 2). The model with random intercept was significantly better that the simpler model (chi square = 217.86, p < 0.001) and had smaller Akaike Information Criterion (AIC) (model 1: df = 10, AIC = 3278, vs. model 2: df = 9, AIC = 3494). Both models showed similar AIC (no interaction: df = 7, AIC = 3279, vs. model 1: df = 10, AIC = 3278) and there was not no significant difference between both models them (chi square = 7.312, p = 0.063), so we removed the time interaction. The model with species interaction had smaller AIC (model 1: df = 10, AIC = 3278 vs. no interaction: df = 6, AIC = 3285) and there was a significant difference between both models (chi square = 15.163, p = 0.004), so we kept the species interaction.
During nest establishment (from 0.5 h to 2 h after setup), there was a significant effect of the number of queens and the number of workers entering inside the nest; there were more workers inside the nest with six queens (z = 4.248, p < 0.001). T. nigerrimun had significantly more workers entering the nest at all times than L. humile (z = 5.441, p < 0.001), but the interaction between the species and the number of queens was also significant (z = −2.898, p = 0.004). The effect of time was also significant (z = 13.270, p < 0.001) because the number of workers entering the nest increased with time since most workers spend most of their time inside the nest with the queens (Figure 2, Table S7). Pairwise comparisons between the number of queens and species combinations showed that the number of queens had a significant effect only for L. humile (Figure 2, Table S8).
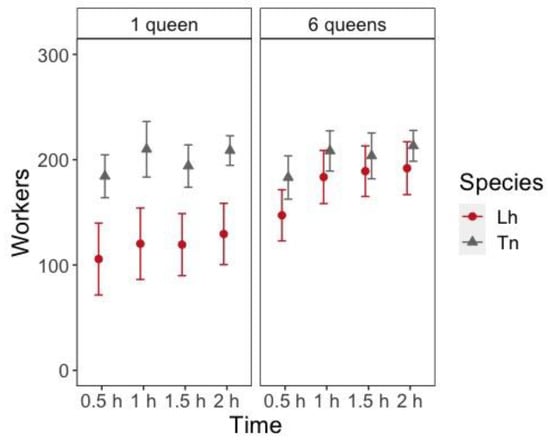
Figure 2.
Number of workers (mean ± 95% Confidence Interval (CI)) inside the nest at different times after placing them in the foraging arena according to the species (Lh = L. humile and Tn = T. nigerrimum) and the number of queens in the colony.
We compared the negative binomial model with random intercept (model 1) with a model without random intercept (model 2). Model 1 was significantly better than model 2 (chi square = 5.753, p = 0.016) and had smaller AIC (model 1: df = 10, AIC = 759, vs. model 2: df = 9, AIC = 763). The model with time interaction had smaller AIC (model 1: df = 10, AIC = 759 vs. no interaction: df = 7, AIC = 764) and there was a significant difference between both models (chi square = 10.775, p = 0.013), so we kept the time interaction. Also, the model with species interaction had smaller AIC (model 1: df = 10, AIC = 759 vs. no interaction: df = 6, AIC = 764) and there was a significant difference between both models (chi square = 12.8415, p = 0.013), so we kept the species interaction.
When nests were established (i.e., 24 h and 7 days after setup), there was a significant effect for the number of queens (z = 5.030, p < 0.001), species (z = 3.372, p < 0.001) and time for the workers to enter inside the nest (z = 4.776, p < 0.001) (Table S8). These differences were mainly driven by the lower number of workers inside one-queen L. humile nests at 24 h after the setup than in each of the other treatments (Figure 3, Table S9).
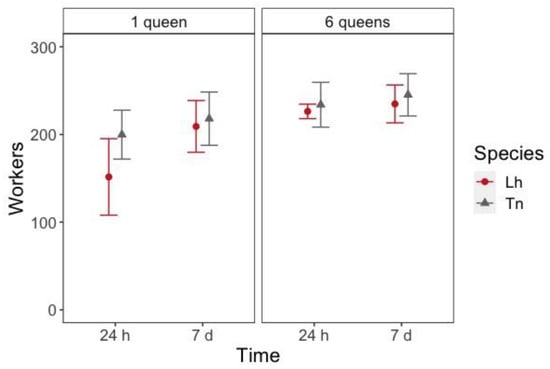
Figure 3.
Number of workers (mean ± 95% Confidence Interval (CI)) inside the nest 24 h and 7 days after placing them in the foraging arena according to the species (Lh = L. humile and Tn = T. nigerrimum) and the number of queens in the colony.
3.3. Workers Outside the Nest
The model “patrolling” without species interaction had a similar AIC than the model with the interactions (no interaction: df = 37, AIC = 248 vs. with interaction: df = 36, AIC = 249), and there was no significant difference between these models (Likelihood Ratio: LR = 0.042, p = 0.838), so we removed the species interaction. The model “nest maintenance” without species interaction had a similar AIC to the model with the interactions (no interaction: df = 4, AIC = 351, vs. with interaction: df = 5, AIC = 352), and there was no significant difference between these models (LR = 0.391, p = 0.532), so we removed the species interaction. The model “feeding” without species interaction had a similar AIC to the model with the interactions (no interaction: df = 4, AIC = 200 vs. with interaction: df = 5, AIC = 200) and there was no significant difference between these models (LR = 1.058, p = 0.304), so we removed the species interaction.
Once nests were established (i.e., 7 days after setup), we quantified the influence of the number of queens and species on the number of workers engaged in three outside nest tasks (i.e., patrolling, nest maintenance, and feeding). For both numbers of queens, T. nigerrimum nests had significantly fewer workers patrolling than L. humile nests did (z = −2.238, p = 0.025) (Figure 4a, Table S10). The number of workers engaged in nest maintenance (z = −1.196, p = 0.232) and feeding (z = −0.830, p = 0.406) was similar for both species (Figure 4b,c, Table S10). We found that six-queen nests had fewer workers engaged in nest maintenance (z = −2.980, p = 0.003) and feeding (z = −2.227, p = 0.026) compared to one-queen nests (Figure 4b,c, Table S10).
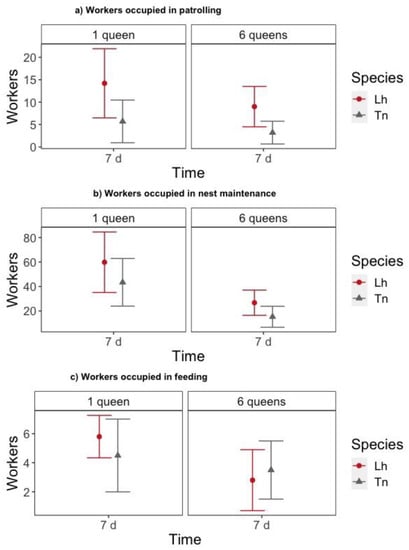
Figure 4.
Number of workers (mean ± 95% Confidence Interval (CI)) outside the nest 7 days after placing them in the foraging arena according to the species (Lh = L. humile and Tn = T. nigerrimum) and the number of queens placed in the colony: (a) workers occupied in patrolling, (b) workers occupied in nest maintenance and (c) workers occupied in feeding.
4. Discussion
4.1. Queens during Nest Establishment
We found that queens belonging to nests with numerous queens entered the nest faster for both species. This result suggests that queens may influence the behavior of other queens during nest establishment. In polygynous species, it has already been demonstrated that queens may affect the reproductive behavior of other queens through pheromones that alter the oviposition rate [,]. To date, however, no study has determined whether specific queen pheromones influence the behavior of other queens during nest establishment.
Moreover, we did not find differences between species in time spent by the first queen to enter inside one-queen nests nor six-queen nests. This could be explained because both species have a similar colony-founding strategy. Indeed, the studied species typically have highly polygynous and polydomous colonies with a lack of aggressiveness that allow them to develop super colonies made up of interconnected nests [].
4.2. Workers Inside the Nest
Our results suggest that for the time period analyzed from 0.5 h to 7 days after setup, the number of workers within the nest was higher in nests with a higher number of queens, especially for T. nigerrimum. Because the number of queens is related to the concentration of queen pheromones [], nests with a higher number of queens could cause a high number of workers to follow them inside the nest [,]. Likely, the less time that workers spend outside the nest, the faster they will start to take care of the brood. Although ant colonies with more queens can grow faster, there might be a critical worker-to-queen ratio for colony growth and reproduction, as seen for some species such as Mycocepurus smithii []. For the invasive L. humile, an overabundance of queens promotes the seasonal execution of less productive queens []. According to our results, it seems that T. nigerrimum is more efficient than L. humile at establishing their nests, because the species depends less on the number of queens.
4.3. Workers Outside the Nest
T. nigerrimum nests had fewer workers engaged in patrolling compared to L. humile nests. Because our colonies were settled and maintained in the same conditions (i.e., humidity, temperature, light, food resources), the different numbers of workers engaged in patrolling could be explained by differences in the behavioral plasticity of species. Indeed, according to external and internal conditions, workers can shift between tasks to optimize colony performance []. Knowledge about the mechanisms underlying the behavioral plasticity of workers, such as genetic and developmental factors, would be the key to better understanding higher level processes such as species coexistence and competition between T. nigerrimum and L. humile colonies in invaded areas such as the Mediterranean basin.
For both species, we found that six-queen nests had fewer workers engaged in nest maintenance and feeding than one-queen nests. It is therefore likely that the number of workers engaged in those tasks could be modulated by the number of queens present in the nests. Indeed, a higher number of queens in the nests would require more workers for inside-nest tasks such as brood rearing and queen attendance rather than outside-nest tasks (i.e., nest maintenance and feeding). Other social characteristics, such as age, have been identified as one of the main factors promoting the task shifts of workers under stable conditions [,].
4.4. Perspectives
One of the main goals of invasion biology is predicting and preventing the future establishment of invasive species. Invasive species cause damage to the environment, and their impact and management have high economic costs []. Although a small fraction of introduced species become invasive [,,], the continual increase in species trade and human-mediated exchange generates many new species introductions worldwide. Consequently, it is of high priority to better understand the processes, underlying causes, and consequences of establishment success. Among invasive species, more than 241 ant species have successfully colonized areas outside of their native range [], and 19 of these species are listed in the Global Invasive Species Database (GISD).
According to the Convention on Biological Diversity [], the most effective approaches to limit the impact of invasive species is to maintain and preserve diverse communities and habitats and prevent any introductions. Nevertheless, once introduced, there is a wide range of chemical, mechanical, and biological methods to limit the spread of invasive species [,]. Among invasive ant species, L. humile continues to spread [] given the absence of an efficient biocontrol agent. In the Mediterranean basin, the presence of native species like T. nigerrimum, which has similar colony attributes and habitat requirements to L. humile, could be a promising approach to limit the spread of the L. humile super colony []. Thus, future studies should assess whether queen numbers can influence nest establishment success in situations in which both L. humile and T. nigerrimum are present.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/11/3/591/s1, Figure S1: Left photo shows the complete experimental set-up before adding the ants, showing the foraging arena attached to the nest through a transparent tube. Right photo shows an example of photos taken after addition of the ants with food and water supplies. The nest was covered with red transparent paper that was lifted only while taking photos; Figure S2: Mean ± 95% CI of the number of workers inside the nest at different times after placing them in the foraging arena according to the species (Lh = L. humile and Tn = T. nigerrimum) and the number of queens in the colony. Table S1: Generalized linear mixed model (GLMM) results for the relationship between workers inside the nest and number of queens (1 or 6), species (Lh = L. humile and Tn = T. nigerrimum), and time (six records: 0.5 h, 1 h, 1.5 h, 2 h, 24 h and 7 days), as well as their interaction as fixed effects. We included nest ID as a random effect. Bold values show p-values < 0.05. Table S2: Model parameters corresponding to model selection. In all cases, we keep models with negative binomial distribution. Table S3: Results obtained from the multivariate Cox model according to species (T. nigerrimum (Tn) and L. humile (Lh)) and number of queens (1-queen and 6 queens). Significant values are presented in bold. Table S4: Results obtained from the univariate Cox model according to species (T. nigerrimum (Tn) and L. humile (Lh)) for 1-queen nests. Significant values are presented in bold. Table S5: Results obtained from the univariate Cox model according to species (T. nigerrimum (Tn) and L. humile (Lh)) for 6-queen nets. Significant values are presented in bold. Table S6: Generalized linear mixed model (GLMM) results for the relationship between workers inside the nest and number of queens (1 or 6), species (Lh = L. humile and Tn = T. nigerrimum), and time (four records: 0.5 h, 1 h, 1.5 hand 2 h), as well as their interaction as fixed effects. We included nest ID as a random effect. Bold values show p-values < 0.05. Table S7: Pairwise comparison for significant interactions between the number of queens and species combination from the model for nest establishment. Bold values show p-values < 0.05. Table S8: Generalized linear mixed model (GLMM) results for the relationship between workers inside the nest and the number of queens (1 or 6), species (Lh = L. humile and Tn = T. nigerrimum), and time (two records: 24 h and 7 days), as well as their interaction as fixed effects. We included nest ID as a random effect. Bold values show p-values < 0.05. Table S9: Pairwise comparison for significant interactions between the number of queens, species, and time combination from the model for established nests. Table S10: Generalized linear model (GLM) results for the relationship between workers outside the nest 7 days after placing them in the foraging arena according to species (Lh = L. humile and Tn = T. nigerrimum), number of queens (1 or 6), and their interaction as fixed effects. Bold values show p-values < 0.05.
Author Contributions
G.L. conceived and planned the experimental setup. G.L. and I.C. carried out the experiments in the laboratory. G.L. and I.C. analyzed the data. I.C. took the lead in writing the manuscript. All authors provided critical feedback on the analysis and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Marie Curie Intra European Fellowship (to G. Luque), financed by the FP7 of the European Commission (contract-N 237862) and by the CNRS and a grant from the French National Research Agency/BiodivERsA: “Alien Scenarios” project (BMBF/PT DLR 01LC1807C). FC was funded by the AXA Research Fund Chair of Invasion Biology of University Paris Saclay.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
We thank Olivier Blight (O.B.) and Melanie Fichaux (M.F.) for their help in collecting the ants in the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grevstad, F.S. Factors influencing the chance of population establishment: Implications for release strategies in biocontrol. Ecol. Appl. 1999, 9, 1439–1447. [Google Scholar] [CrossRef]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mikheyev, A.S.; Tchingnoumba, L.; Henderson, A.; Alonso, A. Effect of propagule pressure on the establishment and spread of the little fire ant Wasmannia auropunctata in a Gabonese oilfield. Divers. Distrib. 2008, 14, 301–306. [Google Scholar] [CrossRef]
- Sagata, K.; Lester, P.J. Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. J. Appl. Ecol. 2009, 46, 19–27. [Google Scholar] [CrossRef]
- Rice, E.S.; Silverman, J. Propagule Pressure and Climate Contribute to the Displacement of Linepithema humile by Pachycondyla chinensis. PLoS ONE 2013, 8, e56281. [Google Scholar] [CrossRef][Green Version]
- Hölldobler, B.; Wilson, E. The Ants; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1990. [Google Scholar]
- Hee, J.J.; Holway, D.A.; Suarez, A.V.; Case, T.J. Role of propagule size in the success of incipient colonies of the invasive Argentine ant. Conserv. Biol. 2000, 14, 559–563. [Google Scholar] [CrossRef]
- Holway, D.A.; Case, T.J. Effects of colony-level variation on competitive ability in the invasive Argentine ant. Anim. Behav. 2001, 61, 1181–1192. [Google Scholar] [CrossRef]
- Luque, G.M.; Giraud, T.; Courchamp, F. Allee effects in ants. J. Anim. Ecol. 2013, 82, 956–965. [Google Scholar] [CrossRef]
- Passera, L.; Aron, S.; Bach, D. Elimination of sexual brood in the Argentine ant Linepithema humile: Queen effect and brood recognition. Entomol. Exp. Appl. 1995, 75, 203–212. [Google Scholar] [CrossRef]
- Abril, S.; Gómez, C. Factors triggering queen executions in the Argentine ant. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Wittenberg, R.; Cock, M.J.W. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; CAB International: Wallingford, Oxon, UK, 2001; Volume 28. [Google Scholar]
- Hoffmann, B.D.; Luque, G.M.; Bellard, C.; Holmes, N.D.; Donlan, C.J. Improving invasive ant eradication as a conservation tool: A review. Biol. Conserv. 2016, 198, 37–49. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef]
- Holway, D.A.; Suarez, A.V.; Case, T.J. Role of abiotic factors in governing susceptibility to invasion: A test with argentine ants. Ecology 2002, 83, 1610–1619. [Google Scholar] [CrossRef]
- Giraud, T.; Pedersen, J.S.; Keller, L. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA 2002, 99, 6075–6079. [Google Scholar] [CrossRef]
- Blight, O.; Provost, E.; Renucci, M.; Tirard, A.; Orgeas, J. A native ant armed to limit the spread of the Argentine ant. Biol. Invasions 2010, 12, 3785–3793. [Google Scholar] [CrossRef]
- Buczkowski, G.; Bennett, G.W. Aggressive interactions between the introduced Argentine ant, Linepithema humile and the native odorous house ant, Tapinoma sessile. Biol. Invasions 2008, 10, 1001–1011. [Google Scholar] [CrossRef]
- Blight, O.; Renucci, M.; Tirard, A.; Orgeas, J.; Provost, E. A new colony structure of the invasive Argentine ant (Linepithema humile) in Southern Europe. Biol. Invasions 2010, 12, 1491–1497. [Google Scholar] [CrossRef]
- Dussutour, A.; Simpson, S.J. Description of a simple synthetic diet for studying nutritional responses in ants. Insectes Soc. 2008, 55, 329–333. [Google Scholar] [CrossRef]
- Hartley, S.; Harris, R.; Lester, P.J. Quantifying uncertainty in the potential distribution of an invasive species: Climate and the Argentine ant. Ecol. Lett. 2006, 9, 1068–1079. [Google Scholar] [CrossRef]
- In, J.; Lee, D.K. Survival analysis: Part I—Analysis of time-to-event. Korean J. Anesthesiol. 2018, 71, 182–191. [Google Scholar] [CrossRef]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Singmann, H. Emmeans: Estimated Marginal Means, aka Least-Squares Means. Am. Stat. 2020, 34, 216–221. [Google Scholar] [CrossRef]
- Oster, G.; Wilson, E. Caste and Ecology in Social Insects; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Bates, D.; Maechler, M. lme4: Mixed-Effect Modeling with R. 2010. Available online: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 8 February 2021).
- Therneau, T.M.; Lumley, T.; Atkinson, E.; Crowson, C. Package “Survival”. 2015. Available online: https://cran.r-project.org/web/packages/survival/survival.pdf (accessed on 8 February 2021).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. 2016. Available online: https://cran.r-project.org/web/packages/ggplot2/ggplot2.pdf (accessed on 8 February 2021).
- Vargo, E.L.; Fletcher, D.J.C. Relationship of queen number and worker size in polygyne colonies of the fire ant Solenopsis invicta. Physiol. Entomol. 1989, 14, 223–232. [Google Scholar] [CrossRef]
- Wilson, E. The Insect Societies; Belknap Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Fang, C.C.; Chang, F.H.; Duong, P.; Kurian, J.; Mueller, U.G. Colony fitness and garden growth in the asexual fungus-growing ant Mycocepurus smithii (Attini, Formicidae). Insectes Soc. 2020, 67, 35–49. [Google Scholar] [CrossRef]
- Robinson, G.E. Division of Labor in Insect Societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef] [PubMed]
- Cassey, P.; Blackburn, T.M.; Russell, G.J.; Jones, K.E.; Lockwood, J.L. Influences on the transport and establishment of exotic bird species: An analysis of the parrots (Psittaciformes) of the world. Glob. Chang. Biol. 2004, 10, 417–426. [Google Scholar] [CrossRef]
- Cassey, P.; Blackburn, T.M.; Jones, K.E.; Lockwood, J.L. Mistakes in the analysis of exotic species establishment: Source pool designation and correlates of introduction success among parrots (Aves: Psittaciformes) of the world. J. Biogeogr. 2004, 31, 277–284. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Strayer, D.L. Determinants of vertebrate invasion success in Europe and North America. Glob. Chang. Biol. 2006, 12, 1608–1619. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Ollier, S.; Liebhold, A.; Keller, L. Recent human history governs global ant invasion dynamics. Nat. Ecol. Evol. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- United Nations. Convention on Biological Diversity; United Nations: New York, NY, USA, 1992. [Google Scholar]
- Rabitsch, W. The hitchhiker’s guide to alien ant invasions. BioControl 2011, 56, 551–572. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).