COMMD1 Exemplifies the Power of Inbred Dogs to Dissect Genetic Causes of Rare Copper-Related Disorders
Simple Summary
Abstract
1. Introduction
2. Inherited Copper Storage Diseases
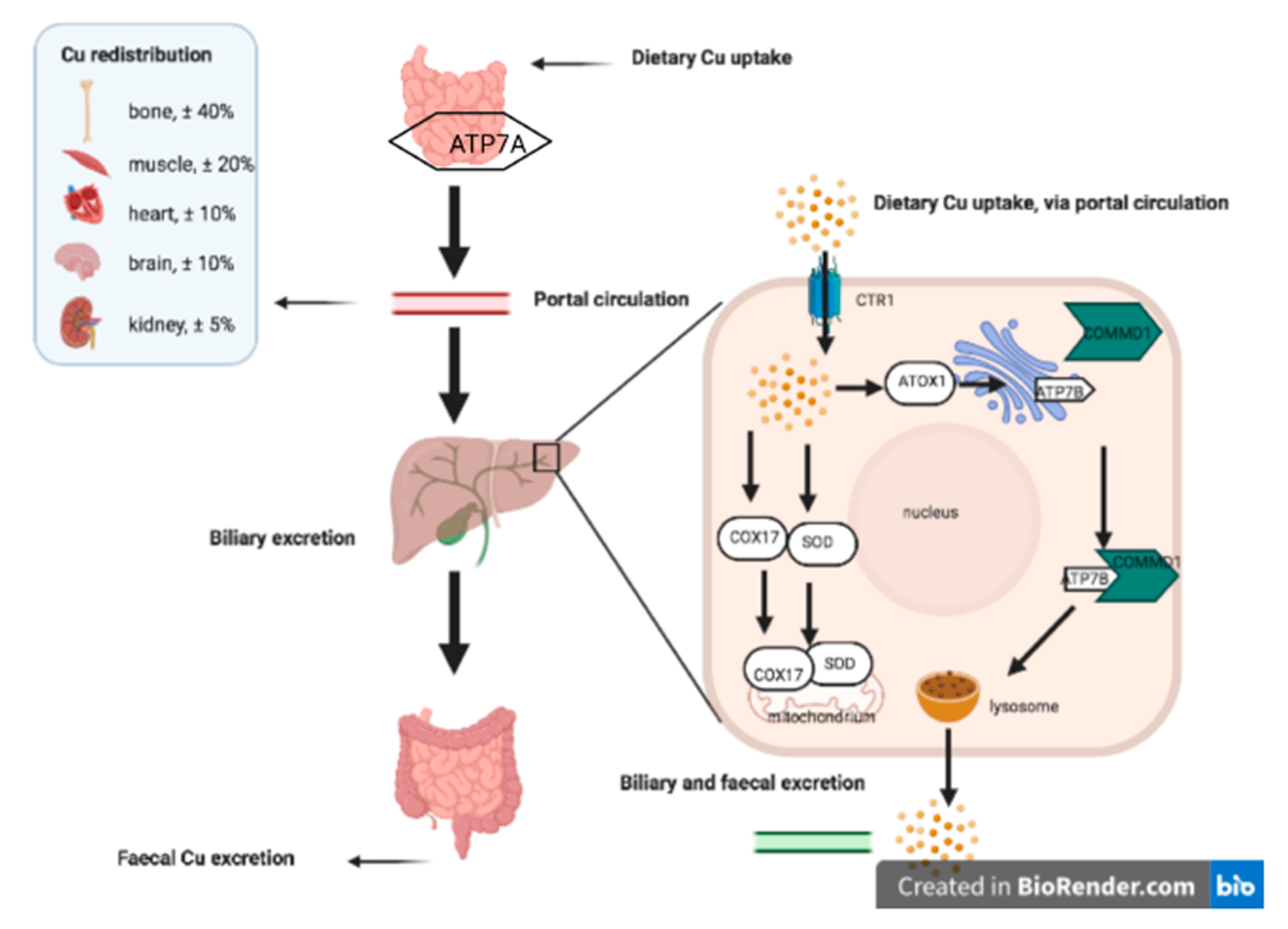
2.1. Copper Homeostasis
2.2. Wilsons Disease
2.3. Menkes Disease
2.4. Very Rare Copper Related Diseases
3. Two Decades of Canine Genetics
4. The Unique Population Structure of Dog Breeds Amplifies Rare Mutations
5. Inherited Copper Storage Diseases Are NOT Rare in Dogs
5.1. COMMD1 Mutations in Bedlington Terriers
5.2. ATP7A and ATP7B Mutations in Labrador Retrievers and Dobermann Dogs
6. Dogs and Cats Are Clearly Different with Regard to Hepatic Copper Accumulation
7. The Mutual Benefits for Men and Dogs in Rare Copper Storage Diseases
8. Dogs as a Test Bed for Humans with Copper Storage Diseases: Copper-Zinc Interactions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakap, S.N.; Deborah, M.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Daniel, M.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Kaela, S.; Singleton, W.M.; Faundez, V. Rare Genetic Diseases: Nature’s Experiments on Human Development. IScience 2020, 23, 101123. [Google Scholar] [CrossRef]
- Inesi, G. Molecular features of copper binding proteins involved in copper homeostasis. Crit. Rev. IUBMB Life 2017, 69, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Gitschier, J. hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. USA 1997, 94, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- Trocello, J.-M.; Brousolle, E.; Girardot-Tinant, N.; Pelosse, M.; Lachaux, A.; Lloyd, C.; Woimant, F. Wilson’s disease, 100 years later. Rev. Neurol. 2013, 169, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-J.; Wu, Z.-Y. Wilson’s disease in China. Neurosci. Bull. 2017, 33, 323–330. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef]
- Lo, C.; Bandmann, O. Epidemiology and introduction to the clinical presentation of Wilson disease. Handb. Clin. Neurol. 2017, 142, 7–17. [Google Scholar]
- Sandahl, T.D.; Laursen, T.L.; Munk, D.E.; Vilstrup, H.; Wiess, K.H.; Ott, P. The prevalence of Wilson’s disease: An update. Hepatology 2020, 71, 722–732. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbers, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Petrukhin, K.; Chernov, I.; Pelleguer, J.L.; Wasco, W.; Ross, B.; Romano, D.M.; Parano, E.; Pavone, L.; Bzustowicz, L.M.; et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993, 5, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Pierson, H.; Nuchenditsi, A.; Byung-Eun, K.; Ralle, M.; Zachos, N.; Huster, D.; Lutsenko, S. The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 2018, 154, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R. Wilson’s disease. Semin. Neurol. 2007, 27, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W. Classification and differential diagnosis of Wilson’s disease. Ann. Transl. Med. 2019, 7, S63. [Google Scholar] [CrossRef] [PubMed]
- Buiakova, O.I.; Xu, J.; Lutsenko, S.; Zeitlin, S.; Das, K.; Das, S.; Ross, B.M.; Mekios, C.; Scheinberg, I.H.; Gilliam, T.C. Null Mutation of the Murine ATP7B (Wilson Disease) Gene Results in Intracellular Copper Accumulation and Late-Onset Hepatic Nodular Transformation. Hum. Mol. Genet. 1999, 8, 1665–1671. [Google Scholar] [CrossRef]
- Reed, E.; Lutsenko, S.; Bandmann, O. Animal models of Wilson disease. J. Neurochem. 2018, 146, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Phenotype-genotype correlations in patients with Wilson’s disease. Ann. N. Y. Acad. Sci. 2014, 1315, 1–5. [Google Scholar] [CrossRef]
- Lutsenko, S. Modifying factors and phenotypic diversity in Wilson’s disease. Ann. N. Y. Acad. Sci. 2014, 1315, 56–63. [Google Scholar] [CrossRef]
- Medici, V.; Weiss, K.H. Genetic and environmental modifiers of Wilson disease. Handb. Clin. Neurol. 2017, 142, 35–41. [Google Scholar]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7, S61. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.; Schaefer, M.; Reichert, J.; Stremmel, W. Analysis of the Human Atox 1 Homologue in Wilson Patients. World J. Gastroenterol. 2008, 14, 2383–2387. [Google Scholar] [CrossRef]
- Weiss, K.H.; Runz, H.; Noe, B.; Gotthardt, D.N.; Merle, U.; Ferenci, P.; Stremmel, W.; Füllekrug, J. Genetic Analysis of BIRC4/XIAP as a Putative Modifier Gene of Wilson Disease. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S233–S240. [Google Scholar] [CrossRef]
- Stuehler, B.; Reichert, J.; Stremmel, W.; Schaefer, M. Analysis of the Human Homologue of the Canine Copper Toxicosis Gene MURR1 in Wilson Disease Patients. J. Mol. Med. 2004, 82, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Rudnicka, M.; Chabik, G.; Przybylkowski, A.; Czlonkowska, A. Genetic variability in the methylenetetrahydrofolate reductase gene (MTHFR) affects clinical expression of Wilson’s disease. J. Hepatol. 2011, 55, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Piguit-Lacroix, G.; Parant, F.; Wilson, C.M.R. Molecualr analysis of Wilson patients: Direct sequencing and MLPA analysis in the ATP7B gene and Atox1 and COMMD1 gene analysis. J. Trace Elem. Med. Biol. 2012, 26, 97–101. [Google Scholar] [CrossRef]
- Menkes, J.H.; Alter, M.; Steigleder, G.K.; Weakley, D.R.; Sung, J.H. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebral degeneration. Pediatrics 1962, 29, 764–779. [Google Scholar] [PubMed]
- Danks, D.M.; Campbell, P.E.; Stevens, B.J.; Mayne, V.; Cartwright, E. Menkes’s kinky hair syndrome. An inherited defect in copper absorption with widespread effects. Pediatrics 1972, 59, 188–201. [Google Scholar]
- Chelly, J.; Tumer, Z.; Tonnesen, T.; Petteson, A.; Ishikawa-Brush, Y.; Tommerup, N.; Norn, N.; Monaco, A.P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 1993, 3, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.F.B.; Livingston, J.; Hall, B.; Paynter, J.A.; Begy, C.; Chandrasekharappa, S.; Lockhart, P.; Grimes, A.; Bhave, M.; Siemieniak, D.; et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993, 3, 20–25. [Google Scholar]
- Vulpe, C.; Levinson, B.; Whitney, S.; Packman, S.; Gitschier, J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993, 3, 7–13. [Google Scholar] [CrossRef]
- Gourdon, P.; Sitsel, O.; Lykkegaard Karlsen, J.; Møller, L.B.; Niassen, P. Structural models of the human copper-type ATPases ATP7A and ATP7B. Biol. Chem. 2012, 393, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tümer, Z.; Møller, L.B. Menkes disease. Eur. J. Hum. Genet. 2010, 18, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Tümer, Z. An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Hum. Mutat. 2013, 34, 417–429. [Google Scholar] [CrossRef]
- Kaler, S.G. Translational research investigations on ATP7A: An important human copper ATPase. Ann. N. Y. Acad. Sci. 2014, 1314, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Smpokou, P.; Samanta, M.; Berry, G.T.; Hecht, L.; Engle, E.C.; Lichter-Konecki, U. Menkes disease in affected females: The clinical disease spectrum. Am. J. Med. Genet. 2015, 167A, 417–420. [Google Scholar] [CrossRef]
- Møller, L.B.; Mogensen, M.; Horn, N. Molecular diagnosis of Menkes disease: Genotype-phenotype correlation. Biochimie 2009, 91, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.S. Role of copper in Indian Childhood Cirrhosis. Am. J. Clin. Nutr. 1998, 67, 1074S–1081S. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Feichtinger, H.; Berger, H.; Muller, W. Endemic Tyrolean Infantile Cirrhosis: An ecogenetic disorder. Lancet 1996, 347, 877–880. [Google Scholar] [CrossRef]
- Scheinberg, I.C.; Sternlieb, I. Wilson Disease and Idiopathic Copper Toxicosis. Am. J. Cin. Nutr. 1996, 63, 842S–845S. [Google Scholar] [CrossRef]
- Wijmenga, C.; Müller, T.; Murli, I.S.; Brunt, T.; Feichtinger, H.; Schönitzer, D.; Houwen, R.H.; Müller, W.; Sandkuijl, L.A.; Pearson, P.L. Endemic Tyrolean infantile cirrhosis is not an allelic variant of Wilson’s disease. Eur. J. Hum. Genet. 1998, 6, 624–628. [Google Scholar] [CrossRef]
- Müller, T.; van de Sluis, B.; Müller, W.; Pearson, P.; Wijmenga, C. Non-Indian childhood cirrhosis. Eur. J. Med. Res. 1999, 4, 293–297. [Google Scholar]
- Müller, T.; van de Sluis, B.; Zhernakova, A.; van Binsbergen, E.; Janecke, A.R.; Bavdekar, A.; Pandit, A.; Weirich-Schwaiger, H.; Witt, H.; Ellemunter, H.; et al. The canine copper toxicosis gene MURR1 does not cause non-Wilsonian hepatic copper toxicosis. J. Hepatol. 2003, 38, 164–168. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, L. Man’s best friend may be companion in cancer research. J. Natl. Cancer Inst. 1993, 85, 1455–1456. [Google Scholar] [CrossRef]
- Hitte, C.; Madeoy, J.; Kirkness, E.F.; Priat, C.; Lorentzen, T.D.; Senger, F.; Thomas, D.; Derrien, T.; Ramirez, C.; Scott, C.; et al. Facilitating genome navigation: Survey sequencing and dense radiation-hybrid gene mapping. Nat. Rev. Genet. 2005, 6, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Ostrander, E.A. Canine genomics and genetics: Running with the pack. PLoS Genet. 2005, 1, e58. [Google Scholar] [CrossRef]
- Ostrander, E.A.; Wayne, R.K. The canine genome. Genome Res. 2005, 15, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J., 3rd; Zody, M.C.; et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005, 438, 803–809. [Google Scholar] [CrossRef]
- Lequarre, A.-S.; Andersson, L.; Andre, C.; Fredholm, M.; Hitte, C.; Leeb, T.; Lohi, H.; Lindblad-Toh, K.; Georges, M. LUPA: A European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet. J. 2011, 189, 155–159. [Google Scholar] [CrossRef]
- Vaysse, A.; Ratnakumaer, A.; Derrien, T.; Axelsson, E.; Pielberg, G.R.; Sigurdsson, S.; Fall, T.; Seppala, E.H.; Hanse, M.S.T.; Lawley, C.T.; et al. Identification of genomic regions associated with phenotype variation between dog breeds using selection mapping. PLoS Genet. 2011, 7, e1002316. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, M.P.; Lundquies, A. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS ONE 2014, 9, e91172. [Google Scholar] [CrossRef] [PubMed]
- Penso-Dolfin, L.; Swofford, R.; Johnson, J.; Alfoedi, J.; Loindblad-Toh, K.; Swarbreck, D.; Moxon, S.; Di Palma, F. An improved microRNA annotation of the canine genome. PLoS ONE 2016, 11, e0153453. [Google Scholar] [CrossRef]
- Wucher, V.; Legeai, F.; Hedan, B.; Rizk, G.; Lagoutee, L.; Leeb, T.; Jagannathan, V.; Cadieu, E.; David, A.; Lohi, H.; et al. FEELnc: A tool for long non-codong RNA annotation and its pplication to the dog transcriptome. Nucl. Acid Res. 2017, 45, e57. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Larson, G.; Kidd, J.M.; vonHoldt, B.M.; Ostrander, E.A.; Zhang, Y.P. Dog10K: The international consortium of canine genome sequencing. Natl. Sci. Rev. 2019, 6, 611–613. [Google Scholar] [CrossRef]
- Fieten, H.; Penning, L.C.; Leegwater, P.A.; Rothuizen, J. New canine models of copper toxicosis: Diagnosis, treatment, and genetics. Ann. N. Y. Acad. Sci. 2014, 1314, 42–48. [Google Scholar] [CrossRef]
- Parker, H.G.; Shearin, A.L.; Ostrander, E.A. Man’s best friend becomes biology’s best in show: Genome analyses in the domestic dog. Annu. Rev. Genet. 2010, 44, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Karlsson, E.K.; Perri, A.; Webster, M.T.; Ho, S.Y.; Peters, J.; Stahl, P.W.; Lingaas, F.; Fredholm, M.; Comstock, K.E.; et al. Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc. Natl. Acad. Sci. USA 2012, 109, 8878–8883. [Google Scholar] [CrossRef]
- van Steenbeek, F.G.; Hytoenen, M.K.; Leegwater, P.A.J.; Lohi, H. The canine era: The rise of a biomedical model. Anim. Genet. 2016, 47, 519–527. [Google Scholar] [CrossRef]
- Fuentalba, I.C.; Aburto, E.M. Animal models of copper-associated liver disease. Comp. Hepatol. 2003, 2, 5. [Google Scholar] [CrossRef]
- Twedt, D.C.; Sternlieb, I.; Gilbertson, S.R. Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J. Am. Vet. Med. Assoc. 1979, 175, 269–275. [Google Scholar] [PubMed]
- Haywood, S.; Rutgers, H.C.; Christian, M.K. Hepatitis and copper accumulation in Skye terriers. Vet. Pathol. 1988, 25, 408–414. [Google Scholar] [CrossRef]
- Thornburg, L.P.; Rottinghaus, G.; Dennis, G.; Crawford, S. The relationship between hepatic copper content and morphologic changes in the liver of West Highland White Terriers. Vet. Pathol. 1996, 33, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, L.P. Histomorphological and immunohistochemical studies of chronic active hepatitis in Doberman Pinschers. Vet. Pathol. 1998, 35, 380–385. [Google Scholar] [CrossRef]
- Webb, C.B.; Twedt, D.C.; Meyer, D.J. Copper-associated liver disease in Dalmatians: A review of 10 dogs (1998–2001). J. Vet. Intern. Med. 2002, 16, 665–668. [Google Scholar] [PubMed]
- Hoffmann, G.; van den Ingh, T.S.; Bode, P.; Rothuizen, J. Copper-associated chronic hepatitis in Labrador Retrievers. J. Vet. Intern. Med. 2006, 20, 856–861. [Google Scholar] [CrossRef] [PubMed]
- van de Sluis, B.J.; Breen, M.; Nanji, M.; van Wolferen, M.; de Jong, P.; Binns, M.M.; Pearson, P.L.; Kuipers, J.; Rothuizen, J.; Cox, D.W.; et al. Genetic mapping of the copper toxicosis locus in Bedlington terriers to dog chromosome 10, in a region syntenic to human chromosome region 2p13-p16. Hum. Mol. Genet. 1999, 8, 501–507. [Google Scholar] [CrossRef]
- van de Sluis, B.; Rothuizen, J.; Pearson, P.L.; van Oost, B.A.; Wijmenga, C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 2002, 11, 165–173. [Google Scholar] [CrossRef]
- Forman, O.P.; Boursnell, M.E.G.; Dunmore, B.J.; Stendall, N.; van de Sluis, B.; Frettwell, N.; Jones, N.; Wijmenga, C.; Rothuizen, J.; van Oost, B.A.; et al. Characterization of the COMMD1 (MURR1) Mutation Causing Copper Toxicosis in Bedlington Terriers. Anim. Genet. 2005, 36, 497–501. [Google Scholar] [CrossRef]
- Ganesh, L.; Burstein, E.; Guha-Niyogi, A.; Louder, M.K.; Mascola, J.R.; Klomp, L.W.; Wijmenga, C.; Duckett, C.S.; Nabel, G.J. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 2003, 426, 853–857. [Google Scholar] [CrossRef]
- Spee, B.; Arends, B.; van Wees, A.M.; Bode, P.; Penning, L.C.; Rothuizen, J. Functional consequences of RNA interference targeting COMMD1 in a canine hepatic cell line in relation to copper toxicosis. Anim. Genet. 2007, 38, 168–170. [Google Scholar] [CrossRef]
- Vonk, W.I.; Bartuzi, P.; de Bie, P.; Kloosterhuis, N.; Wichers, C.G.; Berger, R.; Haywood, S.; Klomp, L.W.; Wijmenga, C.; van de Sluis, B. Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation. PLoS ONE 2011, 6, e29183. [Google Scholar] [CrossRef]
- Nantasanti, S.; Spee, B.; Kruitwagen, H.S.; Chen, C.; Geijsen, N.; Oosterhoff, L.A.; van Wolferen, M.E.; Palaez, N.; Fieten, H.; Wubbolts, R.W.; et al. Disease Modeling and Gene Therapy of Copper Storage Disease in Canine Hepatic Organoids. Stem Cell Rep. 2015, 5, 895–907. [Google Scholar] [CrossRef]
- Su, L.C.; Ravanshad, S.; Owen, C.A., Jr.; McCall, J.T.; Zollman, P.E.; Hardy, R.M. A comparison of copper-loading disease in Bedlington terriers and Wilson’s disease in humans. Am. J. Physiol. 1982, 243, G226–G230. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Penning, L.C. Precilinical models of Wilson’s disease, why dogs are cathy alternatives. Ann. Transl. Med. 2019, 7, S71. [Google Scholar] [CrossRef] [PubMed]
- de Bie, P.; van de Sluis, B.; Burstein, E.; van den Berghe, P.V.; Muller, P.; Berger, R.; Gitlin, J.D.; Wijmenga, C.; Klomp, L.W. Distinct Wilson’s disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology 2007, 133, 1316–1326. [Google Scholar] [CrossRef]
- Weiss, K.H.; Lozoya, J.C.; Tuma, S.; Gotthardt, D.; Reichert, J.; Ehehalt, R.; Stremmel, W.; Fullekrug, J. Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7. Am. J. Pathol. 2008, 173, 1783–1794. [Google Scholar] [CrossRef]
- Vonk, W.I.; de Bie, P.; Wichers, C.G.; van den Berghe, P.V.; van der Plaats, R.; Berger, R.; Wijmenga, C.; Klomp, L.W.; van de Sluis, B. The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression. Cell. Mol. Life Sci. 2012, 69, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Favier, R.P.; Spee, B.; Schotanus, B.A.; van den Ingh, T.S.; Fieten, H.; Brinkhof, B.; Viebahn, C.S.; Penning, L.C.; Rothuizen, J. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS ONE 2012, 7, e42158. [Google Scholar] [CrossRef] [PubMed]
- Favier, R.P.; Spee, B.; Fieten, H.; van den Ingh, T.S.; Schotanus, B.A.; Brinkhof, B.; Rothuizen, J.; Penning, L.C. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficient dogs. J. Trace Elem. Med. Biol. 2015, 29, 347–353. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Oosterhoff, L.A.; van Wolferen, M.E.; Chen, C.; Assawarachan, S.N.; Schneeberger, K.; Kummeling, A.; van Straten, G.; Akkerdaas, I.C.; Vinke, C.R.; et al. Long-term survival of transplanted autologous canine liver organoids in a COMMD1-deficient dog model of metabolic liver disease. Cells 2020, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- de Bie, P.; van de Sluis, B.; Klomp, L.; Wijmenga, C. The many faces of the copper metabolism protein MURR1/COMMD1. J. Hered. 2005, 97, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Maine, G.N.; Burstein, E. COMMD proteins: COMMing to the scene. Cell. Mol. Life Sci. 2007, 64, 1997–2005. [Google Scholar] [CrossRef]
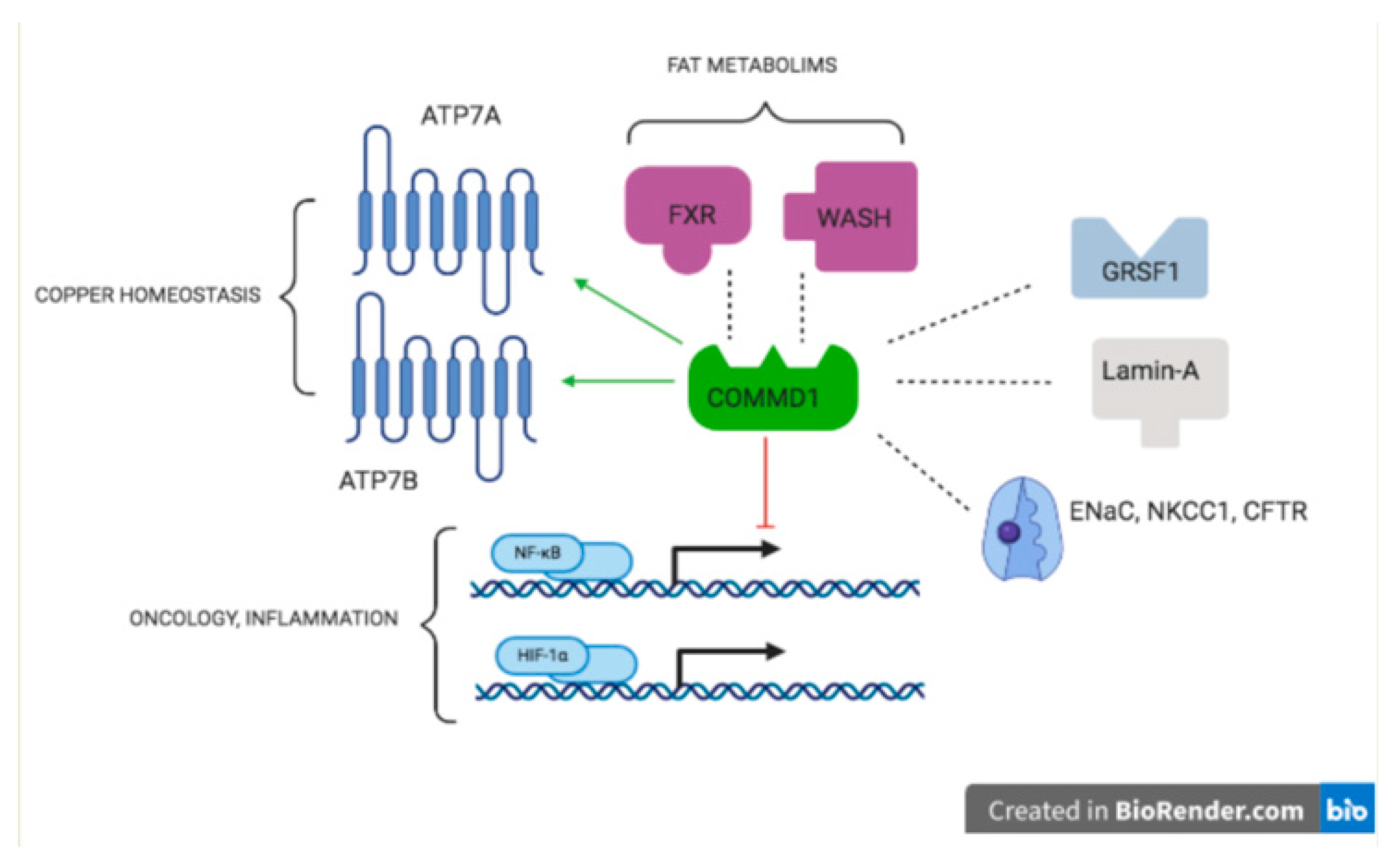
- Fedoseienko, A.; Bartuzi, P.; van de Sluis, B. Functional understanding of the versatile protein copper metabolism MURR1 domain 1 (COMMD1) in copper homeostasis. Ann. N. Y. Acad. Sci. 2014, 1314, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, P.; Hofker, M.H.; van de Sluis, B. Tuning NF-κB activity: A touch of COMMD proteins. Biochim. Biophys. Acta. 2013, 1832, 2315–2321. [Google Scholar] [CrossRef]
- Riera-Romo, M. COMMD1: A Multifunctional Regulatory Protein. J. Cell. Biochem. 2018, 119, 34–51. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Penning, L.C. COMMD1, a multi-potent intracellular protein involved in copper homeostasis, protein trafficking, inflammation, and cancer. J. Trace Elem. Med. Biol. 2021, 65, 126712. [Google Scholar]
- Biasio, W.T.; Chang, T.; McIntosh, C.J.; McDonald, F.J. Identification of Murr1 as a regulator of the human delta epithelial sodium channel. J. Biol. Chem. 2004, 279, 5429–5434. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Butt, A.G.; Swart, M.; Liu, Y.F.; McDonald, F.J. COMMD1 downregulates the epithelial sodium channel through Nedd4-2. Am. J. Physiol. Renal Physiol. 2010, 298, F1445–F1456. [Google Scholar] [CrossRef]
- Smith, L.; Litman, P.; Liedtke, C.M. COMMD1 Interacts with the COOH Terminus of NKCC1 in Calu-3 Airway Epithelial Cells to Modulate NKCC1 Ubiquitination. J. Physiol. Cell. Physiol. 2013, 305, C133–C146. [Google Scholar] [CrossRef] [PubMed]
- Drévillon, L.; Tanguy, G.; Hinzpeter, A.; Arous, N.; de Becdelièvre, A.; Aissat, A.; Tarze, A.; Goossens, M.; Fanen, P. COMMD1-mediated Ubiquitination Regulates CFTR Trafficking. PLoS ONE 2011, 6, e18334. [Google Scholar] [CrossRef]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal receptor trafficking: Retromer and beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef]
- McNally, K.E.; Cullen, P.J. Endosomal Retrieval of Cargo: Retromer Is Not Alone. Trends Cell Biol. 2018, 28, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.; Ganesh, L.; Dick, R.D.; van de Sluis, B.; Wilkinson, J.C.; Klomp, L.W.; Wijmenga, C.; Brewer, G.J.; Nabel, G.J.; Duckett, C.S. A novel role for XIAP in copper homeostasis through regulation of Murr1. EMBO J. 2004, 23, 244–254. [Google Scholar] [CrossRef]
- Maine, G.N.; Burstein, E. COMMD Proteins and the Control of the NF Kappa B Pathway. Cell Cycle 2007, 6, 672–776. [Google Scholar] [CrossRef] [PubMed]
- Mufti, A.R.; Burstein, E.; Duckett, C.S. XIAP: Cell Death Regulation Meets Copper Homeostasis. Arch. Biochem. Biophys. 2007, 463, 168–174. [Google Scholar] [CrossRef]
- van de Sluis, B.; Mao, X.; Zhai, Y.; Groot, A.J.; Vermeulen, J.F.; van der Wall, E.; van Diest, P.J.; Hofker, M.H.; Wijmenga, C.; Klomp, L.W.; et al. COMMD1 Disrupts HIF-1alpha/beta Dimerization and Inhibits Human Tumor Cell Invasion. J. Clin. Investig. 2010, 120, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.; Louhimo, R.; Koivula, S.; Chen, P.; Rantanen, V.; Holte, H.; Delabie, J.; Karjalainen-Lindsberg, M.-L.; Björkholm, M. Deregulation of COMMD1 Is Associated with Poor Prognosis in Diffuse Large B-cell Lymphoma. PLoS ONE 2014, 9, e91031. [Google Scholar] [CrossRef]
- Bartuzi, P.; Billadeau, D.D.; Favier, R.P.; Rong, S.; Dekker, D.; Fedoseienko, A.; Fieten, H.; Wijers, M.; Levels, H.; Huijkman, N.; et al. CCC- And WASH-mediated Endosomal Sorting of LDLR Is Required for Normal Clearance of Circulating LDL. Nat. Commun. 2016, 7, 10961. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Krawczak, C.A.; Singla, A.; Starokadomskyy, P.; Deng, Z.; Osborne, D.G.; Li, H.; Dick, C.J.; Gomez, T.S.; Koenecke, M.; Zhang, J.-S.; et al. COMMD1 Is Linked to the WASH Complex and Regulates Endosomal Trafficking of the Copper Transporter ATP7A. Mol. Biol. Cell 2015, 26, 91–103. [Google Scholar] [CrossRef]
- Wooton-Kee, C.R.; Jian, A.K.; Wagner, M.; Grusak, M.A.; Finegold, M.J.; Lutsenko, S.; Moore, D.D. Elevated copper impairs hepatic nuclear receptor function in Wilson’s diseases. J. Clin. Investig. 2015, 125, 3449–3460. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Koganti, L.; Muchenditsi, A.; Pendyala, V.S.; Huso, D.; Hankin, J.; Murphy, R.C.; Huster, D.; Merle, U.; Mangels, C.; et al. Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B(-/-) (wilson disease) mice. Hepatology 2016, 63, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chien, H.; van Wolferen, M.E.; Kruitwagen, H.S.; Oosterhoff, L.A.; Penning, L.C. Reduced FXR target gene expression in copper-laden livers of COMMD1-deficient dogs. Vet. Sci. 2019, 6, 78. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Zhou, J.; Peng, Q.; Zheng, H.; Yuan, Y.; Cui, H.; Zhao, W.; Sun, X.; Zhou, Z.; et al. Identification of COMMD1 as a Novel Lamin a Binding Partner. Mol. Med. Rep. 2019, 20, 1790–1796. [Google Scholar] [CrossRef]
- Dumoulin, B.; Ufer, C.; Stehling, S.; Heydeck, D.; Kuhun, H.; Sofi, S. Identification of the COMMD-domain containing protein 1 as specific binding partner for the guanine-rich RNA sequence binding factor 1. Biochim. Biophys. Acta 2020, 1864, 129678. [Google Scholar] [CrossRef]
- Fieten, H.; Gill, Y.; Martin, A.J.; Concilli, M.; Dirksen, K.; van Steenbeek, F.G.; Spee, B.; van den Ingh, T.S.; Martens, E.C.; Festa, P.; et al. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: A new canine model for copper-metabolism disorders. Dis. Models Mech. 2016, 9, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mandigers, P.J.J.; Watson, A.L.; van den Ingh, T.S.G.A.M.; Leegwater, P.A.J.; Fieten, H. Association of the ATP7A and ATP7B with hepatic copper accumulation in Dobermann dogs. J. Vet. Intern. Med. 2019, 33, 1646–1652. [Google Scholar] [CrossRef]
- Wu, X.; Mandigers, P.J.J.; Fieten, H.; Leegwater, P.A. Evaluation of COMMD1 in copper toxicosis in Labrador retrievers and Dobermans. Vet. J. 2020, 265, 105561. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; den Boer, E.R.; Vos-Lohuis, M.; van Steenbeek, F.G.; Monroe, G.R.; Nijman, I.J.; Leegwater, P.A.J.; Fieten, H. Investigation of genetic modifiers of coper toxicosis in Labrador retrievers. Life 2020, 10, 266. [Google Scholar] [CrossRef]
- Haynes, J.S.; Wade, P.R. Hepatopathy associated with excessive hepatic copper in a Siamese cat. Vet. Pathol. 1995, 43, 427–429. [Google Scholar] [CrossRef]
- Meertens, N.M.; Bokhove, C.A.; van den Ingh, T.S. Copper-associated chronic hepatitis and cirrhosis in a European Shorthair cat. Vet. Pathol. 2005, 42, 97–100. [Google Scholar] [CrossRef]
- Andreani, G.; Cottignoli, S.; Perfetti, B.; Kismali, G.; Carpene, E.; Isani, G. Trace elements and methallothionein in liver and kidney of Felis catus. Biol. Trace Elem. Res. 2010, 137, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, J.C.; Newkirk, K.M.; Reel, D.M.; Reed, A. Hepatic copper and iron accumulation and histologic findings in 104 feline liver biopsies. J. Vet. Diagn. Investig. 2012, 24, 656–661. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Liver and kidney concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in cats. BMC Vet. Res. 2014, 10, 163. [Google Scholar] [CrossRef]
- Yamkate, P.; Gold, R.M.; Xenoulis, P.G.; Steiger, K.; Twedt, D.C.; Suchodolski, J.S.; Stiener, J.M.; Lidburry, J.A. Assessment of copper accumulation in archived liver specimens from cats. J. Feline Med. Surg. 2020, in press. [Google Scholar] [CrossRef]
- Bernard, J.M.; Newkirk, K.M.; McRee, A.E.; Whittemore, J.C.; Ramsay, E.C. Hepatic lesions in 90 captive nondomestic felids presented for autopsy. Vet. Pathol. 2015, 52, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Thomason, R.L.; Lockhart, J.M.; Loughry, W.J.; Bielmyer-Fraser, G.K. Metal accumulation in bobcats in the Southeastern USA. Environ. Monit. Asess. 2016, 188, 565. [Google Scholar] [CrossRef] [PubMed]
- Hough, S.E.; Lockhart, J.M.; Loughry, W.J.; Bielmyer-Frase, G.K. Comparative metal analysis in a special assemblage of mammals from the Southeastern USA. Environ. Monit. Asess. 2020, 192, 306. [Google Scholar] [CrossRef]
- Asada, H.; Kojima, M.; Nagahara, T.; Goto-Koshino, Y.; Chambers, J.K.; Nakagawa, T.; Yokoyama, N.; Uchida, K.; Tsujimoto, H.; Ohono, K. Hepatic copper accumulation in a young cat with familial variations in the ATP7B gene. J. Vet. Intern. Med. 2019, 33, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Asada, H.; Chambers, J.K.; Kojima, M.; Goto-Koshino, Y.; Nakagawa, T.; Yokoyama, N.; Uchida, K.; Tsujimoto, H.; Ohno, K. Variations in ATP7B in cats with primary copper-associated hepatopathy. J. Feline Med. Surg. 2020, 22, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Vapalahti, K.; Virtala, A.-M.; Joensuu, T.A.; Tiira, K.; Tahtinen, J.; Lohi, J. Health and behavioral survey of over 8000 Finnish cats. Front. Vet. Sci. 2016, 3, 70. [Google Scholar] [CrossRef]
- Poldervaart, J.H.; Favier, R.P.; Penning, L.C.; van den Ingh, T.S.; Rothuizen, J. Primary hepatitis in dogs: A retrospective review (2002–2006). J. Vet. Intern. Med. 2009, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kruitwagen, H.S.; Spee, B.; Fieten, H.; van Steenbeek, F.G.; Schotanus, B.A.; Penning, L.C. Translation from mice to men: Are dogs a dodgy intermediate? Eur. Med. J. Hepatol. 2014, 1, 48–54. [Google Scholar]
- Fieten, H.; Biourge, V.C.; Watson, A.L.; Leegwater, P.A.J.; van den Ingh, T.S.G.A.M.; Rothuizen, J. Dietary Management of Labrador Retrievers with Subclinical Hepatic Copper Accumulation. J. Vet. Intern. Med. 2015, 29, 822–827. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Schall, W.; Yuzbasiyan-Gurkan, V.; Mullamey, T.P.; Pace, C.; Lindgren, J.; Thomas, M.; Padgett, G. Use of zinc acetate to treat copper toxicosis in dogs. J. Am. Vet. Med. Assoc. 1992, 201, 564–568. [Google Scholar] [PubMed]
- Zhu, X.-Q.; Li, L.-Y.; Yang, W.-M.; Wang, Y. Combined dimercaptosuccinic acid and zinc treatment in neurological Wilson’s disease patients with penicillamine-induced allergy or early neurological deterioration. Biosci. Rep. 2020, 40, BSR20200654. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbee, R.J.; Penning, L.C. COMMD1 Exemplifies the Power of Inbred Dogs to Dissect Genetic Causes of Rare Copper-Related Disorders. Animals 2021, 11, 601. https://doi.org/10.3390/ani11030601
Corbee RJ, Penning LC. COMMD1 Exemplifies the Power of Inbred Dogs to Dissect Genetic Causes of Rare Copper-Related Disorders. Animals. 2021; 11(3):601. https://doi.org/10.3390/ani11030601
Chicago/Turabian StyleCorbee, Ronald Jan, and Louis C. Penning. 2021. "COMMD1 Exemplifies the Power of Inbred Dogs to Dissect Genetic Causes of Rare Copper-Related Disorders" Animals 11, no. 3: 601. https://doi.org/10.3390/ani11030601
APA StyleCorbee, R. J., & Penning, L. C. (2021). COMMD1 Exemplifies the Power of Inbred Dogs to Dissect Genetic Causes of Rare Copper-Related Disorders. Animals, 11(3), 601. https://doi.org/10.3390/ani11030601






