A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Design and Diets
2.3. Metagenomics and Microbial Profiling Analysis
2.4. Histomorphology of the Duodenum
2.5. Short-Chain Fatty Acids (SCFAs) Evaluation in Broilers Caecum Chymus
2.6. Measurements of the Productivity Parameters
2.7. Evaluation of Broiler Carcass Traits
2.8. Evaluation of Chemical, Physical and Technological Characteristics of Broiler Breast Meat Samples
2.9. Statistical Analysis
3. Results
3.1. Microbial Profiling Analysis
3.2. Histomorphology Parameters of Broilers Duodenum
3.3. Short-Chain Fatty Acids Concentrations in Broilers’ Caecum Chymus
3.4. Parameters of the Broiler Chickens’ Production Performance
3.5. Carcass Traits
3.6. Chemical, Physical, and Technological Characteristics of Broiler Breast Meat Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salah, A.S.; Ahmed-Farid, O.A.; El-Tarabany, M.S. Carcass yields, muscle amino acid and fatty acid profiles, and antioxidant indices of broilers supplemented with synbiotic and/or organic acids. J. Anim. Physiol. Anim. Nutr. 2019, 103, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, U.; Cengiz, Ö.; Raza, I.; Kuter, E.; Chacher, M.; Iqbal, Z.; Umar, S.; Çakir, S. Sodium butyrate in chicken nutrition: The dynamics of performance, gut microbiota, gut morphology, and immunity. World’s Poult. Sci. J. 2016, 72, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Van Immerseel, F.; Boyen, F.; Gantois, I.; Timbermont, L.; Bohez, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005, 84, 1851–1856. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 440–446. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Panda, A.K.; Rao, S.V.R.; Raju, M.V.L.N.; Sunder, G.S. Effect of Butyric Acid on Performance, Gastrointestinal Tract Health and Carcass Characteristics in Broiler Chickens. Asian-Austral. J. Anim. Sci. 2009, 22, 1026–1031. [Google Scholar] [CrossRef]
- Shahidi, S.; Maziar, Y.; Delaram, N.Z. Influence of dietary organic acids supplementation on reproductive performance of freshwater Angelfish (Pterophyllum scalare). Glob. Vet. 2014, 13, 373–377. [Google Scholar] [CrossRef]
- Khatibjoo, A.; Mahmoodi, M.; Fattahnia, F.; Akbari-Gharaei, M.; Shokri, A.-N.; Soltani, S. Effects of dietary short- and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 2018, 46, 492–498. [Google Scholar] [CrossRef]
- Del Alamo, A.G.; De Los Mozos, J.; Van Dam, J.T.P.; De Ayala, P.P. The use of short and medium chain fatty acids as an alternative to antibiotic growth promoters in broilers infected with malabsorption syndrome. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 317–320. [Google Scholar]
- Dierick, N.; Decuypere, J.; Molly, K.; Van Beek, E.; Vanderbeke, E. The combined use of triacylglycerols containing medium-chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition: I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Livest. Prod. Sci. 2002, 75, 129–142. [Google Scholar] [CrossRef]
- Hermans, D.; Martel, A.; Van Deun, K.; Verlinden, M.; Van Immerseel, F.; Garmyn, A.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult. Sci. 2010, 89, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, S.A.; El-Sanhoury, M.H.; El-Mednay, N.M.; Abdel-Azeem, F. Thyroid Activity, Some Blood Constituents, Organs Morphology and Performance of Broiler Chicks Fed Supplemental Organic Acids. Int. J. Poult. Sci. 2008, 7, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of Dietary Supplementation of Organic Acids on Performance, Intestinal Histomorphology, and Serum Biochemistry of Broiler Chicken. Vet. Med. Int. 2010, 2010, 479485. [Google Scholar] [CrossRef] [Green Version]
- Isabel, B.; Santos, Y. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. J. Appl. Poult. Res. 2009, 18, 472–476. [Google Scholar] [CrossRef]
- Ao, T.; Cantor, A.H.; Pescatore, A.J.; Ford, M.J.; Pierce, J.L.; Dawson, K.A. Effect of enzyme supplementation and acidification of diets on nutrient digestibility and growth performance of broiler chicks. Poult. Sci. 2009, 88, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Azhar, K.; Zulkifli, I.; Bejo, M.H.; Kamyab, A. Effects of nonantibiotic feed additives on performance, nutrient retention, gut pH, and intestinal morphology of broilers fed different levels of energy. J. Appl. Poult. Res. 2011, 20, 121–128. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 21st ed.; AOAC: Arlington, VA, USA, 2019. [Google Scholar]
- Zarghi, H.; Golian, A.; Kermanshah, H.; Raji, A.R.; Heravi, A.R. The Effect of Triticale and Enzyme in Finisher Diet on Performance, Gut Morphology and Blood Chemistry of Broiler Chickens. J. Anim. Vet. Adv. 2010, 9, 2305–2314. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jönsson, J. Åke Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Marche, G.; Room, C.D. Dissection of Poultry Carcasses-Chicken, Duck, Turkey; INRA: Paris, France, 2000; ISBN 2-7380-0941-7 (CD). [Google Scholar]
- ISO 937:1974. Meat and Meat Products—Determination of Nitrogen Content—Kjeldahl Method. 1974. Available online: https://www.iso.org/standard/5356.html (accessed on 15 December 2020).
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, E.E.; Stanley, D.; Hughes, R.J.; Moore, R.J. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. PeerJ 2017, 5, e3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Fravalo, P.; Yergeau, É.; Arsenault, J.; Lahaye, L.; Letellier, A. Chicken Caecal Microbiome Modifications Induced by Campylobacter jejuni Colonization and by a Non-Antibiotic Feed Additive. PLoS ONE 2015, 10, e0131978. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef] [Green Version]
- Lan, P.T.N.; Sakamoto, M.; Sakata, S.; Benno, Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. Int. J. Syst. Evol. Microbiol. 2006, 56, 2853–2859. [Google Scholar] [CrossRef] [Green Version]
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.L.T.; Chung, C.-Y.; Kuo, C.-H.; Kuo, T.-F.; Yang, C.-W.; Yang, W.-C. Beneficial Effect of Bidens pilosa on Body Weight Gain, Food Conversion Ratio, Gut Bacteria and Coccidiosis in Chickens. PLoS ONE 2016, 11, e0146141. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, B.R.C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Yu, Z.; Wang, C.; Zhang, Q.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Pilot Safety Evaluation of a Novel Strain of Bacteroides ovatus. Front. Genet. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L. Enterotoxigenic Bacteroides fragilis: A Rogue among Symbiotes. Clin. Microbiol. Rev. 2009, 22, 349–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A. Current Perspectives of the Chicken Gastrointestinal Tract and Its Microbiome. Comput. Struct. Biotechnol. J. 2019, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, L.; Doyle, M.P. Potential Competitive Exclusion Bacteria from Poultry Inhibitory to Campylobacter jejuni and Salmonella. J. Food Prot. 2007, 70, 867–873. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, K.; Tohno, M.; Miura, Y.; Kamada, T.; Ikegami, S.; Kitazawa, H. Immunomodulation in gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult. Sci. 2009, 88, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Dunham, H.J.; Williams, C.; Edens, F.W.; Casas, I.A.; Dobrogosz, W.J. Lactobacillus reuteri immunomodulation of stressor-associated diseases in newly hatched chickens and turkeys. Poult. Sci. 1993, 72, 103. [Google Scholar]
- Torshizi, M.K.; Moghaddam, A.; Rahimi, S.; Mojgani, N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010, 51, 178–184. [Google Scholar] [CrossRef]
- Ojala, T.; Kuparinen, V.; Koskinen, J.P.; Alatalo, E.; Holm, L.; Auvinen, P.; Edelman, S.; Westerlund-Wikström, B.; Korhonen, T.K.; Paulin, L.; et al. Genome Sequence of Lactobacillus crispatus ST1. J. Bacteriol. 2010, 192, 3547–3548. [Google Scholar] [CrossRef] [Green Version]
- Tamrakar, R.; Yamada, T.; Furuta, I.; Cho, K.; Morikawa, M.; Yamada, H.; Sakuragi, N.; Minakami, H. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect. Dis. 2007, 7, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Futur. Microbiol. 2010, 5, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Al Jassim, R.A.; Scott, P.T.; Trebbin, A.L.; Trott, D.; Pollitt, C.C. The genetic diversity of lactic acid producing bacteria in the equine gastrointestinal tract. FEMS Microbiol. Lett. 2005, 248, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hald, T. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008—Part A: Campylobacter and Salmonella prevalence estimates. EFSA J. 2010, 8, 1503. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Amaretti, A. Probiotic Properties of Bifidobacterial; Caister Academic Press: Norfolk, UK, 2010; pp. 97–123. [Google Scholar]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Khan, R.U.; Tufarelli, V.; Passantino, A. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G.; Jiang, W.; Lamont, S.; Lillehoj, H.S.; Beker, A.; Teeter, R.G.; et al. Butyrate Enhances Disease Resistance of Chickens by Inducing Antimicrobial Host Defense Peptide Gene Expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef]
- Yadav, A.K.; Tyagi, A.; Kumar, A.; Panwar, S.; Grover, S.; Saklani, A.C.; Hemalatha, R.; Batish, V.K. Adhesion of Lactobacilli and their anti-infectivity potential. Crit. Rev. Food Sci. Nutr. 2017, 57, 2042–2056. [Google Scholar] [CrossRef]
- Awad, W.A.; Dublecz, F.; Hess, C.; Khayal, B.; Aschenbach, J.R.; Hess, M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016, 95, 2259–2265. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, H.; Wang, X.; Xia, W.; Lv, W.; Xiao, Y.; Zou, X. Early Intervention With Cecal Fermentation Broth Regulates the Colonization and Development of Gut Microbiota in Broiler Chickens. Front. Microbiol. 2019, 10, 1422. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; Reyes-Gavilan, C.G.D.L. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorganic Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved Shifts in the Gut Microbiota Due to Gastric Bypass Reduce Host Weight and Adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.-C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998, 80, S147–S171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wu, J.; Li, J.V.; Zhou, N.Y.; Tang, H.; Wang, Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Arboleya, S.; Hernandez-Barranco, A.M.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Interactions between Bifidobacterium and Bacteroides species in co-fermentations are affected by carbon sources, including exopolysaccharides produced by bifidobacteria. Appl. Environ. Microbiol. 2013, 79, 7518–7524. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Rios-Covián, D.; Sánchez, B.; Martínez, N.; Cuesta, I.; Hernández-Barranco, A.M.; de los Reyes-Gavilan, C.G.; Gueimonde, M. A proteomic approach towards understanding the cross talk between Bacteroides fragilis and Bifidobacterium longum in co-culture. Can. J. Microbiol. 2016, 62, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef]
- Sultan, A.; Ullah, T.; Khan, S.; Khan, R.U. Effect of organic acid supplementation on the performance and ileal microflora of broiler during finishing period. Pak. J. Zool. 2015, 47, 635–639. [Google Scholar]
- García, V.; Catalá-Gregori, P.; Hernández, F.; Megías, M.D.; Madrid, J. Effect of Formic Acid and Plant Extracts on Growth, Nutrient Digestibility, Intestine Mucosa Morphology, and Meat Yield of Broilers. J. Appl. Poult. Res. 2007, 16, 555–562. [Google Scholar] [CrossRef]
- Senkoylu, N.; Samli, H.E.; Kanter, M.; Agma, A. Influence of a combination of formic and propionic acids added to wheat- and barley-based diets on the performance and gut histomorphology of broiler chickens. Acta Vet. Hung. 2007, 55, 479–490. [Google Scholar] [CrossRef]
- Salah, A.S.; El-Tarabany, M.S.; Ali, M.A. Impact of dietary supplementation with a synbiotic, organic acids or their combination on growth performance, carcass traits, economic efficiency, jejunum histomorphometry and some blood indices of broiler chickens. Anim. Prod. Sci. 2019, 59, 1318. [Google Scholar] [CrossRef]
- Smulikowska, S.; Czerwiński, J.; Mieczkowska, A. Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010, 94, 15–23. [Google Scholar] [CrossRef]
- Çınar, M.; Çatlı, A.; Küçükyılmaz, K.; Bozkurt, M. The effect of single or combined dietary supplementation of prebiotics, organic acid and probiotics on performance and slaughter characteristics of broilers. S. Afr. J. Anim. Sci. 2009, 39, 197–205. [Google Scholar] [CrossRef]
- Brzóska, F.; Sliwiński, B.; Michalik-Rutkowska, O. Effect of Dietary Acidifier on Growth, Mortality, Post-Slaughter Parameters and Meat Composition of Broiler Chickens. Ann. Anim. Sci. 2013, 13, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Kim, J.H.; Kil, D.Y. Dietary organic acids for broiler chickens: A review. Rev. Colomb. Cienc. Pecu. 2015, 28, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [Green Version]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.; Müller, C.; Carling, D.; Khan, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339. [Google Scholar] [CrossRef]
- Cruz, C.E.B.; Freitas, E.R.; Aguiar, G.C.; Braz, N.D.M.; Trevisan, M.T.S. Calcium anacardate in the diet of broiler chickens: The effects on growth and bone quality. Rev. Ciência Agronômica 2019, 50, 329–337. [Google Scholar] [CrossRef]
- Chiang, S.H.; Huang, K.H.; Lee, H.F. Effects of medium chain triglyceride on energy metabolism, growth and body fat in broilers. J. Chin. Soc. Anim. Sci. 1990, 19, 11–19. [Google Scholar]
- Du Toit, E.; Browne, L.; Irving-Rodgers, H.; Massa, H.M.; Fozzard, N.; Jennings, M.P.; Peak, I.R. Effect of GPR84 deletion on obesity and diabetes development in mice fed long chain or medium chain fatty acid rich diets. Eur. J. Nutr. 2018, 57, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nagasawa, A.; Hase, T.; Tokimitsu, I.; Shimasaki, H.; Itakura, H. Dietary Tea Catechins Reduce Development of Obesity Accompanied with Gene Expression of Lipid-metabolizing Enzymes in Mice. J. Oleo Sci. 2001, 50, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Odle, J.; Benevenga, N.J.; Crenshaw, T.D. Utilization of Medium-Chain Triglycerides by Neonatal Piglets: Chain Length of Even- and Odd-Carbon Fatty Acids and Apparent Digestion/Absorption and Hepatic Metabolism. J. Nutr. 1991, 121, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.C.; Ingenbleek, Y.; Frey, A. The usefulness of dietary medium-chain triglycerides in body weight control: Fact or fancy? J. Lipid Res. 1996, 37, 708–726. [Google Scholar] [CrossRef]
- Adeyemi, K.D.; Shittu, R.M.; Sabow, A.B.; Ebrahimi, M.; Sazili, A.Q. Influence of Diet and Postmortem Ageing on Oxidative Stability of Lipids, Myoglobin and Myofibrillar Proteins and Quality Attributes of Gluteus Medius Muscle in Goats. PLoS ONE 2016, 11, e0154603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabow, A.B.; Sazili, A.Q.; Aghwan, Z.A.; Zulkifli, I.; Goh, Y.M.; Ab Kadir, M.Z.A.; Nakyinsige, K.; Kaka, U.; Adeyemi, K.D. Changes of microbial spoilage, lipid-protein oxidation and physicochemical properties during post mortem refrigerated storage of goat meat. Anim. Sci. J. 2016, 87, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Salwani, M.; Adeyemi, K.; Sarah, S.; Vejayan, J.; Zulkifli, I.; Sazili, A. Skeletal muscle proteome and meat quality of broiler chickens subjected to gas stunning prior slaughter or slaughtered without stunning. CyTA J. Food 2016, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, K.; Popp, J.; Becker, A.; Hankel, J.; Visscher, C.; Klein, G.; Meemken, D. Lauric acid as feed additive—An approach to reducing Campylobacter spp. in broiler meat. PLoS ONE 2017, 12, e0175693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
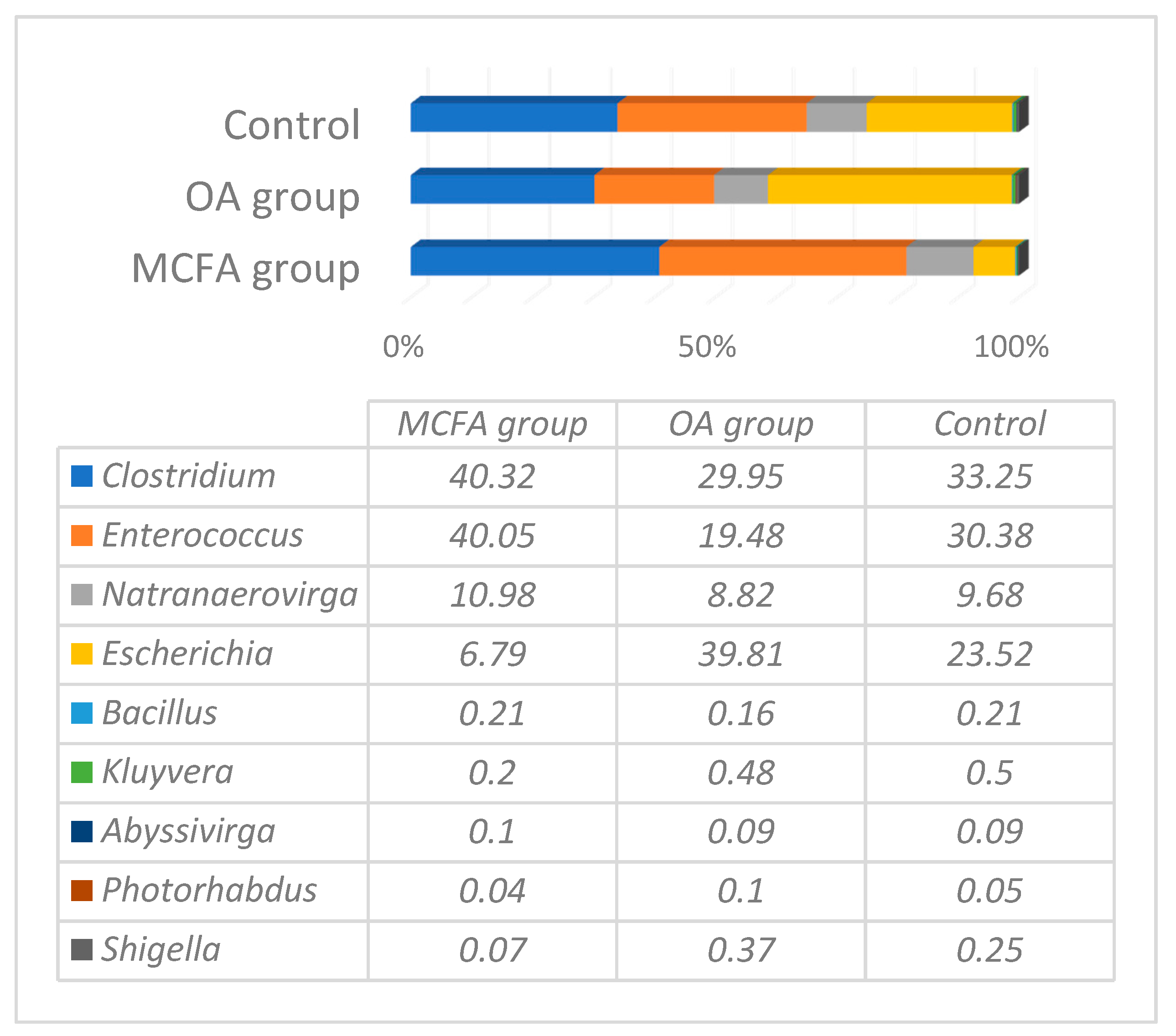
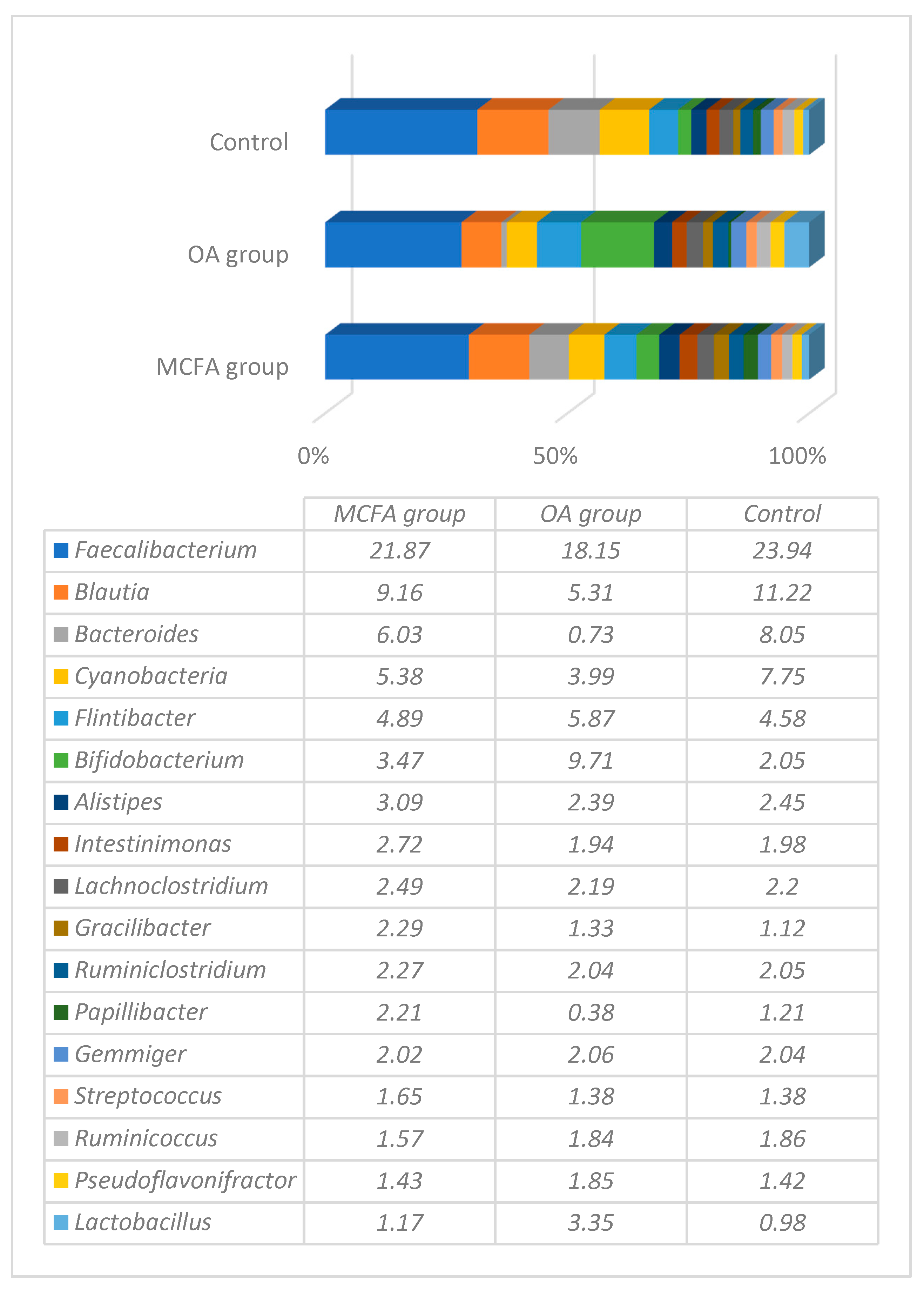
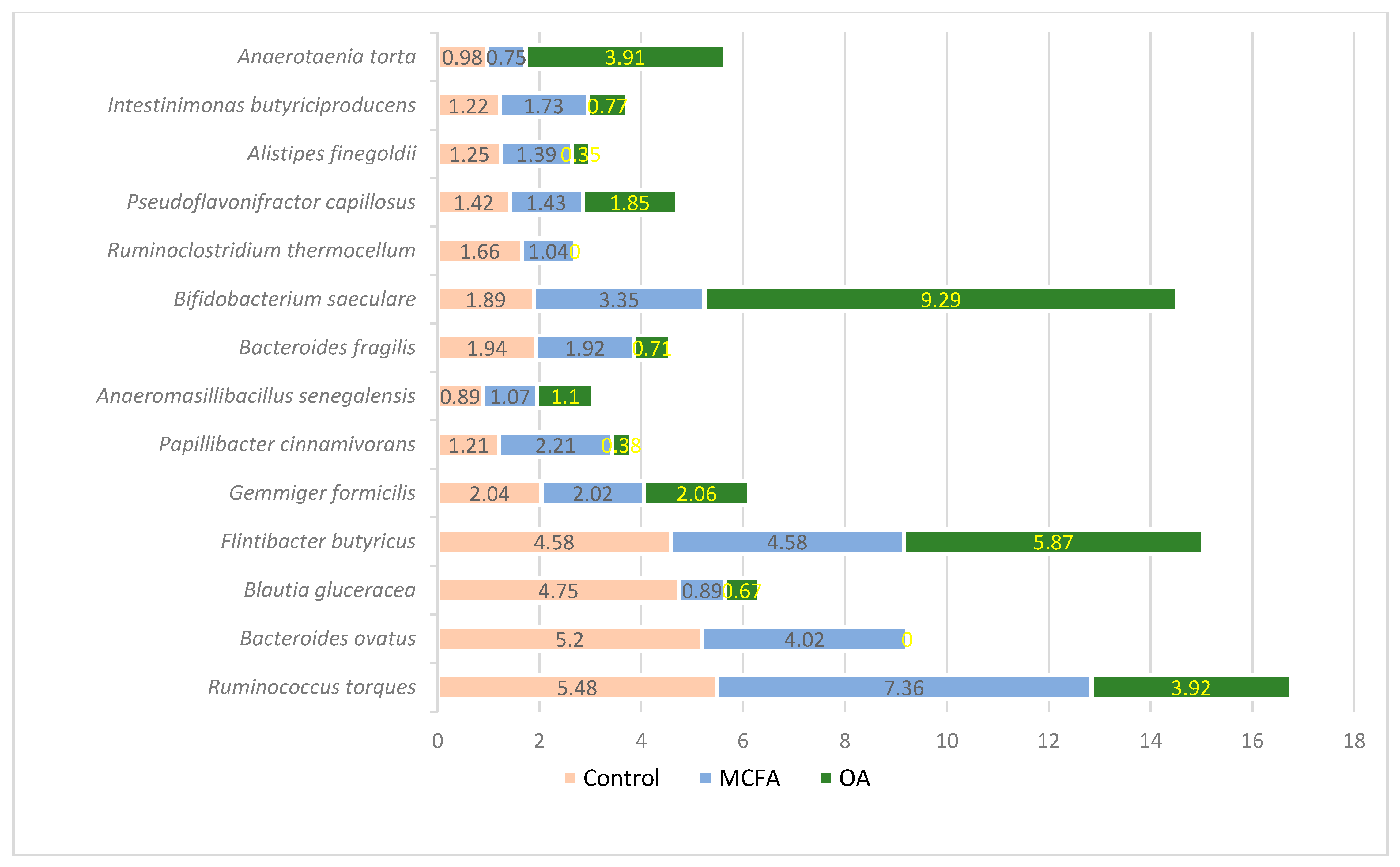
| Ingredients and Calculated Values | Prestarter | Starter | Grower | Finisher | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1–7 days) | (8–21 days) | (22–35 days) | (36–42 days) | |||||||||
| Dietary Treatments | ||||||||||||
| Ingredients (%) | CON | MCFAs | OAs | CON | MCFAs | OAs | CON | MCFAs | OAs | CON | MCFAs | OAs |
| Soybean meal | 39.11 | 39.11 | 39.11 | 35.16 | 35.16 | 35.16 | 26.59 | 26.59 | 26.59 | 25.91 | 25.91 | 25.91 |
| Maize | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 | 35.00 | 35.00 | 35.00 |
| Wheat | 22.47 | 22.27 | 22.27 | 25.35 | 25.15 | 25.15 | 33.11 | 32.91 | 32.91 | 26.57 | 28.57 | 28.57 |
| Sunflower oil | 3.58 | 3.58 | 3.58 | 5.01 | 5.01 | 5.01 | 6.16 | 6.16 | 6.16 | 6.33 | 6.33 | 6.33 |
| Limestone | 1.64 | 1.64 | 1.64 | 1.52 | 1.52 | 1.52 | 1.23 | 1.23 | 1.23 | 1.22 | 1.22 | 1.22 |
| Monocalcium phosphate | 1.16 | 1.16 | 1.16 | 0.98 | 0.98 | 0.98 | 0.55 | 0.55 | 0.55 | 0.57 | 0.57 | 0.57 |
| MHA | 0.46 | 0.46 | 0.46 | 0.44 | 0.44 | 0.44 | 0.52 | 0.52 | 0.52 | 0.49 | 0.49 | 0.49 |
| Lysine sulphate | 0.43 | 0.43 | 0.43 | 0.42 | 0.42 | 0.42 | 0.69 | 0.69 | 0.69 | 0.66 | 0.66 | 0.66 |
| Wheat flour | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium sulphate | 0.20 | 0.20 | 0.20 | 0.17 | 0.17 | 0.17 | 0.15 | 0.15 | 0.15 | 0.14 | 0.14 | 0.14 |
| L-Threonine | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.21 | 0.21 | 0.21 | 0.19 | 0.19 | 0.19 |
| Sodium chloride | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Choline chloride | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Coccidiostatic | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.06 | |||
| Phytase EC 5L (liquid) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Rovabio Advance L2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Premix | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.12 | 0.12 | 0.12 |
| OAs | 0.20 | 0.20 | 0.20 | 0.20 | ||||||||
| MCFAs | 0.20 | 0.20 | 0.20 | 0.20 | ||||||||
| Calculated Values (%) | ||||||||||||
| MEN (MJ/kg) | 12.43 | 12.43 | 12.43 | 12.56 | 12.56 | 12.56 | 13.11 | 13.11 | 13.11 | 13.23 | 13.23 | 13.23 |
| Crude protein | 20.02 | 20.00 | 20.00 | 19.02 | 19.00 | 19.00 | 18.02 | 18.00 | 18.00 | 17.52 | 17.50 | 17.50 |
| Crude fat | 6.04 | 5.43 | 5.45 | 7.18 | 6.84 | 6.86 | 8.59 | 8.23 | 8.25 | 8.40 | 8.34 | 8.37 |
| Crude ash | 6.64 | 6.56 | 6.62 | 6.04 | 6.07 | 6.13 | 5.22 | 5.21 | 5.28 | 5.13 | 5.13 | 5.16 |
| Crude fiber | 2.40 | 2.60 | 2.59 | 2.52 | 2.50 | 2.53 | 2.80 | 2.80 | 2.82 | 2.78 | 2.80 | 2.79 |
| Ca | 0.89 | 0.89 | 0.89 | 0.78 | 0.78 | 0.78 | 0.74 | 0.74 | 0.74 | 0.69 | 0.69 | 0.69 |
| P | 0.68 | 0.68 | 0.68 | 0.62 | 0.62 | 0.62 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 |
| Av. P | 0.46 | 0.46 | 0.46 | 0.44 | 0.44 | 0.44 | 0.41 | 0.41 | 0.41 | 0.38 | 0.38 | 0.38 |
| Na | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| K | 1.00 | 1.00 | 1.00 | 0.94 | 0.94 | 0.94 | 0.85 | 0.85 | 0.85 | 0.84 | 0.84 | 0.84 |
| Cl | 0.18 | 0.18 | 0.18 | 0.16 | 0.16 | 0.16 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Lysine | 1.20 | 1.20 | 1.20 | 1.19 | 1.19 | 1.19 | 1.07 | 1.07 | 1.07 | 1.08 | 1.08 | 1.08 |
| Av. Lysine | 1.16 | 1.16 | 1.16 | 1.08 | 1.08 | 1.08 | 0.96 | 0.96 | 0.96 | 0.93 | 0.93 | 0.93 |
| Methionine | 0.51 | 0.51 | 0.51 | 0.48 | 0.48 | 0.48 | 0.46 | 0.46 | 0.46 | 0.44 | 0.44 | 0.44 |
| Av. methionine | 0.48 | 0.48 | 0.48 | 0.45 | 0.45 | 0.45 | 0.42 | 0.42 | 0.42 | 0.40 | 0.40 | 0.40 |
| Methionine + cysteine | 1.03 | 1.03 | 1.03 | 0.95 | 0.95 | 0.95 | 0.90 | 0.90 | 0.90 | 0.85 | 0.85 | 0.85 |
| Av. methionine + cysteine | 0.90 | 0.90 | 0.90 | 0.82 | 0.82 | 0.82 | 0.79 | 0.79 | 0.79 | 0.73 | 0.73 | 0.73 |
| Tryptophane | 0.20 | 0.20 | 0.20 | 0.18 | 0.18 | 0.18 | 0.16 | 0.16 | 0.16 | 0.15 | 0.15 | 0.15 |
| Av. Tryptophane | 0.18 | 0.18 | 0.18 | 0.16 | 0.16 | 0.16 | 0.15 | 0.15 | 0.15 | 0.14 | 0.14 | 0.14 |
| Lactobacillus Species | Chicken Groups and Amount of Lactobacillus Species, % | ||
|---|---|---|---|
| CON | MCFAs | OAs | |
| L. salivarius | 34 | 30 | 19 |
| L. crispatus | 22 | 28 | 39 |
| L. kitasatonis | 12 | 9 | 8 |
| L. reuteri | 8 | 7 | 7 |
| L. johnsonii | 14 | 11 | 7 |
| –L. vaginalis | 0.8 | 0.9 | 6 |
| L. agilis | 1 | 0 | 3 |
| L. acidophilus | 1 | 0 | 2 |
| L. helveticus | 0.1 | 0.6 | 2 |
| L. delbrueckii | 0 | 2 | 1 |
| L. jenseni | 1 | 2 | 0.9 |
| L. avarius | 0.4 | 1 | 0.9 |
| L. rogosae | 0 | 0 | 0.7 |
| L. ingluviei | 0 | 0 | 0.4 |
| L. ruminis | 0 | 0.6 | 0.4 |
| L. mucosae | 0 | 0.3 | 0.3 |
| L. fermentum | 0.2 | 0.3 | 0.2 |
| L. acidopiscis | 0 | 0 | 0.2 |
| L. taiwanensis | 0 | 0.9 | 0.2 |
| L. gassen | 0 | 0 | 0.2 |
| L. gallinarum | 0 | 0 | 0.2 |
| L. amilolyticus | 0.4 | 0 | 0.2 |
| L. animalis | 0.4 | 0.9 | 0.1 |
| L. porcinae | 0 | 0 | 0.1 |
| L. iners | 0 | 0.3 | 0.1 |
| L. casei | 0 | 0 | 0.1 |
| L. oris | 2 | 0.9 | 0.1 |
| L. pontis | 1 | 0.3 | 0.1 |
| L. kafiranofaciens | 0 | 0 | 0.1 |
| L. frumenti | 0.2 | 0 | 0.1 |
| L. kisonensis | 0 | 0 | 0.1 |
| L. acidophilus | 0 | 2 | 0.1 |
| L. amylovorus | 0.4 | 0.3 | 0 |
| L. hellongjiangensis | 0.4 | 0.3 | 0 |
| L. gasseri | 0 | 0.3 | 0 |
| unclasified | 0.6 | 0.9 | 0.1 |
| Number of species | 19 | 22 | 32 |
| Dietary Treatments | ||
|---|---|---|
| CON | MCFAs | OAs |
| Broilers duodenum villus height (VH), µm | ||
| 2467.7 ± 374.5 a | 2499.7 ± 380.3 a | 2824.6 ± 412.1 b |
| Broilers duodenum crypt depth (CD), µm | ||
| 585.3 ± 84.7 a | 511.7 ± 136.2 a | 504.3 ± 99.5 b |
| Broilers duodenum villus height and crypt depth ratio | ||
| 4.22 | 4.89 | 5.60 |
| Short-Chain Fatty Acids Concentration in Broilers Caecum, (µmol/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Acetic | Propionic | Isobutyric | Butyric | Isovaleric | Valeric | Isocaproic | Caproic | n-Heptanoic |
| CON | 42.25 ± 0.62 a | 9.84 ± 0.27 a | 4.30 ± 0.12 a | 6.72 ± 0.18 a | 3.16 ± 0.06 a | 3.82 ± 0.03 a | 1.21 ± 0.06 | 0.73 ± 0.23 | 0.66 ± 0.01 |
| MCFAs | 46.39 ± 0.17 b | 10.13 ± 0.08 b | 4.82 ± 0.15 b | 6.89 ± 0.11 b | 3.17 ± 0.08 b | 3.92 ± 0.07 b | 1.26 ± 0.06 | 0.98 ± 0.29 | 0.66 ± 0.01 |
| OAs | 39.42 ± 1.47 a | 9.19 ± 0.17 a | 4.61 ± 0.07 a | 6.33 ± 0.16 a | 3.12 ± 0.09 a | 3.79 ± 0.10 a | 1.28 ± 0.03 | 0.82 ± 0.01 | 0.66 ± 0.01 |
| p | 0.01 | 0.001 | 0.01 | 0.001 | 0.01 | 0.01 | ns | ns | ns |
| Growth Performance Parameters | Dietary Treatments | |||
|---|---|---|---|---|
| CON | MCFAs | OAs | p | |
| 0 day | ||||
| Body weight, g | 39.50 ± 0.35 | 39.50 ± 0.39 | 39.50 ± 0.35 | ns |
| period (1–7 days) | ||||
| Body weight, g | 161.67 ± 2.04 a | 165.33 ± 0.71 a | 167.33 ± 0.64 b | 0.0001 |
| Mortality, % | 0.87 ± 0.19 a | 0.72 ± 0.02 a | 0.54 ± 0.01 b | 0.0001 |
| 8–14 days | ||||
| Body weight, g | 421.00 ± 0.71 a | 432.00 ± 1.22 b | 418.00 ± 1.58 a | 0.0001 |
| Mortality, % | 1.40 ± 0.12 a | 1.27 ± 0.07 a | 0.90 ± 0.12 b | 0.001 |
| 15–21 days | ||||
| Body weight, g | 836.67 ± 4.02 a | 846.67 ± 4.43 b | 836.33 ± 1.98 a | 0.01 |
| Mortality, % | 2.01 ± 0.04 a | 1.58 ± 0.01 a | 1.27 ± 0.02 b | 0.0001 |
| 22–28 days | ||||
| Body weight, g | 1443.00 ± 1.5 | 1459.33 ± 1.8 | 1458.00 ± 0.7 | ns |
| Mortality, % | 1.95 ± 0.19 | 1.69 ± 0.03 | 1.50 ± 0.16 | ns |
| 29–42 days | ||||
| Body weight, g | 2043.67 ± 1.7 a | 2053.11 ± 2.1 b | 2031.0 ± 1.1 a | 0.001 |
| Mortality, % | 1.92 ± 0.06 a | 1.85 ± 0.02 a | 1.61 ± 0.01 b | 0.001 |
| 1–42 days | ||||
| Body weight, g | 2420.67 ± 1.6 a | 2483.0 ± 2.7 a | 2562.3 ± 1.5 b | 0.0001 |
| Mortality, % | 1.63 ± 1.9 a | 1.53 ± 2.1 a | 1.17 ± 2.1 b | 0.0001 |
| FCR, g/ kg | 1.65 ± 0.05 a | 1.68 ± 0.01 a | 1.61 ± 0.01 b | 0.010 |
| Carcass Traits | CON | MCFAs | OAs | p |
|---|---|---|---|---|
| Carcass weight, g | 2043.48 ± 99.0 | 2055.3 ± 172.3 | 2106.9 ± 238.9 | ns |
| Both wings weight, g | 191.44 ± 6.8 | 197.4 ± 8.3 | 202.9 ± 17.2 | ns |
| Both legs muscle with skin and bone, g | 534.98 ± 37.1 | 568.7 ± 56.0 | 586.8 ± 58.4 | ns |
| Both thigh muscles with skin and bone, g | 293.44 ± 26.2 a | 305.4 ± 34.8 a | 317.7 ± 39.2 b | 0.01 |
| Both shin muscles with skin and bone, g | 261.54 ± 22.1 a | 263.1 ± 26.8 a | 269.5 ± 27.8 b | 0.001 |
| Both legs muscle without skin and bone, g | 375.06 ± 32.7 | 395.0 ± 42.2 | 410.6 ± 50.0 | ns |
| Both thigh muscles without skin and bone, g | 204.42 ± 24.9 | 231.0 ± 30.7 | 237.5 ± 40.2 | ns |
| Both shin muscles without skin and bone, g | 170.64 ± 19.8 a | 163.9 ± 14.8 a | 173.0 ± 13.1 b | 0.01 |
| Breast muscles without skin, g | 573.48 ± 64.1 | 651.6 ± 57.1 | 630.8 ± 68.2 | ns |
| Abdominal fat weight, g | 29.06 ± 4.6 | 18.8 ± 7.2 | 21.6 ± 4.2 | ns |
| Chest ridge length, cm | 10.26 ± 0.4 | 13.1 ± 2.1 | 14.3 ± 1.6 | ns |
| Length of Femur bone, cm | 7.60 ± 0.4 a | 8.3 ± 0.5 b | 7.4 ± 0.5 a | 0.0001 |
| Length of Tibia bone, cm | 10.48 ± 0.5 a | 11.0 ± 0.7 a | 11.1 ± 0.7 b | 0.01 |
| Carcass bones without wings and legs, g | 554.26 ± 40.2 a | 564.7 ± 53.2 a | 611.4 ± 49.6 b | 0.001 |
| Breast muscle yield (%) | 26.75 ± 2.57 a | 32.00 ± 0.79 b | 29.93 ± 1.60 a | 0.01 |
| Leg muscle yield (%) | 17.50 ± 1.63 | 19.21 ± 0.76 | 19.48 ± 0.35 | ns |
| Abdominal fat yield (%) | 1.16 ± 0.18 b | 0.92 ± 0.30 a | 1.00 ± 0.27 a | 0.01 |
| Groups | DM % | pH | Color Coordinates | DL % | WHC % | CL % | SF kg/cm | IF % | Ash % | Protein Content % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||||
| CON | 25.24 ± 0.46 | 5.96 ± 0.03 | 70.28 ± 1.89 | 13.18 ± 0.75 | 13.25 ± 1.86 a | 1.62 ± 0.18 | 64.73 ± 1.84 a | 12.77 ± 0.66 | 1.02 ± 0.15 | 1.56 ± 0.32 | 1.22 ± 0.05 | 22.47 ± 0.39 |
| MCFs | 25.11 ± 0.97 | 6.02 ± 0.11 | 69.44 ± 4.04 | 11.58 ± 0.77 | 15.20 ± 1.29 b | 2.05 ± 0.64 | 66.28 ± 2.03 b | 12.82 ± 3.71 | 0.90 ± 0.18 | 2.93 ± 0.64 | 1.46 ± 0.10 | 20.72 ± 0.58 |
| OAs | 25.41 ± 1.49 | 6.02 ± 0.12 | 68.39 ± 2.70 | 12.30 ± 1.13 | 12.00 ± 2.22 a | 1.83 ± 0.49 | 64.88 ± 1.79 a | 11.87 ± 2.55 | 1.55 ± 0.61 | 3.11 ± 0.93 | 1.33 ± 0.20 | 20.96 ± 1.01 |
| p | ns | ns | ns | ns | 0.01 | ns | 0.001 | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauksiene, A.; Ruzauskas, M.; Gruzauskas, R.; Zavistanaviciute, P.; Starkute, V.; Lele, V.; Klupsaite, D.; Klementaviciute, J.; Bartkiene, E. A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids. Animals 2021, 11, 610. https://doi.org/10.3390/ani11030610
Dauksiene A, Ruzauskas M, Gruzauskas R, Zavistanaviciute P, Starkute V, Lele V, Klupsaite D, Klementaviciute J, Bartkiene E. A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids. Animals. 2021; 11(3):610. https://doi.org/10.3390/ani11030610
Chicago/Turabian StyleDauksiene, Agila, Modestas Ruzauskas, Romas Gruzauskas, Paulina Zavistanaviciute, Vytaute Starkute, Vita Lele, Dovile Klupsaite, Jolita Klementaviciute, and Elena Bartkiene. 2021. "A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids" Animals 11, no. 3: 610. https://doi.org/10.3390/ani11030610
APA StyleDauksiene, A., Ruzauskas, M., Gruzauskas, R., Zavistanaviciute, P., Starkute, V., Lele, V., Klupsaite, D., Klementaviciute, J., & Bartkiene, E. (2021). A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids. Animals, 11(3), 610. https://doi.org/10.3390/ani11030610









