Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
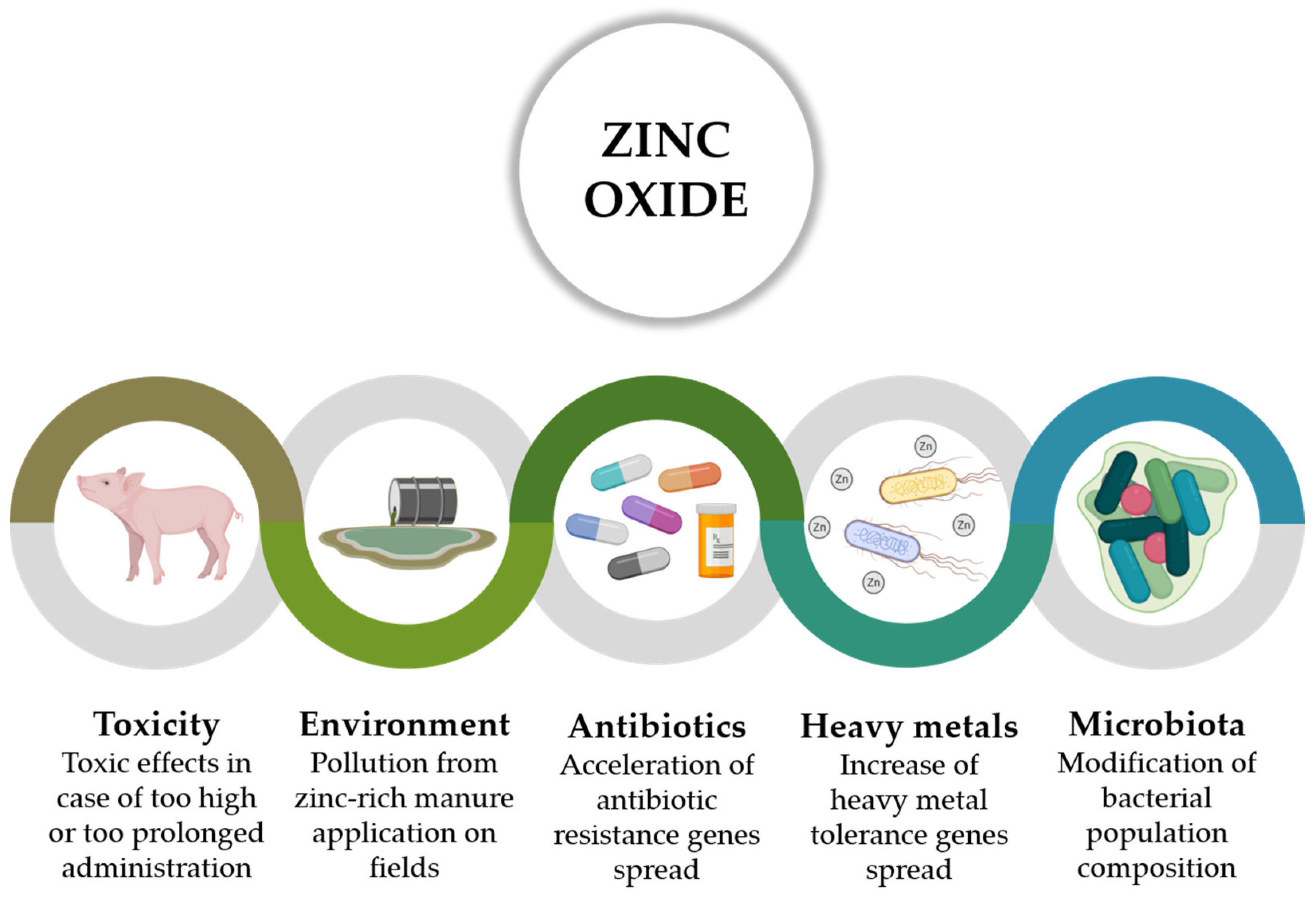
2. Zinc Oxide
2.1. Mechanism of Action of ZnO
2.2. Risks Related to Pharmacological Levels of ZnO
2.3. Why ZnO?
3. Alternatives to ZnO
3.1. Adjusting Diet Composition: Protein and Fiber
3.2. Organic Acids
3.3. Essential Oils and Nature Identical Compounds
3.4. Polyphenol-Rich Extracts
3.5. Prebiotics, Probiotics, and Symbiotics
3.6. Others
3.6.1. Antimicrobial Peptides
3.6.2. Bacteriophages
3.6.3. Egg Yolk Antibodies and Spray-Dried Plasma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO’s Animal Production and Health Division Meat & Meat Products. Available online: http://www.fao.org/ag/againfo/themes/en/meat/backgr_sources.html (accessed on 22 December 2020).
- FAO; OECD Meat. OECD-FAO Agricultural Outlook 2020–2028; OECD Publishing: Paris, France, 2020; pp. 162–173. ISBN 9789264582958. [Google Scholar]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Recén, B. When to wean—Observations from free-ranging domestic pigs. Appl. Anim. Behav. Sci. 1989, 23, 49–60. [Google Scholar] [CrossRef]
- Bøe, K. The process of weaning in pigs: When the sow decides. Appl. Anim. Behav. Sci. 1991, 30, 47–59. [Google Scholar] [CrossRef]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Colson, V.; Martin, E.; Orgeur, P.; Prunier, A. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiol. Behav. 2012, 107, 59–64. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front. Vet. Sci. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning Anorexia May Contribute to Local Inflammation in the Piglet Small Intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef]
- Castillo, M.; Martín-Orúe, S.M.; Nofrarías, M.; Manzanilla, E.G.; Gasa, J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet. Microbiol. 2007, 124, 239–247. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl.) 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Cordonnier, C.; Livrelli, V.; Van de Wiele, T.; Blanquet-Diot, S. Enterotoxigenic and Enterohemorrhagic Escherichia coli: Survival and Modulation of Virulence in the Human Gastrointestinal Tract. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Amidou, S., Ed.; InTech: London, UK, 2017; pp. 3–24. [Google Scholar]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 1–47. [Google Scholar] [CrossRef]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef]
- Sloup, V.; Jankovská, I.; Nechybová, S.; Peřinková, P.; Langrová, I. Zinc in the Animal Organism: A Review. Sci. Agric. Bohem. 2017, 48, 13–21. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Benjamin Cummings: San Francisco, CA, USA, 2011; ISBN 9781408204238. [Google Scholar]
- Poulsen, H.D. Zinc oxide for weanling piglets. Acta Agric. Scand. A Anim. Sci. 1995, 45, 159–167. [Google Scholar] [CrossRef]
- EMA; CVMP. EMA—Zinc Oxide—Annex II—Scientific Conclusions and Grounds for the Refusal of the Marketing Authorisation and for Withdrawal of the Existing Marketing Authorisations; EMEA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hill, G.M.; Mahan, D.C.; Carter, S.D.; Cromwell, G.L.; Ewan, R.C.; Harrold, R.L.; Lewis, A.J.; Miller, P.S.; Shurson, G.C.; Veum, T.L.; et al. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J. Anim. Sci. 2001, 79, 934–941. [Google Scholar] [CrossRef]
- Davin, R.; Manzanilla, E.G.; Klasing, K.C.; Pérez, J.F. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2013, 97, 6–12. [Google Scholar] [CrossRef]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef]
- Hu, C.; Song, J.; Li, Y.; Luan, Z.; Zhu, K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 2013, 110, 681–688. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Song, J.; Luan, Z.S. Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Anim. Feed Sci. Technol. 2013, 181, 65–71. [Google Scholar] [CrossRef]
- Johansen, M.; Jørgensen, L.; Schultz, M.S. Effect of Zinc and Organic Acids on Diarrhoea in the Weaner Period; SEGES: Copenhagen, Denmark, 2007. [Google Scholar]
- Poulsen, H. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J. Anim. Feed Sci. 1998, 7, 135–142. [Google Scholar] [CrossRef]
- Hu, C.; Song, J.; You, Z.; Luan, Z.; Li, W. Zinc Oxide–Montmorillonite Hybrid Influences Diarrhea, Intestinal Mucosal Integrity, and Digestive Enzyme Activity in Weaned Pigs. Biol. Trace Elem. Res. 2012, 149, 190–196. [Google Scholar] [CrossRef]
- Case, C.L.; Carlson, M.S. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 2002, 80, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.D.; Baker, D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 1993, 71, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Sanz Fernandez, M.-V.; Torrison, J.; Wilson, M.E.; Baumgard, L.H.; Gabler, N.K. Dietary organic zinc attenuates heat stress–induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 2015, 93, 4702–4713. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Tugnoli, B.; Vitari, F.; Domeneghini, C.; Morlacchini, M.; Piva, A.; Prandini, A. Low doses of microencapsulated zinc oxide improve performance and modulate the ileum architecture, inflammatory cytokines and tight junctions expression of weaned pigs. Animal 2015, 9, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lv, H.; Chen, Z.; Wang, L.; Wu, X.; Chen, Z.; Zhang, W.; Liang, R.; Jiang, Z. Dietary Zinc Oxide Modulates Antioxidant Capacity, Small Intestine Development, and Jejunal Gene Expression in Weaned Piglets. Biol. Trace Elem. Res. 2017, 175, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Carlson, M.S.; Hill, G.M.; Link, J.E. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: Effect on metallothionein and mineral concentrations. J. Anim. Sci. 1999, 77, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Sunzel, B.; Holm, S.; Elmros, T.; Hallmans, G.; Sjöberg, S. Antibacterial effect of zinc oxide in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Surjawidjaja, J.E.; Hidayat, A.; Lesmana, M. Growth inhibition of enteric pathogens by zinc sulfate: An in vitro study. Med. Princ. Pract. 2004, 13, 286–289. [Google Scholar] [CrossRef]
- Højberg, O.; Canibe, N.; Poulsen, H.D.; Hedemann, M.S.; Jensen, B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl. Environ. Microbiol. 2005, 71, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Katouli, M.; Melin, L.; Jensen-Waern, M.; Wallgren, P.; Möllby, R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J. Appl. Microbiol. 1999, 87, 564–573. [Google Scholar] [CrossRef]
- Mores, N.; Cristani, J.; Piffer, I.A.; Barioni, W.J.; Lima, G.M.M. Effects of zinc oxide on postweaning diarrhea control in pigs experimentally infected with E. coli. Arq. Bras. Med. Veterinária e Zootec. 1998, 50, 513–523. [Google Scholar]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, H.R.; Miller, H.M.; Shaw, M.-A. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol. Immunol. 2011, 48, 2113–2121. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc and the Immune System. In Nutrition and Immunity; Springer International Publishing: Cham, Switzerland, 2019; pp. 127–158. [Google Scholar]
- Ercan, M.T.; Bor, N.M. Phagocytosis by macrophages in zinc-deficient rats. Int. J. Radiat. Appl. Instrum. 1991, 18, 765–768. [Google Scholar] [CrossRef]
- Wirth, J.J.; Fraker, P.J.; Kierszenbaum, F. Zinc requirement for macrophage function: Effect of zinc deficiency on uptake and killing of a protozoan parasite. Immunology 1989, 68, 114–119. [Google Scholar]
- Rolles, B.; Maywald, M.; Rink, L. Influence of zinc deficiency and supplementation on NK cell cytotoxicity. J. Funct. Foods 2018, 48, 322–328. [Google Scholar] [CrossRef]
- Honscheid, A.; Rink, L.; Haase, H. T-Lymphocytes: A Target for Stimulatory and Inhibitory Effects of Zinc Ions. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 132–144. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kloubert, V.; Blaabjerg, K.; Dalgaard, T.S.; Poulsen, H.D.; Rink, L.; Wessels, I. Influence of zinc supplementation on immune parameters in weaned pigs. J. Trace Elem. Med. Biol. 2018, 49, 231–240. [Google Scholar] [CrossRef]
- Ou, D.; Li, D.; Cao, Y.; Li, X.; Yin, J.; Qiao, S.; Wu, G. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J. Nutr. Biochem. 2007, 18, 820–826. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Li, D.; Yue, T.; Fang, Q.; Ni, J.; Zhou, X.; Wu, G. Dietary supplementation with zinc oxide stimulates ghrelin secretion from the stomach of young pigs. J. Nutr. Biochem. 2009, 20, 783–790. [Google Scholar] [CrossRef]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Jensen, B.B.; Poulsen, H.D. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J. Anim. Sci. 2006, 84, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.F.; Becker, D.E.; Terrill, S.W.; Jensen, A.H. Zinc Toxicity in the Weanling Pig. J. Anim. Sci. 1959, 18, 836–842. [Google Scholar] [CrossRef]
- Burrough, E.R.; De Mille, C.; Gabler, N.K. Zinc overload in weaned pigs: Tissue accumulation, pathology, and growth impacts. J. Vet. Diagnostic Investig. 2019, 31, 537–545. [Google Scholar] [CrossRef]
- Martin, L.; Pieper, R.; Schunter, N.; Vahjen, W.; Zentek, J. Performance, organ zinc concentration, jejunal brush border membrane enzyme activities and mRNA expression in piglets fed with different levels of dietary zinc. Arch. Anim. Nutr. 2013, 67, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sugie, K.; Inukai, N.; Eguchi, O.; Oyamada, T.; Sawada, H.; Yamanaka, N.; Shibahara, T. Chronic pancreatitis in farmed pigs fed excessive zinc oxide. J. Vet. Diagn. Investig. 2020, 32, 689–694. [Google Scholar] [CrossRef]
- Poulsen, H.D.; Larsen, T. Zinc excretion and retention in growing pigs fed increasing levels of zinc oxide. Livest. Prod. Sci. 1995, 43, 235–242. [Google Scholar] [CrossRef]
- Meyer, T.A.; Lindemann, M.D.; Cromwell, G.L.; Monegue, H.J.; Inocencio, N. Effects of Pharmacological Levels of Zinc as Zinc Oxide on Fecal Zinc and Mineral Excretion in Weanling Pigs. Prof. Anim. Sci. 2002, 18, 162–168. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Lofts, S.; Boxall, A.B.A. Pre-assessment of environmental impact of zinc and copper used in animal nutrition. EFSA Support. Publ. 2010, 7, 1–138. [Google Scholar] [CrossRef]
- Gräber, I.; Hansen, J.F.; Olesen, S.E.; Petersen, J.; Øtergaard, H.S.; Krogh, L. Accumulation of copper and zinc in Danish agricultural soils in intensive pig production areas. Geogr. Tidsskr. 2005, 105, 15–22. [Google Scholar] [CrossRef]
- Bak, J.L.; Jensen, J.; Larsen, M.M. Belysning af Kobber- og Zinkindholdet i Jord—Indhold og Udvikling i Kvadratnettet og Måling på Udvalgte Brugstyper; Aarhus Universitet, Institut for Bioscience: Aarhus, Denmark, 2015; Volume 159, ISBN 9788771560350. [Google Scholar]
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed—Development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25. [Google Scholar] [CrossRef]
- Slifierz, M.J.; Friendship, R.; Weese, J.S. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant staphylococcus aureus in pigs: A randomized controlled trial. Zoonoses Public Health 2015, 62, 301–308. [Google Scholar] [CrossRef]
- Vahjen, W.; Pietruszyńska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015, 7, 3–7. [Google Scholar] [CrossRef]
- Bednorz, C.; Oelgeschläger, K.; Kinnemann, B.; Hartmann, S.; Neumann, K.; Pieper, R.; Bethe, A.; Semmler, T.; Tedin, K.; Schierack, P.; et al. The broader context of antibiotic resistance: Zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int. J. Med. Microbiol. 2013, 303, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef] [PubMed]
- Johanns, V.C.; Ghazisaeedi, F.; Epping, L.; Semmler, T.; Lübke-Becker, A.; Pfeifer, Y.; Bethe, A.; Eichhorn, I.; Merle, R.; Walther, B.; et al. Effects of a Four-Week High-Dosage Zinc Oxide Supplemented Diet on Commensal Escherichia coli of Weaned Pigs. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Slifierz, M.J.; Park, J.; Friendship, R.M.; Weese, J.S. Zinc-resistance gene CzrC identified in methicillin-resistant Staphylococcus hyicus isolated from pigs with exudative epidermitis. Can. Vet. J. La Rev. Vet. Can. 2014, 55, 489–490. [Google Scholar]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Zhu, Q.; Xu, J.; Chen, Z.; Jiang, Z. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef]
- Li, B.T.; Van Kessel, A.G.; Caine, W.R.; Huang, S.X.; Kirkwood, R.N. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Can. J. Anim. Sci. 2001, 81, 511–516. [Google Scholar] [CrossRef]
- Vahjen, W.; Pieper, R.; Zentek, J. Bar-Coded Pyrosequencing of 16S rRNA Gene Amplicons Reveals Changes in Ileal Porcine Bacterial Communities Due to High Dietary Zinc Intake. Appl. Environ. Microbiol. 2010, 76, 6689–6691. [Google Scholar] [CrossRef]
- Pieper, R.; Vahjen, W.; Neumann, K.; Van Kessel, A.G.; Zentek, J. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2012, 96, 825–833. [Google Scholar] [CrossRef]
- Vahjen, W.; Pieper, R.; Zentek, J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J. Anim. Sci. 2011, 89, 2430–2439. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Vahjen, W.; Zentek, J. The impact of dietary zinc oxide on the bacterial diversity of the small intestinal microbiota of weaned piglets. J. Vet. Sci. Technol. 2014, 5. [Google Scholar] [CrossRef]
- EFSA; FEEDAP. Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2016, 12. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision of 26.6.2017 Concerning, in the Framework of Article 35 of Directive 2001/82/EC of the European Parliament and of the Council, the Marketing Authorisations for Veterinary Medicinal Products Containing “Zinc Oxide” to be Ad; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1334/2003 of 25 July 2003 Amending the Conditions for Authorisation of a Number of Additives in Feedingstuffs Belonging to the Group of Trace Elements; Official Journal of the European Union: Brussels, Belgium, 2003. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2016/1095 of 6 July 2016; Official Journal of the European Union: Brussels, Belgium, 2016. [Google Scholar]
- Wedekind, K.J.; Baker, D.H. Zinc bioavailability in feed-grade sources of zinc. J. Anim. Sci. 1990, 68, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee for Animal Nutrition of the European Commission. Opinion of the Scientific Committee for Animal Nutrition on the Use of Zinc in Feedingstuffs; European Commission—Health & Consumer Protection Directorate General: Brussels, Belgium, 2003. [Google Scholar]
- Schell, T.C.; Kornegay, E.T. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine, Zn-lysine, or ZnSO4. J. Anim. Sci. 1996, 74, 1584. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Hasman, H. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet. Microbiol. 2004, 100, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Henry, P.R.; Ammerman, C.B.; Miles, R.D.; Littell, R.C. Relative bioavailability of basic zinc sulfate and basic zinc chloride for chicks. J. Appl. Poult. Res. 2000, 9, 513–517. [Google Scholar] [CrossRef]
- Batal, A.B.; Parr, T.M.; Baker, D.H. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 2001, 80, 87–90. [Google Scholar] [CrossRef]
- Mavromichalis, I.; Webel, D.M.; Parr, E.N.; Baker, D.H. Growth-promoting efficacy of pharmacological doses of tetrabasic zinc chloride in diets for nursery pigs. Can. J. Anim. Sci. 2001, 81, 387–391. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y. Beneficial effects of tetrabasic zinc chloride for weanling piglets and the bioavailability of zinc in tetrabasic form relative to ZnO. Anim. Feed Sci. Technol. 2007, 135, 75–85. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, T.; Zhao, J.; Liu, L.; He, P.; Zhang, S.; Zhang, L. Moderate tetrabasic zinc chloride supplementation improves growth performance and reduces diarrhea incidence in weaned pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 264–276. [Google Scholar] [CrossRef]
- Nitrayova, S.; Windisch, W.; von Heimendahl, E.; Müller, A.; Bartelt, J. Bioavailability of zinc from different sources in pigs. J. Anim. Sci. 2012, 90, 185–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Ward, T.L.; Ji, F.; Peng, C.; Zhu, L.; Gong, L.; Dong, B. Effects of zinc sources and levels of zinc amino acid complex on growth performance, hematological and biochemical parameters in weanling pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, K.J.; Hortin, A.E.; Baker, D.H. Methodology for assessing zinc bioavailability: Efficacy estimates for zinc-methionine, zinc sulfate, and zinc oxide. J. Anim. Sci. 1992, 70, 178–187. [Google Scholar] [CrossRef]
- Bouwhuis, M.A.; Sweeney, T.; Mukhopadhya, A.; Thornton, K.; McAlpine, P.O.; O’Doherty, J.V. Zinc methionine and laminarin have growth-enhancing properties in newly weaned pigs influencing both intestinal health and diarrhoea occurrence. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 101, 1273–1285. [Google Scholar] [CrossRef]
- Woodworth, J.C.; Tokach, M.D.; Nelssen, J.L.; Goodband, R.D.; Quinn, P.R.O.; Fakler, T.M. Interactive Effects of Diet Complexity, Zinc Source and Feed-grade Antibiotics on Weanling Pig Growth Performance. J. Anim. Vet. Adv. 2005, 4, 688–693. [Google Scholar]
- Hollis, G.R.; Carter, S.D.; Cline, T.R.; Crenshaw, T.D.; Cromwell, G.L.; Hill, G.M.; Kim, S.W.; Lewis, A.J.; Mahan, D.C.; Miller, P.S.; et al. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. J. Anim. Sci. 2005, 83, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Van Noten, N.; Degroote, J.; Romeo, A.; Vermeir, P.; Michiels, J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019, 103, 231–241. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Y.; Wang, Z.; Zhou, A.; He, M.; Mao, L.; Zou, H.; Peng, Q.; Xue, B.; Wang, L.; et al. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br. J. Nutr. 2014, 111, 2123–2134. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Ying, Z.; He, J.; Zhou, L.; Zhang, L.; Zhong, X.; Wang, T. Effects of Dietary Zinc Oxide Nanoparticles on Growth, Diarrhea, Mineral Deposition, Intestinal Morphology, and Barrier of Weaned Piglets. Biol. Trace Elem. Res. 2018, 185, 364–374. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Su, W.; Ying, Z.; He, J.; Zhang, L.; Zhong, X.; Wang, T. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS ONE 2017, 12, e0181136. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Chen, J.; Zhang, Y.; Liang, X.; Ni, H.; Zhang, B.; Yin, Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 2017, 12, e0182550. [Google Scholar] [CrossRef]
- Hu, C.H.; Gu, L.Y.; Luan, Z.S.; Song, J.; Zhu, K. Effects of montmorillonite-zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs. Anim. Feed Sci. Technol. 2012, 177, 108–115. [Google Scholar] [CrossRef]
- Cho, J.H.; Liu, S.D.; Yun, W.; Kim, K.S.; Kim, I.H. Effect of supplemented microencapsulated zinc oxide and organic acids and pure botanicals on growth performance, nutrient digestibility, blood profiles, feces microflora, and zinc level of feces in weanling pigs. Can. J. Anim. Sci. 2019, 99, 66–73. [Google Scholar] [CrossRef]
- Lei, X.J.; Kim, I.H. Low dose of coated zinc oxide is as effective as pharmacological zinc oxide in promoting growth performance, reducing fecal scores, and improving nutrient digestibility and intestinal morphology in weaned pigs. Anim. Feed Sci. Technol. 2018, 245, 117–125. [Google Scholar] [CrossRef]
- Dong, X.; Xu, Q.; Wang, C.; Zou, X.; Lu, J. Supplemental-coated zinc oxide relieves diarrhoea by decreasing intestinal permeability in weanling pigs. J. Appl. Anim. Res. 2019, 47, 362–368. [Google Scholar] [CrossRef]
- Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Pluske, J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012, 173, 3–16. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Jondreville, C.; Matte, J.J.; Seve, B. Importance of sanitary environment for growth performance and plasma nutrient homeostasis during the post-weaning period in piglets. Arch. Anim. Nutr. 2006, 60, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef]
- Gibbons, R.A.; Sellwood, R.; Burrows, M.; Hunter, P.A. Inheritance of resistance to neonatal E. coli diarrhoea in the pig: Examination of the genetic system. Theor. Appl. Genet. 1977, 51, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, I.G.W.; Bouw, J. Inheritance of K88-mediated adhesion of Escherichia coli to jejunal brush borders in pigs: A genetic analysis. Vet. Res. Commun. 1987, 11, 509–518. [Google Scholar] [CrossRef]
- Fontanesi, L.; Bertolini, F.; Dall’Olio, S.; Buttazzoni, L.; Gallo, M.; Russo, V. Analysis of Association between the MUC4 g.8227C>G Polymorphism and Production Traits in Italian Heavy Pigs Using a Selective Genotyping Approach. Anim. Biotechnol. 2012, 23, 147–155. [Google Scholar] [CrossRef]
- Rasschaert, K.; Verdonck, F.; Goddeeris, B.M.; Duchateau, L.; Cox, E. Screening of pigs resistant to F4 enterotoxigenic Escherichia coli (ETEC) infection. Vet. Microbiol. 2007, 123, 249–253. [Google Scholar] [CrossRef]
- Nicolai, R.W.; Sørensen, T.; Bækbo, P. Weaning without Zinc Oxide—Field Experiences; SEGES: Copenhagen, Denmark, 2019. [Google Scholar]
- Halas, D.; Heo, J.M.; Hansen, C.F.; Kim, J.C.; Hampson, D.J.; Mullan, B.P.; Pluske, J.R. Organic acids, prebiotics and protein level as dietary tools to control the weaning transition and reduce post-weaning diarrhoea in piglets. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Maribo, H.; Kjeldsen, N.; Pluske, J.R. Effects of dietary protein level and zinc oxide supplementation on the incidence of post-weaning diarrhoea in weaner pigs challenged with an enterotoxigenic strain of Escherichia coli. Livest. Sci. 2010, 133, 210–213. [Google Scholar] [CrossRef]
- Molist, F.; Gómez de Segura, A.; Pérez, J.F.; Bhandari, S.K.; Krause, D.O.; Nyachoti, C.M. Effect of wheat bran on the health and performance of weaned pigs challenged with Escherichia coli K88+. Livest. Sci. 2010, 133, 214–217. [Google Scholar] [CrossRef]
- Fernandes, C.D.; Resende, M.; Rodrigues, L.M.; Garbossa, C.A.P.; Costa, L.B.; Ferreira, R.A.; de Abreu, M.L.T.; Cantarelli, V.S. Dietary fiber and zinc additives on performance and intestinal health of Escherichia coli challenged piglets. Sci. Agric. 2020, 77. [Google Scholar] [CrossRef]
- Molist, F.; Hermes, R.G.; De Segura, A.G.; Martí-N-Orúe, S.M.; Gasa, J.; Manzanilla, E.G.; Pérez, J.F. Effect and interaction between wheat bran and zinc oxide on productive performance and intestinal health in post-weaning piglets. Br. J. Nutr. 2011, 105, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Tsiloyiannis, V.K.; Kyriakis, S.C.; Vlemmas, J.; Sarris, K. The effect of organic acids on the control of porcine post-weaning diarrhoea. Res. Vet. Sci. 2001, 70, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Rossi, B.; Toschi, A.; Piva, A.; Grilli, E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D. Antibacterial and antidiarrheal activities of plant products against enterotoxinogenic Escherichia coli. Toxins (Basel) 2013, 5, 2009–2041. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T. The effects of plant polyphenols on enterotoxigenic Escherichia coli adhesion and toxin binding. Livest. Sci. 2010, 133, 101–103. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef]
- Xiao, H.; Shao, F.; Wu, M.; Ren, W.; Xiong, X.; Tan, B.; Yin, Y. The application of antimicrobial peptides as growth and health promoters for swine. J. Anim. Sci. Biotechnol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Mine, Y.; Kovacs-Nolan, J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: A review. J. Med. Food 2002, 5, 159–169. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Polo, J.; Torrallardona, D. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc. Health Manag. 2016, 2, 16. [Google Scholar] [CrossRef]
- Pieper, R.; Villodre Tudela, C.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef]
- Wellock, I.J.; Fortomaris, P.D.; Houdijk, J.G.M.; Kyriazakis, I. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: Health. Animal 2008, 2, 834–842. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Yue, L.Y.; Qiao, S.Y. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livest. Sci. 2008, 115, 144–152. [Google Scholar] [CrossRef]
- Kjeldsen, N.J.; Lynegaard, J.C.; Bache, J.K. Low Protein for Weaned Pigs Can Reduce Diarrhoea; SEGES: Copenhagen, Denmark, 2019. [Google Scholar]
- Wan, K.; Li, Y.; Sun, W.; An, R.; Tang, Z.; Wu, L.; Chen, H.; Sun, Z. Effects of dietary calcium pyruvate on gastrointestinal tract development, intestinal health and growth performance of newly weaned piglets fed low-protein diets. J. Appl. Microbiol. 2020, 128, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 2008, 62, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, S.H.; Zeng, X.F.; Liu, H.; Qiao, S.Y. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-Australas. J. Anim. Sci. 2015, 28, 1742–1750. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.; et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, L.A.F.; Thomaz, M.C.; Watanabe, P.H.; Ruiz, U.D.S.; Amorim, A.B.; Daniel, E.; Silva, S.Z.D. Purified cellulose, soybean hulls and citrus pulp as a source of fiber for weaned piglets. Sci. Agric. 2015, 72, 400–410. [Google Scholar] [CrossRef][Green Version]
- Molist, F.; de Segura, A.G.; Gasa, J.; Hermes, R.G.; Manzanilla, E.G.; Anguita, M.; Pérez, J.F. Effects of the insoluble and soluble dietary fibre on the physicochemical properties of digesta and the microbial activity in early weaned piglets. Anim. Feed Sci. Technol. 2009, 149, 346–353. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Pérez, J.F.; Hermes, R.G.; Molist, F.; Jiménez-Díaz, R.; Martín-Orúe, S.M. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Br. J. Nutr. 2014, 111, 633–642. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Hermes, R.G.; Jiménez-Díaz, R.; Pérez, J.F.; Martín-Orúe, S.M. Screening of extracts from natural feed ingredients for their ability to reduce enterotoxigenic Escherichia coli (ETEC) K88 adhesion to porcine intestinal epithelial cell-line IPEC-J2. Vet. Microbiol. 2013, 167, 494–499. [Google Scholar] [CrossRef]
- Hansen, C.F.; Riis, A.L.; Bresson, S.; Højbjerg, O.; Jensen, B.B. Feeding organic acids enhances the barrier function against pathogenic bacteria of the piglet stomach. Livest. Sci. 2007, 108, 206–209. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: Towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Namkung, H.; Li, M.; Gong, J.; Yu, H.; Cottrill, M.; De Lange, C.F.M. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can. J. Anim. Sci. 2004, 84, 697–704. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Bosi, P.; Oswald, I.; Mengheri, E. Alternatives to in-feed antibiotics in pigs: Evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results. Anim. Res. 2005, 54, 203–218. [Google Scholar] [CrossRef]
- Ferrara, F.; Tedin, L.; Pieper, R.; Meyer, W.; Zentek, J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 101, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Hanczakowska, E.; Szewczyk, A.; Okoñ, K. Effects of dietary caprylic and capric acids on piglet performance and mucosal epithelium structure of the ileum. J. Anim. Feed Sci. 2011, 20, 556–565. [Google Scholar] [CrossRef]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 2002, 1, 35–41. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Foerster, C.J.; Piva, A. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J. Anim. Sci. 2016, 94, 433–436. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, B.; Gan, Z.; Song, D.; Lu, Z.; Yi, H.; Wu, Y.; Wang, Y.; Du, H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Rossi, B.; Giovagnoni, G.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins (Basel) 2020, 12, 468. [Google Scholar] [CrossRef]
- Bosi, P.; Sarli, G.; Casini, L.; De Filippi, S.; Trevisi, P.; Mazzoni, M.; Merialdi, G. The influence of fat protection of calcium formate on growth and intestinal defence in Escherichia coli K88-challenged weanling pigs. Anim. Feed Sci. Technol. 2007, 139, 170–185. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Q.; Guan, W.; Lin, X.; Wang, Y.; Song, H.; Zhang, Y. Immune response of piglets receiving mixture of formic and propionic acid alone or with either capric acid or bacillus licheniformis after Escherichia coli challenge. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zeghib, A.; Kabouche, A.; Laggoune, S.; Calliste, C.A.; Simon, A.; Bressolier, P.; Aouni, M.; Duroux, J.L.; Kabouche, Z. Antibacterial, antiviral, antioxidant and antiproliferative activities of thymus guyonii essential oil. Nat. Prod. Commun. 2017, 12, 1651–1654. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yoo, J.S.; Kim, H.J.; Wang, Y.; Chen, Y.J.; Cho, J.H.; Kim, I.H. Effects of dietary supplementation with blended essential oils on growth performance, nutrient digestibility, blood profiles and fecal characteristics in weanling Pigs. Asian-Australas. J. Anim. Sci. 2010, 23, 607–613. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.Y.; Piao, X.S. Essential oil blend could decrease diarrhea prevalence by improving antioxidative capability for weaned pigs. Animals 2019, 9, 847. [Google Scholar] [CrossRef]
- Jiang, X.R.; Li, X.L.; Awati, A.; Bento, H.; Zhang, H.J.; Bontempo, V. Effect of an essential oils blend on growth performance, and selected parameters of oxidative stress and antioxidant defence of Escherichia coli challenged piglets. J. Anim. Feed Sci. 2017, 26, 38–43. [Google Scholar] [CrossRef]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Nezhadali, A.; Nabavi, M.; Rajabian, M.; Akbarpour, M.; Pourali, P.; Amini, F. Chemical variation of leaf essential oil at different stages of plant growth and in vitro antibacterial activity of Thymus vulgaris Lamiaceae, from Iran. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 87–92. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Fruit extracts to control pathogenic Escherichia coli: A sweet solution. Heliyon 2020, 6, e03410. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Namkung, W.; Thiagarajah, J.R.; Phuan, P.; Verkman, A.S. Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010, 24, 4178–4186. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T. Selection of Escherichia coli heat-labile toxin (LT) inhibitors using both the gm1-elisa and the cAMP Vero cell assay. Foodborne Pathog. Dis. 2013, 10, 603–607. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compound-nature, occurrence, dietary intake and effects. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Spees, A.M.; Wangdi, T.; Lopez, C.A.; Kingsbury, D.D.; Xavier, M.N.; Winter, S.E.; Tsolis, R.M.; Bäumler, A.J. Streptomycin-Induced Inflammation Enhances Escherichia coli Gut Colonization Through Nitrate Respiration. MBio 2013, 4, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, J.; Mahfuz, S.; Piao, X. Effects of Hydrolysable Tannins as Zinc Oxide Substitutes on Antioxidant Status, Immune Function, Intestinal Morphology, and Digestive Enzyme Activities in Weaned Piglets. Animals 2020, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human Milk Oligosaccharides Inhibit the Adhesion to Caco-2 Cells of Diarrheal Pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef]
- Sarabia-Sainz, H.M.; Armenta-Ruiz, C.; Sarabia-Sainz, J.A.I.; Guzmán-Partida, A.M.; Ledesma-Osuna, A.I.; Vázquez-Moreno, L.; Montfort, G.R.C. Adhesion of enterotoxigenic Escherichia coli strains to neoglycans synthesised with prebiotic galactooligosaccharides. Food Chem. 2013, 141, 2727–2734. [Google Scholar] [CrossRef]
- Hermes, R.G.; Manzanilla, E.G.; Martín-Orúe, S.M.; Pérez, J.F.; Klasing, K.C. Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88). Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 479–488. [Google Scholar] [CrossRef]
- Stuyven, E.; Cox, E.; Vancaeneghem, S.; Arnouts, S.; Deprez, P.; Goddeeris, B.M. Effect of β-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 2009, 128, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Huang, Z.; Luo, J.; Luo, Y.; et al. Alginate oligosaccharide alleviates enterotoxigenic: Escherichia coli-induced intestinal mucosal disruption in weaned pigs. Food Funct. 2018, 9, 6401–6413. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli and probiotics in swine: What the bleep do we know? Biosci. Microbiota Food Health 2017, 36, 75–90. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Mengheri, E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2006, 95, 1177. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Mengheri, E. The Novel Porcine Lactobacillus sobrius Strain Protects Intestinal Cells from Enterotoxigenic Escherichia coli K88 Infection and Prevents Membrane Barrier Damage. J. Nutr. 2007, 137, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.Q.; Liu, H.Y.; Lai, T.; Ma, J.L.; Wang, J.F.; Zhu, Y.H. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet. Microbiol. 2010, 141, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Nordeste, R.; Tessema, A.; Sharma, S.; Kovač, Z.; Wang, C.; Morales, R.; Griffiths, M.W. Molecules produced by probiotics prevent enteric colibacillosis in pigs. BMC Vet. Res. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Trckova, M.; Faldyna, M.; Alexa, P.; Zajacova, Z.S.; Gopfert, E.; Kumprechtova, D.; Auclair, E.; D’Inca, R. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 2014, 92, 767–774. [Google Scholar] [CrossRef]
- Krause, D.O.; Bhandari, S.K.; House, J.D.; Nyachoti, C.M. Response of nursery pigs to a synbiotic preparation of starch and an anti-Escherichia coli K88 probiotic. Appl. Environ. Microbiol. 2010, 76, 8192–8200. [Google Scholar] [CrossRef]
- Guerra-Ordaz, A.A.; González-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M. Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef]
- Badia, R.; Zanello, G.; Chevaleyre, C.; Lizardo, R.; Meurens, F.; Martínez, P.; Brufau, J.; Salmon, H. Effect of Saccharomyces cerevisiae var. Boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88). Vet. Res. 2012, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mandal, S.; Tomar, S.K. Effect of Lactobacillus rhamnosus NCDC 298 with FOS in Combination on Viability and Toxin Production of Enterotoxigenic Escherichia coli. Probiotics Antimicrob. Proteins 2019, 11, 23–29. [Google Scholar] [CrossRef]
- Bradshaw, J.P. Cationic Antimicrobial Peptides. BioDrugs 2003, 17, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- Cutler, S.A.; Lonergan, S.M.; Cornick, N.; Johnson, A.K.; Stahl, C.H. Dietary inclusion of colicin E1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob. Agents Chemother. 2007, 51, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huang, Y.; Sun, S.; Wu, Z.; Wu, S.; Yin, Z.; Bao, W. The impact of BPI expression on Escherichia coli f18 infection in porcine kidney cells. Animals 2020, 10, 2118. [Google Scholar] [CrossRef]
- Johnson, R.P.; Gyles, C.L.; Huff, W.E.; Ojha, S.; Huff, G.R.; Rath, N.C.; Donoghue, A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008, 9, 201–215. [Google Scholar] [CrossRef]
- Cha, S.B.; Yoo, A.N.; Lee, W.J.; Shin, M.K.; Jung, M.H.; Shin, S.W.; Cho, Y.W.; Yoo, H.S. Effect of bacteriophage in enterotoxigenic Escherichia coli (ETEC) infected pigs. J. Vet. Med. Sci. 2012, 74, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Kim, S.J.; Park, B.C.; Han, J.H. Effects of dietary supplementation of bacteriophages against enterotoxigenic Escherichia coli (ETEC) K88 on clinical symptoms of post-weaning pigs challenged with the ETEC pathogen. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 101, 88–95. [Google Scholar] [CrossRef]
- Jamalludeen, N.; Johnson, R.P.; Shewen, P.E.; Gyles, C.L. Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 2009, 136, 135–141. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Cao, Z.; Wang, L.; Li, X.; Li, S.; Xu, Y. Bacteriophages as antimicrobial agents against major pathogens in swine: A review. J. Anim. Sci. Biotechnol. 2015, 6, 39. [Google Scholar] [CrossRef]
- Colomer-Lluch, M.; Imamovic, L.; Jofre, J.; Muniesa, M. Bacteriophages carrying antibiotic resistance genes in fecal waste from cattle, pigs, and poultry. Antimicrob. Agents Chemother. 2011, 55, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.R.; Jin, L.Z.; Kim, J.W.; Fang, L.; Frohlich, A.A.; Baidoo, S.K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med. Microbiol. 1999, 23, 283–288. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: A review. J. Anim. Sci. Biotechnol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Jin, L.Z.; Baidoo, S.K.; Marquardt, R.R.; Frohlich, A.A. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol. Med. Microbiol. 1998, 21, 313–321. [Google Scholar] [CrossRef]
- Chernysheva, L.V.; Friendship, R.M.; Abvp, D.; Dewey, C.E.; Gyles, C.L. The effect of dietary chicken egg-yolk antibodies on the clinical response in weaned pigs challenged with a K88+ Escherichia coli isolate. J. Swine Health Prod. 2004, 12, 119–122. [Google Scholar]
- Owusu-Asiedu, A.; Nyachoti, C.M.; Baidoo, S.K.; Marquardt, R.R.; Yang, X. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody. J. Anim. Sci. 2003, 81, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Asiedu, A.; Nyachoti, C.M.; Marquardt, R.R. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J. Anim. Sci. 2003, 81, 1790–1798. [Google Scholar] [CrossRef]
- Yi, G.F.; Carroll, J.A.; Allee, G.L.; Gaines, A.M.; Kendall, D.C.; Usry, J.L.; Toride, Y.; Izuru, S. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88 +-challenged weaned pigs. J. Anim. Sci. 2005, 83, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Bosi, P.; Casini, L.; Finamore, A.; Cremokolini, C.; Merialdi, G.; Trevisi, P.; Nobili, F.; Mengheri, E. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2004, 82, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.I.; Kim, I.H.; Nyachoti, C.M. Gut Health of Pigs: Challenge Models and Response Criteria with a Critical Analysis of the Effectiveness of Selected Feed Additives—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 909–924. [Google Scholar] [CrossRef] [PubMed]

| ZnO Feeding Alternatives | Advantages | Disadvantages | References |
|---|---|---|---|
| Low protein diets | ↓ proteolytic bacteria population ↓ pathogenic E. coli ↓ PWD symptoms ↓ pro-inflammatory cytokines | ↓ pig productivity | [124,125] |
| High fiber diets | ↓ PWD symptoms ↓ E. coli shedding ↓ E. coli adhesion ↑ SCFA production in digesta ↓ retention time of digesta | Few comparisons with pharmacological ZnO | [126,127,128] |
| Organic acids | Powerful antimicrobial activity Potential complete pharmacological Zn elimination ↑ gastric acidity ↑ nutrient digestibility ↑ growth performance ↓ PWD symptoms ↓ harmful coliforms ↓ inflammation ↑ intestinal morphology | Lack of comparisons with pharmacological ZnO Different activities between acids | [129,130] |
| Essential oils and nature identical compounds | Powerful antimicrobial, antioxidant, and anti-inflammatory activity ↑ growth performance ↑ diet digestibility ↓ PWD symptoms ↓ harmful coliforms ↓ bacterial virulence gene expression ↑ intestinal morphology | High variability in efficacy among different EO and molecules | [131,132,133] |
| Polyphenol-rich extracts | Considerable antimicrobial activity Ion chelating capacity High antioxidant activity ↓ PWD symptoms ↓ bacterial virulence gene expression ↓ bacterial adhesion to enterocytes ↓ bacterial toxin action ↑ intestinal morphology ↑ digestive enzymes activity | Few studies comparing polyphenols to pharmacological ZnO Polyphenols mechanism of action not yet fully elucidated | [134,135,136] |
| Antimicrobial peptides | Powerful antimicrobial activity ↓ bacterial resistance acquisition ↓ bacterial adhesion ↑ growth performance ↓ PWD symptoms ↑ intestinal morphology ↓ harmful coliforms ↑ immune response parameters | Lack of comparisons with pharmacological ZnO Need to investigate AMP pharmacokinetics | [137,138] |
| Egg yolk antibodies | ↑ growth performance ↓ PWD symptoms ↓ bacterial adhesion to enterocytes | Lack of comparisons with pharmacological ZnO High cost Stability issues in the gastrointestinal tract | [139] |
| Spray-dried plasma | ↑ growth performance ↓ PWD symptoms ↓ E. coli shedding ↑ intestinal morphology ↓ pro-inflammatory cytokines | Lack of comparisons with pharmacological ZnO High cost Complex production Potential presence of pathogens in SDP | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. https://doi.org/10.3390/ani11030642
Bonetti A, Tugnoli B, Piva A, Grilli E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals. 2021; 11(3):642. https://doi.org/10.3390/ani11030642
Chicago/Turabian StyleBonetti, Andrea, Benedetta Tugnoli, Andrea Piva, and Ester Grilli. 2021. "Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets" Animals 11, no. 3: 642. https://doi.org/10.3390/ani11030642
APA StyleBonetti, A., Tugnoli, B., Piva, A., & Grilli, E. (2021). Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals, 11(3), 642. https://doi.org/10.3390/ani11030642







