Monitoring and Controlling House Mouse, Mus musculus domesticus, Infestations in Low-Income Multi-Family Dwellings
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Initial Inspection and Resident Interview
2.3. House Mouse Management: Design and Implementation
2.4. Follow-up Mouse Inspections at 6 and 12 Months
2.5. Data Analysis
3. Results
3.1. Initial Inspection and Resident Interview
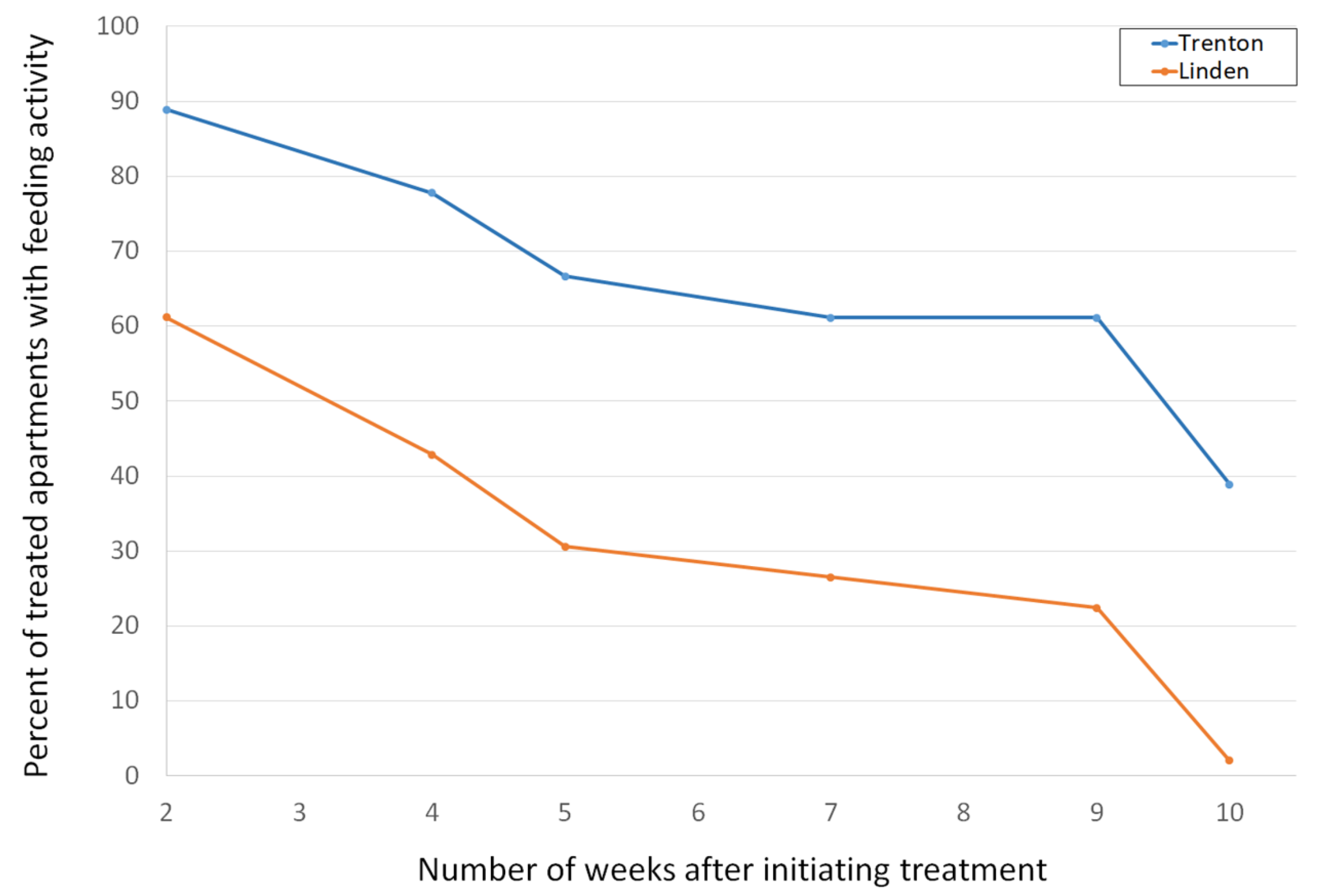
3.2. House Mouse Management Program Effectiveness
3.3. Detection of Mouse Infestations by Chocolate Spread and Two Non-Toxic Baits
3.4. Consumption among Chocolate Spread and the Two Toxic Baits
3.5. Rates of Apartments with House Mouse Activity between Lower and Upper Floors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Childs, J.E.; McLafferty, S.L.; Sadek, R.; Miller, G.L.; Khan, A.S.; DuPree, E.R.; Advani, R.; Glass, G.E. Epidemiology of rodent bites and prediction of rat infestation in New York City. Am. J. Epidemiol. 1998, 148, 78–87. [Google Scholar] [CrossRef]
- Pocock, M.J.; Hauffe, H.C.; Searle, J.B. Dispersal in house mice. Biol. J. Linn. Soc. 2005, 84, 565–583. [Google Scholar] [CrossRef]
- Murphy, R.G.; Williams, R.H.; Hughes, J.M.; Hide, G.; Ford, N.J.; Oldbury, D.J. The urban house mouse (Mus domesticus) as a reservoir of infection for the human parasite Toxoplasma gondii: An unrecognized public health issue? Int. J. Environ. Health Res. 2008, 18, 177–185. [Google Scholar] [CrossRef]
- IBIS-World. IBIS World Industry Report 56171: Pest Control in the U.S. 2011. Available online: http://clients1.ibisworld.com/reports/us/industry/default.aspx?entid=1495: 41: (accessed on 22 December 2020).
- Williams, S.H.; Che, X.; Garcia, J.; Klena, J.D.; Lee, B.; Muller, D.; Ulrich, W.; Corrigan, R.M.; Nichol, S.T.; Jain, K.; et al. Viral diversity of house mice in New York City. Mol. Biol. 2018, 9, e01354-17. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Paulick, A.; Guo, C.; Lee, B.; Muller, D.; Uhlemann, A.-C.; Lowy, F.D.; Corrigan, R.M.; Lipkin, I. New York City house mice (Mus musculus) as potential reservoirs for pathogenic bacteria and antimicrobial resistance determinants. Mol. Biol. 2018, 9, e0062418. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.P. Aspects of mouse behaviour related to control. Mamm. Rev. 1973, 3, 58–63. [Google Scholar] [CrossRef]
- Childs, J.; Glass, G.; LeDuc, J. Rodent sightings and contacts in an inner-city population of Baltimore, Maryland, USA. Bull. Soc. Vector Ecol. 1991, 16, 245–255. [Google Scholar]
- Wang, C.; El-Nour, M.M.A.; Bennett, G.W. Survey of pest infestation, asthma, and allergy in low-income housing. J. Commun. Health 2008, 33, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.A.; Murphy, R.G. Investigating residents’ perceptions of urban rodents in Manchester. In Rats, Mice and People: Rodent Biology and Management; Singleton, G.R., Hinds, L.A., Krebs, C.J., Spratt, D.M., Eds.; ACIAR, Bruce ACT: Canberra, Australia, 2003; Volume 96, pp. 473–476. [Google Scholar]
- Corrigan, R.M. Rats and Mice. In The Mallis Handbook of Pest Control, 10th ed.; Hedges, S., Moreland, D., Eds.; GIE Publications: Cleveland, OH, USA, 2011; pp. 11–148. [Google Scholar]
- Berry, R.J. House mouse Mus domesticus. In The handbook of British Mammals; Corbet, G.B., Harris, S., Eds.; Blackwell Science, Inc.: Oxford, UK, 1991; pp. 239–247. [Google Scholar]
- MacKay, J. Datasheet; Mus domesticus (house mouse). In Invasive Species Compendium: Detailed Coverage of Invasive Species Threatening Livelihoods and the Environment Worldwide; CAB International: Wallingford, UK, 2010; Available online: https://www.cabi.org/isc/datasheet/35218 (accessed on 12 December 2020).
- Andrzejewski, R.; Petrusewicz, K.; Walkowa, W. Absorption of newcomers by a population of white mice. Ekol. Polska. 1963, 11, 223–240. [Google Scholar]
- Lund, M. Commensal Rodents. In Rodent Pests and Their Control; Buckle, A.P., Smith, R.H., Eds.; CAB: Wallingford, UK, 1994; pp. 23–44. [Google Scholar]
- Seto, K.C.; Fragkias, M.; Guneralp, B.; Reilly, M.K. A meta-analysis of global urban land expansion. PLoS ONE 2011, 6, e23777. [Google Scholar] [CrossRef] [PubMed]
- Hygnstrom, S.E. House mouse damage to insulation II. Int. Biodeterior. Biodegrad. 1995, 36, 143–150. [Google Scholar] [CrossRef]
- Jahrling, P.; Peters, C. Lymphocytic choriomeningitis virus; A neglected pathogen of man. Arch. Pathol. Lab. Med. 1992, 116, 486–488. [Google Scholar] [PubMed]
- Gratz, N. Rodents as carriers of disease. In Rodent Pests and Their Control; Buckle, A.P., Smith, R.H., Eds.; CAB International: Wallingford, UK; Oxon, UK, 1994; pp. 85–108. [Google Scholar]
- Mills, J.N.; Childs, J.E. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg. Infect. Dis. 1998, 4, 529. [Google Scholar] [CrossRef] [PubMed]
- Phipatanakul, W. Rodent allergens. Curr. Allergy Asthm. R. 2002, 2, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Mcnally, R.J.; Steketee, G.S. The etiology and maintenance of severe animal phobias. Bahav. Res. Ther. 1985, 23, 431–435. [Google Scholar] [CrossRef]
- Kaukeinen, D. Rodent control in practice: Householders, pest control operators and municipal authorities. In Rodent Pests and Their Control; Buckle, A.P., Smith, R.H., Eds.; CAB International: Wallingford, UK, 1994; pp. 249–271. [Google Scholar]
- Meyer, A.; Drummond, D. Improving rodent control strategies in Lambeth. Environ. Health 1980, 88, 77–78. [Google Scholar]
- Cooper, R.; Wang, C.; Singh, N. Evaluation of a model community-wide bed bug management program in affordable housing. Pest. Manag. Sci. 2015, 72, 45–56. [Google Scholar] [CrossRef] [PubMed]
- DeLong, K.T. Population ecology of feral house mice. Ecology 1967, 48, 611–634. [Google Scholar] [CrossRef]
- Krebs, C.J.; Kenney, A.J.; Singleton, G.R. Movements of feral house mice in agricultural landscapes. Aust. J. Zool. 1995, 43, 293–302. [Google Scholar] [CrossRef]
- Gray, S.J.; Jensen, S.P.; Hurst, J.L. Effects of resource distribution on activity and territory defense in house mice, Mus domesticus. Anim. Behav. 2002, 63, 531–539. [Google Scholar] [CrossRef]
- Murphy, R.G.; Williams, R.H.; Hide, G. Population biology of the urban mouse (Mus domesticus) in the UK. In Proceedings of the Fifth International Conference on Urban Pests, Singapore, 11–13 July 2005; Lee, C., Robinson, W.H., Eds.; pp. 351–355. [Google Scholar]
- Stoloff, J.A. A Brief History of Public Housing. In Proceedings of the Annual Meeting of the American Sociological Association, San Francisco, CA, USA, 14 August 2004; Available online: http://www.allacademic.com/meta/p108852_index.html (accessed on 4 June 2020).
- Wang, C.; Bischoff, E.; Eiden, A.L.; Zha, C.; Cooper, R.; Graber, J.M. Residents attitudes and home sanitation predict presence of German cockroaches (Blattodea: Ectobiidae) in apartments for low income senior residents. J. Econ. Entomol. 2019, 112, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research, Inc. Rodenticides Market Size, Share & Trends Analysis Report by Mode of Application (Pellets, Blocks, Powder & Spray), by Product, by End Use (Pest Control Companies, Household, Agriculture), and Segment Forecasts, 2018–2025; Report ID: GVR-2-68038-499-4; Grand View Research, Inc.: San Francisco, CA, USA, 2018. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide; Version 9.3, Cary, North Carolina. 2011. Available online: https://support.sas.com/documentation/onlinedoc/stat/930/ (accessed on 2 December 2020).
- Wang, C.; Singh, N.; Zha, C.; Cooper, R. Bed bugs: Prevalence in low-income communities, resident’s reactions, and implementation of a low-cost inspection protocol. J. Med. Entomol. 2016, 53, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M. Rodent pest management in homes and apartment complexes. In Rodent Control: A Practical Guide for Pest Management Professionals; Moreland, D., Ed.; GIE Inc.: Cleveland, OH, USA, 2001; pp. 189–207. [Google Scholar]
- Gangloff-Kaufmann, J.; Frye, M. The Scientific Coalition on Pest Exclusion: Project Report. 2017. Available online: https://go.rutgers.edu/xv9ng6en (accessed on 2 December 2020).
- Calhoun, J. The study of wild animals under controlled conditions. Ann. N. Y. Acad. Sci. 1950, 51, 1113–1122. [Google Scholar] [CrossRef]
- Brown, R.Z. Biological Factors in Domestic Rodent Control; No. 773; USPHS Publication: Washington, DC, USA, 1960. [Google Scholar]
- Connor, J.L. Genetic mechanisms controlling the domestication of a wild house mouse population (Mus musculus L.). J. Comp. Physiol. Psychol. 1975, 89, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Kronenberger, J.P.; Medioni, J. Food neophobia in wild and laboratory mice, Mus musculus domesticus. Behav. Process. 1985, 11, 53–60. [Google Scholar] [CrossRef]
- Espinosa-Carrasco, J.; Burokos, A.; Fructuoso, M.; Erb, I.; Martin-Garcia, E.; Gutiérrez-Martos, M.; Notredam, C.; Maldanado, R.; Dierssen, M. Time-course and dynamics of obesity-related behavioral changes induced by energy-dense foods in mice. Addict. Biol. 2018, 23, 531–543. [Google Scholar] [CrossRef]
- Burokos, A.; Martin-Garcia, E.; Espinosa-Carrasco, J.; Erb, I.; McDonald, J.; Notredam, C.; Dierssen, M.; Maldanado, R. Extinction and reinstatement of an operant responding maintained by food in different models of obesity. Addict. Biol. 2018, 23, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Southern, H.N. Control of Rats and Mice: House Mice; Clarendon Press: Oxford, UK, 1954; Volume III. [Google Scholar]
- Meehan, A.P. Rodenticide activity of bromadiolone—A new anticoagulant. Proc. Vert. Pest. Conf. 1978, 8, 122–126. [Google Scholar]
- Lechevin, J.C.; Poche, R.M. Activity of LM 2219 (difethialone) a new anticoagulant rodenticide, in common rodents. Proc. Vert. Pest. Conf. 1988, 13, 59–63. [Google Scholar]
- Crowcroft, P. Spatial distribution of feeding activity in the wild house mouse (Mus musculus L.). Ann. Appl. Biol. 1959, 47, 150–155. [Google Scholar] [CrossRef]
- Frantz, S.C.; Padula, C.M. A laboratory test method for evaluating the efficacy of glueboards for trapping house mice. In Vertebrate Pest Control and Management Materials: Fourth Symposium; Kaukeinen, D.E., Ed.; ASTM STP 817: Philadelphia, PA, USA, 1983; pp. 209–225. [Google Scholar]
- Corrigan, R.M. The efficacy of glue traps against wild populations of house mice. Proc. Vertebr. Pest. Conf. 1998, 18, 268–275. [Google Scholar]




| Question | Number (Percentage) | ||
|---|---|---|---|
| Agree | Disagree | Not Sure | |
| Do mice cause disease? | 136 (80%) | 6 (4%) | 28 (16%) |
| Are mice easy to eliminate? | 40 (24%) | 88 (52%) | 40 (24%) |
| Do mice prefer dirty apartments? | 91 (54%) | 57 (34%) | 19 (11%) |
| Commercial Baits Fed Upon When Chocolate Was Consumed | Number of Occurrences When Bait Options Were Fed Upon | Proportion of Occurrences When Bait Options Were Fed Upon |
|---|---|---|
| Neither bait had feeding activity | 82 | 70% |
| One of the baits was fed upon | 24 | 20% |
| Both baits were fed upon | 12 | 10% |
| Total | 118 | 100% |
| Building | Period | Number of Apartments | % of Apartments Infested in Lower Floors | % of Apartments Infested in Upper Floors | Statistics | Odds Ratio |
|---|---|---|---|---|---|---|
| Trenton | Month 0 | 216 | 37.1% | 3.1% | χ2 = 44.4, p < 0.0001 | 18.2× |
| Trenton | Month 6 | 213 | 28.9% | 4.0% | χ2 = 25.1, p < 0.0001 | 9.8× |
| Trenton | Month 12 | 210 | 31.4% | 7.4% | χ2 = 16.6, p = 0.0003 | 5.7× |
| Linden | Month 0 | 180 | 46.9% | 23.0% | χ2 = 7.6, p = 0.0059 | 3.0× |
| Linden | Month 6 | 157 | 20.8% | 0.0% | χ2 = 28.6, p < 0.0001 | * |
| Linden | Month 12 | 178 | 0.0% | 2.1% | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sked, S.; Abbar, S.; Cooper, R.; Corrigan, R.; Pan, X.; Ranabhat, S.; Wang, C. Monitoring and Controlling House Mouse, Mus musculus domesticus, Infestations in Low-Income Multi-Family Dwellings. Animals 2021, 11, 648. https://doi.org/10.3390/ani11030648
Sked S, Abbar S, Cooper R, Corrigan R, Pan X, Ranabhat S, Wang C. Monitoring and Controlling House Mouse, Mus musculus domesticus, Infestations in Low-Income Multi-Family Dwellings. Animals. 2021; 11(3):648. https://doi.org/10.3390/ani11030648
Chicago/Turabian StyleSked, Shannon, Salehe Abbar, Richard Cooper, Robert Corrigan, Xiaodan Pan, Sabita Ranabhat, and Changlu Wang. 2021. "Monitoring and Controlling House Mouse, Mus musculus domesticus, Infestations in Low-Income Multi-Family Dwellings" Animals 11, no. 3: 648. https://doi.org/10.3390/ani11030648
APA StyleSked, S., Abbar, S., Cooper, R., Corrigan, R., Pan, X., Ranabhat, S., & Wang, C. (2021). Monitoring and Controlling House Mouse, Mus musculus domesticus, Infestations in Low-Income Multi-Family Dwellings. Animals, 11(3), 648. https://doi.org/10.3390/ani11030648








