Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. RNA Isolation
2.3. RNA Sequencing
2.4. NGS Data Analysis and Statistical Approach
2.5. Validation of NGS Data Using Real Time PCR
3. Results
3.1. Next Generating Sequencing Basic Characteristic
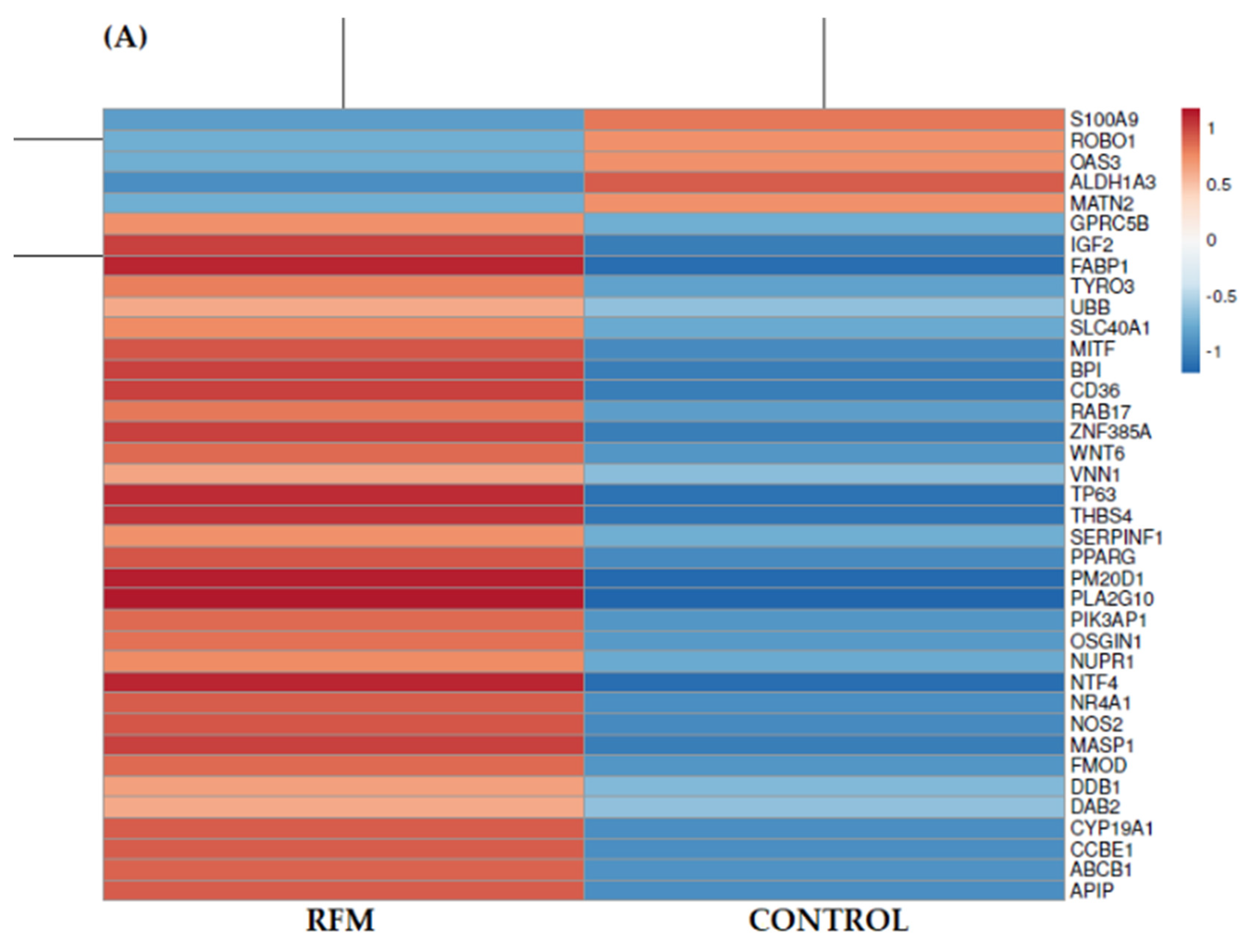
3.2. Identification of Differentially Expressed Genes (DEGs)
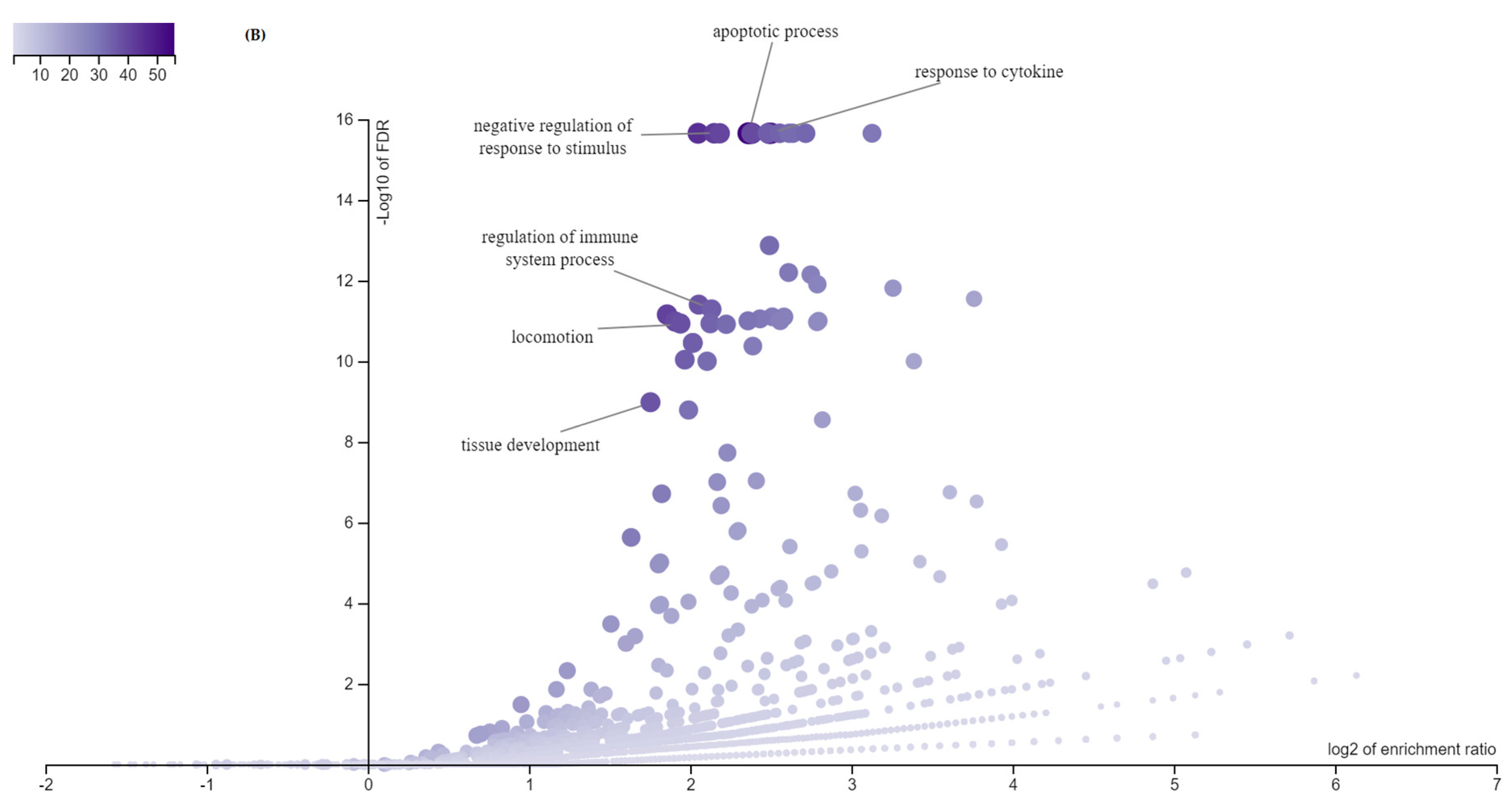
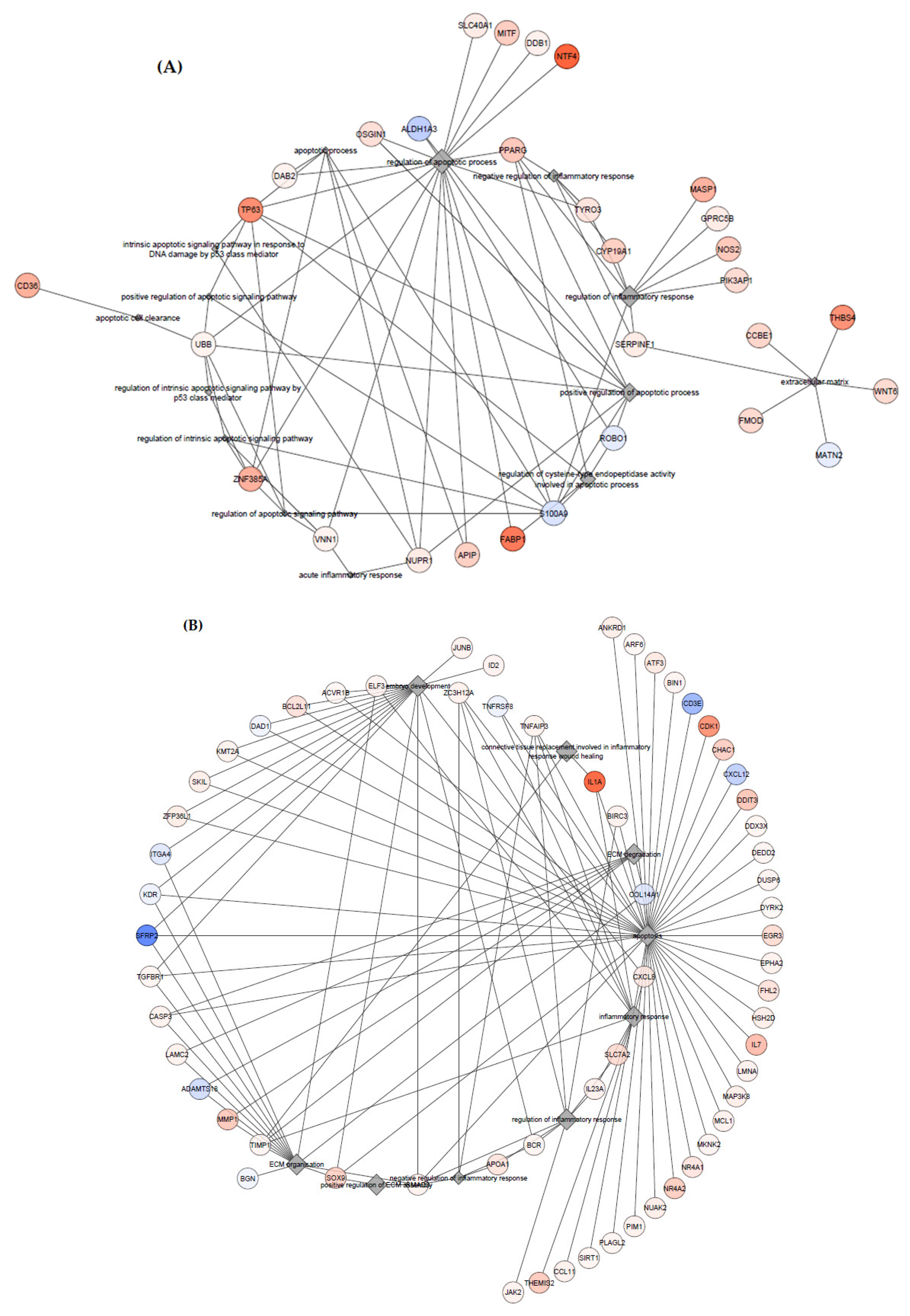
3.3. Gene Ontology—GO Analysis
3.4. KEGG Pathways Enrichment Analysis
3.5. Validation Results
4. Discussion
4.1. Differences in the Transcriptomes of the Allantochorion and Endometrium between RFM and Control Mares
4.2. Inflammatory Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, W.R.; Wilsher, S. A Review of implantation and early placentation in the mare. Placenta 2009, 30, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Rodriguez, J.S.; Sanz, M.G.; Coutinho da Silva, M.A. A clinical approach to the diagnosis and treatment of retained fetal membranes with an emphasis placed on the critically ill mare. J. Equine Vet. Sci. 2013, 33, 570–579. [Google Scholar] [CrossRef]
- Warnakulasooriya, D.N.; Marth, C.D.; McLeod, J.A.; Hanlon, D.W.; Krekeler, N. Treatment of retained fetal membranes in the mare-a practitioner survey. Front. Vet. Sci. 2018, 5, 6–8. [Google Scholar] [CrossRef]
- Marcellin, L.; Schmitz, T.; Messaoudene, M.; Chader, D.; Parizot, C.; Jacques, S.; Delaire, J.; Gogusev, J.; Schmitt, A.; Lesaffre, C.; et al. Immune modifications in fetal membranes overlying the cervix precede parturition in humans. J. Immunol. 2017, 198, 1345–1356. [Google Scholar] [CrossRef]
- Chavan, A.R.; Griffith, O.W.; Wagner, G.P. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- Dilly, M.; Hambruch, N.; Shenavai, S.; Schuler, G.; Froehlich, R.; Haeger, J.D.; Ozalp, G.R.; Pfarrer, C. Expression of matrix metalloproteinase (MMP)-2, MMP-14 and tissue inhibitor of matrix metalloproteinase (TIMP)-2 during bovine placentation and at term with or without placental retention. Theriogenology 2011, 75, 1104–1114. [Google Scholar] [CrossRef]
- Stygar, D.; Wang, H.; Vladic, Y.S.; Ekman, G.; Eriksson, H.; Sahlin, L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol. Reprod. 2002, 67, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Sevinga, M.; Barkema, H.W.; Stryhn, H.; Hesselink, J.W. Retained placenta in friesian mares: Incidence, and potential risk factors with special emphasis on gestational length. Theriogenology 2004, 61, 851–859. [Google Scholar] [CrossRef]
- Rapacz-Leonard, A.; Kankofer, M.; Leonard, M.; Wawrzykowski, J.; Dąbrowska, M.; Rasś, A.; Paździor-Czapula, K.; Janowski, T. Differences in extracellular matrix remodeling in the placenta of mares that retain fetal membranes and mares that deliver fetal membranes physiologically. Placenta 2015, 36, 1167–1177. [Google Scholar] [CrossRef]
- Rapacz-Leonard, A.; Raś, A.; Całka, J.; Janowski, T.E. Expression of oxytocin receptors is greatly reduced in the placenta of heavy mares with retained fetal membranes due to secondary uterine atony. Equine Vet. J. 2015, 47, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Janowski, T. Expression of proinflammatory cytokines IL-1β, IL-6 and TNFα in the retained placenta of mares. Theriogenology 2019, 126, 1–7. [Google Scholar] [CrossRef]
- Benedictus, L.; Jorritsma, R.; Knijn, H.M.; Vos, P.L.A.M.; Koets, A.P. Chemotactic activity of cotyledons for mononuclear leukocytes related to occurrence of retained placenta in dexamethasone induced parturition in cattle. Theriogenology 2011, 76, 802–809. [Google Scholar] [CrossRef]
- Boos, A.; Janssen, V.; Mülling, C. Proliferation and apoptosis in bovine placentomes during pregnancy and around induced and spontaneous parturition as well as in cows retaining the fetal membranes. Reproduction 2003, 126, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Pozor, M. Equine Placenta—A clinician’s perspective. part 1: Normal placenta—Physiology and evaluation. Equine Vet. Educ. 2016, 28, 327–334. [Google Scholar] [CrossRef]
- Pozor, M. Equine Placenta—A clinician’s perspective. part 2: Abnormalities. Equine Vet. Educ. 2016, 28, 396–404. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 23 November 2020).
- Dodt, M.; Roehr, J.T.; Ahmed, R.; Dieterich, C. FLEXBARFlexible barcode and adapter processing for next-generation sequencing platforms. Biology 2012, 1, 895–905. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. Genome Analysis HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
- Bower, K.M. When to use Fisher’s Exact Test. Am. Soc. Qual. 2003, 2, 35–37. [Google Scholar]
- Ahn, K.; Bae, J.-H.; Nam, K.-H.; Lee, C.-E.; Park, K.-D.; Lee, H.-K.; Cho, B.-W.; Kim, H.-S. Identification of reference genes for normalization of gene expression in thoroughbred and jeju native horse (jeju pony) tissues. Genes Genom. 2011, 33, 245–250. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in Real-Time RT-PCR. Nucleic Acid Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Böhmdorfer, G.; Wierzbicki, A.T. Control of chromatin structure by long noncoding RNA. Trends Cell Biol. 2015, 25, 623–632. [Google Scholar] [CrossRef]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding rna controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, Y.; Yang, Y.; Yang, Y.-C.T.; Wang, Z.; Yuan, J.; Yang, Y.; Hua, C.; Fan, X.; Niu, G.; et al. Comprehensive analysis of long non-coding RNAs highlights their spatio-temporal expression patterns and evolutional conservation in Sus Scrofa. Sci. Rep. 2017, 7, 43166. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, M.; Lopucki, M.; Stachowicz, N.; Morawska, D.; Kankofer, M. Extracellular matrix proteins in healthy and retained placentas, comparing hemochorial and synepitheliochorial placentas. Placenta 2017, 50, 19–24. [Google Scholar] [CrossRef]
- Hassan, S.S.; Romero, R.; Haddad, R.; Hendler, I.; Khalek, N.; Tromp, G.; Diamond, M.P.; Sorokin, Y.; Malone, J. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am. J. Obstet. Gynecol. 2006, 195, 778–786. [Google Scholar] [CrossRef]
- Kamemori, Y.; Wakamiya, K.; Nishimura, R.; Hosaka, Y.; Ohtani, S.; Okuda, K. Expressions of apoptosis-regulating factors in bovine retained placenta. Placenta 2011, 32, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Paździor, K.; Rapacz, A.; Rotkiewicz, T.; Raś, A. Proliferation and apoptosis in fetal membranes and endometrium during placental retention in heavy draft mares. J. Equine Vet. Sci. 2012, 32, 80–84. [Google Scholar] [CrossRef]
- Arikat, S.; Novince, R.W.; Mercer, B.M.; Kumar, D.; Fox, J.M.; Mansour, J.M.; Moore, J.J. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am. J. Obstet. Gynecol. 2006, 194, 211–217. [Google Scholar] [CrossRef]
- Strohl, A.; Kumar, D.; Novince, R.; Shaniuk, P.; Smith, J.; Bryant, K.; Moore, R.M.; Novak, J.; Stetzer, B.; Mercer, B.M.; et al. Decreased adherence and spontaneous separation of fetal membrane layers—Amnion and choriodecidua—A possible part of the normal weakening process. Placenta 2010, 31, 18–24. [Google Scholar] [CrossRef][Green Version]
- Attupuram, N.M.; Kumaresan, A.; Narayanan, K.; Kumar, H. Cellular and molecular mechanisms involved in placental separation in the bovine: A review. Mol. Reprod. Dev. 2016, 83, 287–297. [Google Scholar] [CrossRef]
- Geng, J.; Huang, C.; Jiang, S. Roles and regulation of the matrix metalloproteinase system in parturition. Mol. Reprod. Dev. 2016, 83, 276–286. [Google Scholar] [CrossRef]
- Nastase, M.V.; Young, M.F.; Schaefer, L. Biglycan: A multivalent proteoglycan providing structure and signals. J. Histochem. Cytochem. 2012, 60, 963–975. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Horgan, C.E.; Carr, O.; Owens, R.T.; Iozzo, R.V.; Lechner, B.E. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol. 2014, 35, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Uldbjerg, N.; Ekman, G.; Malmström, A.; Olsson, K.; Ulmsten, U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am. J. Obstet. Gynecol. 1983, 147, 662–666. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 33. [Google Scholar] [CrossRef]
- Goldman, S.; Weiss, A.; Eyali, V.; Shalev, E. Differential activity of the gelatinases (Matrix Metalloproteinases 2 and 9) in the fetal membranes and decidua, associated with labour. Mol. Hum. Reprod. 2003, 9, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Oddsdóttir, C.; Riley, S.C.; Leask, R.; Shaw, D.J.; Aurich, C.; Palm, F.; Fowden, A.L.; Ricketts, S.W.; Watson, E.D. Dynamics of activities of matrix metalloproteinases-9 and -2, and the tissue inhibitors of MMPs in fetal fluid compartments during gestation and at parturition in the mare. Theriogenology 2011, 75, 1130–1138. [Google Scholar] [CrossRef]
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J. Histochem. Cytochem. 2018, 66, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Roedig, H.; Nastase, M.V.; Frey, H.; Moreth, K.; Zeng-Brouwers, J.; Poluzzi, C.; Hsieh, L.T.H.; Brandts, C.; Fulda, S.; Wygrecka, M.; et al. Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol. 2019, 77, 4–22. [Google Scholar] [CrossRef]
- Gröne Bonrouhi, H.-J.; Burgdorf, S.; Young, M.F.; Ziya Kaya, L.; Porubsky, S.; Meisner, M.; Zoran Popovic, M.V.; Wang, S.; Papatriantafyllou, M. Autoimmune perimyocarditis via MyD88 and TRIF pathways and triggers potentially antigen-specific T cell activation the proteoglycan biglycan enhances. J. Immunol. Ref. 2011, 187, 6217–6226. [Google Scholar]
- Mayer, C.; Adam, M.; Glashauser, L.; Dietrich, K.; Schwarzer, J.U.; Köhn, F.M.; Strauss, L.; Welter, H.; Poutanen, M.; Mayerhofer, A. Sterile inflammation as a factor in human male infertility: Involvement of toll like receptor 2, biglycan and peritubular cells. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Schöniger, S.; Gräfe, H.; Wipplinger, M.; Schoon, H.A. Expression of toll-like receptors 2, 4 and 6 in the equine chorioallantois. Vet. Immunol. Immunopathol. 2018, 206, 49–53. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S.; Lappas, M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2019, 79, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hadley, E.E.; Richardson, L.S.; Torloni, M.R.; Menon, R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am. J. Reprod. Immunol. 2018, 79, 1–11. [Google Scholar] [CrossRef]
- Pieters, J. MHC class II restricted antigen presentation. Curr. Opin. Immunol. 1997, 9, 89–96. [Google Scholar] [CrossRef]
- Witonsky, S.; Buechner-Maxwell, V.; Santonastasto, A.; Pleasant, R.; Werre, S.; Wagner, B.; Ellison, S.; Lindsay, D. Can levamisole upregulate the equine cell-mediated macrophage (M1) dendritic cell (DC1) T-helper 1 (CD4 Th1) T-cytotoxic (CD8) immune response in vitro? J. Vet. Intern. Med. 2019, 33, 889–896. [Google Scholar] [CrossRef]
- Luciano, A.A.; Yu, H.; Jackson, L.W.; Wolfe, L.A.; Bernstein, H.B. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS ONE 2011, 6, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, J.F.; Li, S.; Damlaj, O.; Dave, V.P. Expression of fully assembled TCR-CD3 complex on double positive thymocytes: Synergistic role for the PRS and ER retention motifs in the intra-cytoplasmic tail of CD3ε. Int. Immunol. 2009, 21, 1317–1327. [Google Scholar] [CrossRef]
- Buxadé, M.; Encabo, H.H.; Riera-Borrull, M.; Quintana-Gallardo, L.; López-Cotarelo, P.; Tellechea, M.; Martínez-Martínez, S.; Redondo, J.M.; Martín-Caballero, J.; Flores, J.M.; et al. Macrophage-specific MHCII expression is regulated by a remote CIITa enhancer controlled by NFAT5. J. Exp. Med. 2018, 215, 2901–2918. [Google Scholar] [CrossRef] [PubMed]
- Xaus, J.; Comalada, M.; Barrachina, M.; Herrero, C.; Goñalons, E.; Soler, C.; Lloberas, J.; Celada, A. The expression of MHC class II genes in macrophages is cell cycle dependent. J. Immunol. 2000, 165, 6364–6371. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Vega-Sanchez, R.; Castillo-Castrejon, M.; Romero, R.; Cubeiro-Arreola, K.; Vadillo-Ortega, F. Evidence for a role for the adaptive immune response in human term parturition. Am. J. Reprod. Immunol. 2013, 69, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of complement activation and placental dysfunction: A potential target to treat preeclampsia? Front. Immunol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Wang, C.C.; Yim, K.W.; Poon, T.C.W.; Choy, K.W.; Chu, C.Y.; Lui, W.T.; Lau, T.K.; Rogers, M.S.; Leung, T.N. Innate immune response by ficolin binding in apoptotic placenta is associated with the clinical syndrome of preeclampsia. Clin. Chem. 2007, 53, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Franzke, C.W.; Yang, F.; Romero, R.; Girardi, G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am. J. Pathol. 2011, 179, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Huang, H.-L.; Chen, Y.-H. Ovarian steroid-regulated synthesis and secretion of complement C3 and factor B in mouse endometrium during the natural estrous cycle and pregnancy period. Biol. Reprod. 2002, 66, 322–332. [Google Scholar] [CrossRef]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential role of complement in pregnancy: From implantation to parturition and beyond. Front. Immunol. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]


| Gene Symbol | Gene Name | Accession Number | FC | Adjusted p-Value | |
|---|---|---|---|---|---|
| endometrium | LncRNA | Long non-coding RNA | ENSECAG00000033289 | 249 | *** |
| Novel gene | Novel gene | ENSECAG00000040328 | 202,7 | *** | |
| CPA4 | Carboxypeptidase A4 | ENSECAG00000018730 | 179 | *** | |
| LncRNA | Long non-coding RNA | ENSECAG00000032862 | 173.8 | *** | |
| GPRIN2 | G protein regulated inducer of neurite outgrowth 2 | ENSECAG00000004381 | 163.7 | *** | |
| allantochorion | EGR4 | early growth response 4 | ENSECAG00000010604 | 183 | ns (0.001) |
| LRRN4 | leucine rich repeat neuronal 4 | ENSECAG00000020607 | 102.7 | **** | |
| PI16 | peptidase inhibitor 16 | ENSECAG00000024845 | 102 | * | |
| CPLX1 | complexin 1 | ENSECAG00000010131 | 86 | ns (0.002) | |
| ALKAL2 | ALK and LTK ligand 2 | ENSECAG00000029965 | 68.9 | **** |
| DEG | Gene Accession Number | Correlation Coefficient |
|---|---|---|
| CASP3 | ENSECAG00000022197 | 0.4 * |
| DRB | ENSECAG0000001293 | 0.1 |
| SMAD3 | ENSECAG00000020259 | 0.4 |
| TIMP1 | ENSECAG00000014259 | 0.6 * |
| TNFRSF8 | ENSECAG00000014108 | 0.2 |
| CXCL12 | ENSECAG00000019551 | 1 * |
| BGN | ENSECAG00000018717 | 0.8 * |
| Bcl2l | ENSECAG00000032970 | 0.9 * |
| lncRNA | ENSECAG00000033289 | 0.9 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, J.; Ropka-Molik, K.; Piórkowska, K.; Szmatoła, T.; Kowalczyk-Zięba, I.; Wocławek-Potocka, I.; Siemieniuch, M. Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease. Animals 2021, 11, 675. https://doi.org/10.3390/ani11030675
Jaworska J, Ropka-Molik K, Piórkowska K, Szmatoła T, Kowalczyk-Zięba I, Wocławek-Potocka I, Siemieniuch M. Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease. Animals. 2021; 11(3):675. https://doi.org/10.3390/ani11030675
Chicago/Turabian StyleJaworska, Joanna, Katarzyna Ropka-Molik, Katarzyna Piórkowska, Tomasz Szmatoła, Ilona Kowalczyk-Zięba, Izabela Wocławek-Potocka, and Marta Siemieniuch. 2021. "Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease" Animals 11, no. 3: 675. https://doi.org/10.3390/ani11030675
APA StyleJaworska, J., Ropka-Molik, K., Piórkowska, K., Szmatoła, T., Kowalczyk-Zięba, I., Wocławek-Potocka, I., & Siemieniuch, M. (2021). Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease. Animals, 11(3), 675. https://doi.org/10.3390/ani11030675





