Unusual Presentation of Aortic Valve Infective Endocarditis in a Dog: Aorto-Cavitary Fistula, Tricuspid Valve Endocarditis, and Third-Degree Atrioventricular Block
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials, Methods, and Results
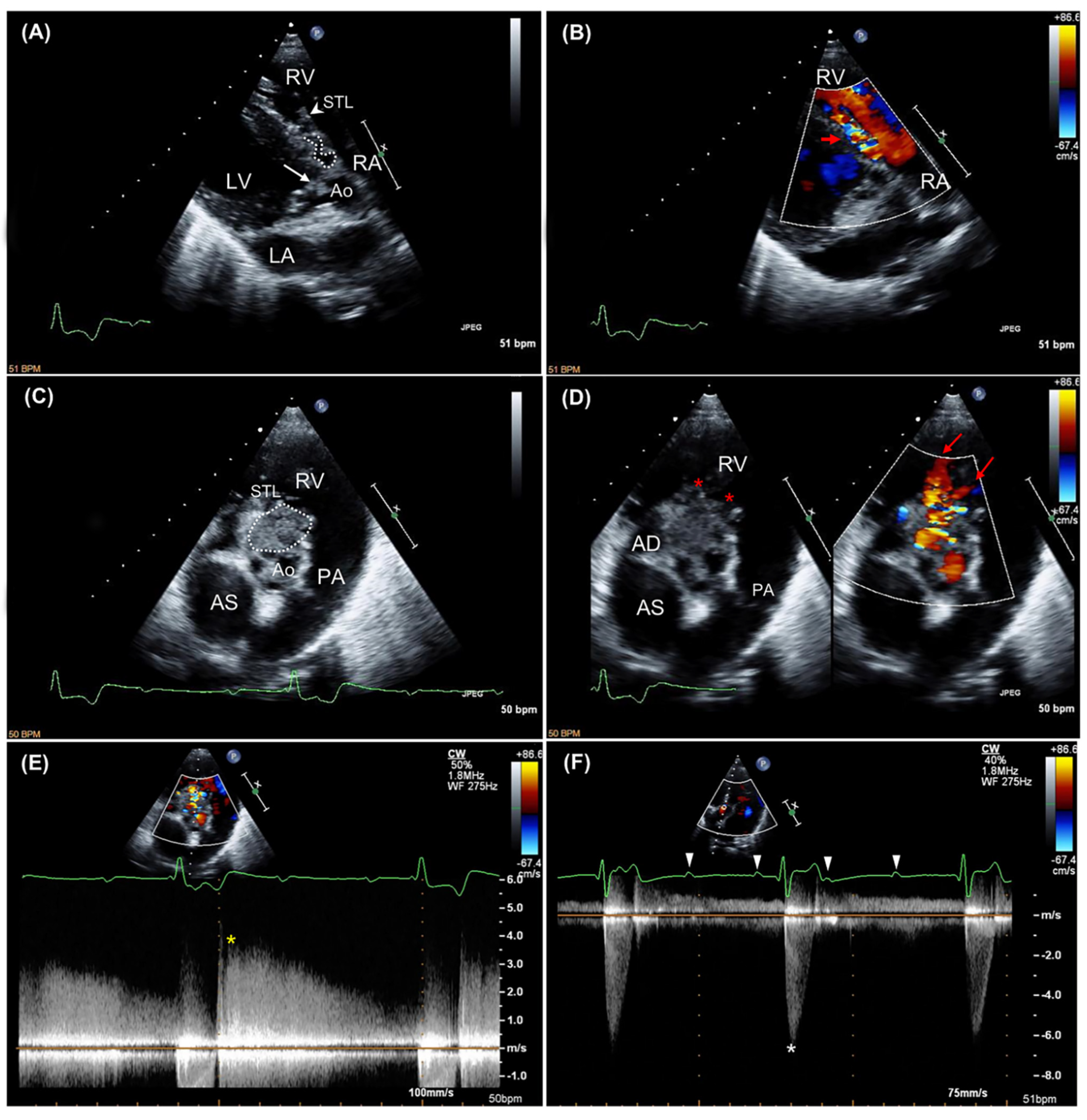
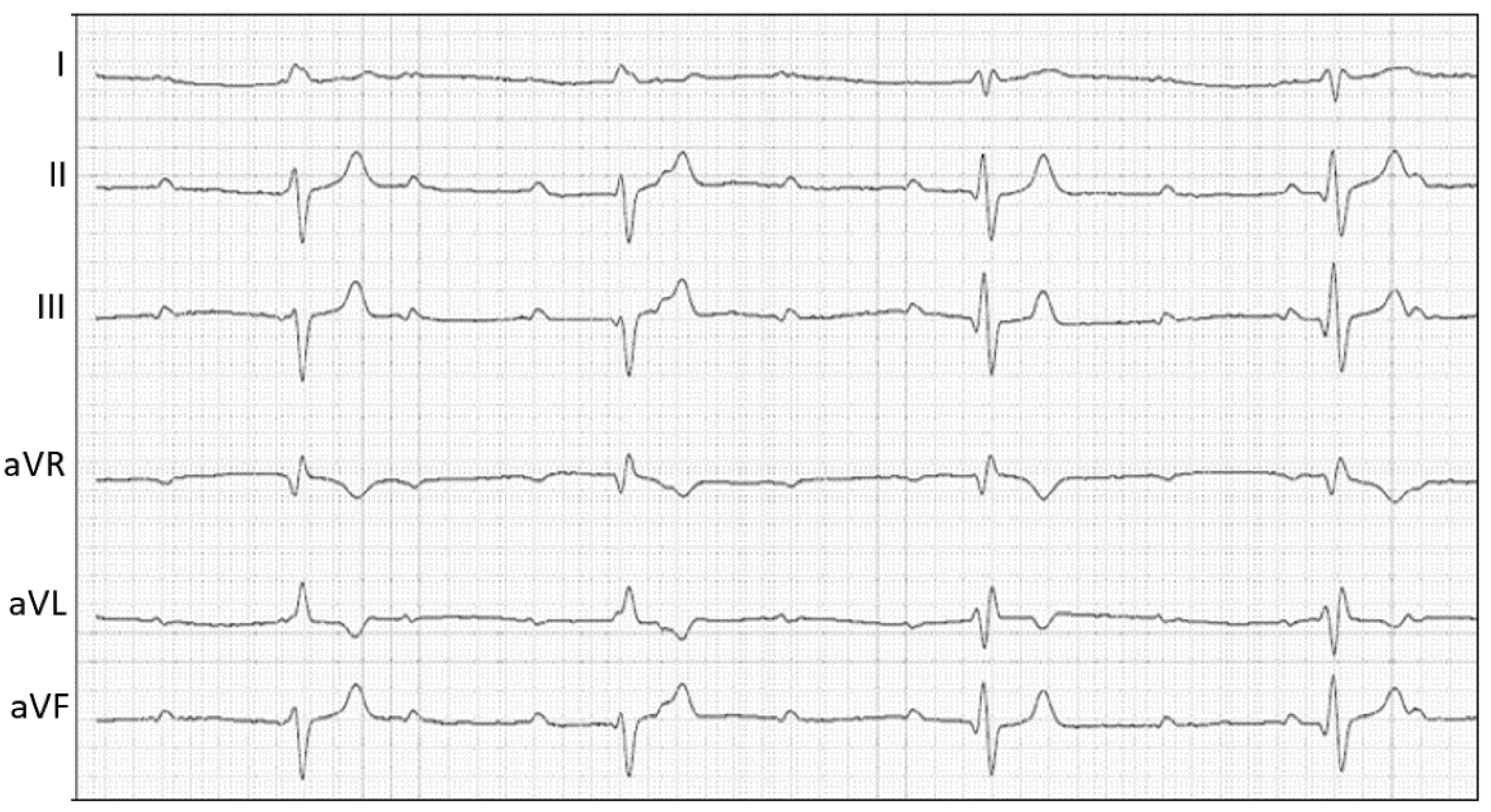
2.1. Case Description and Clinical Investigations
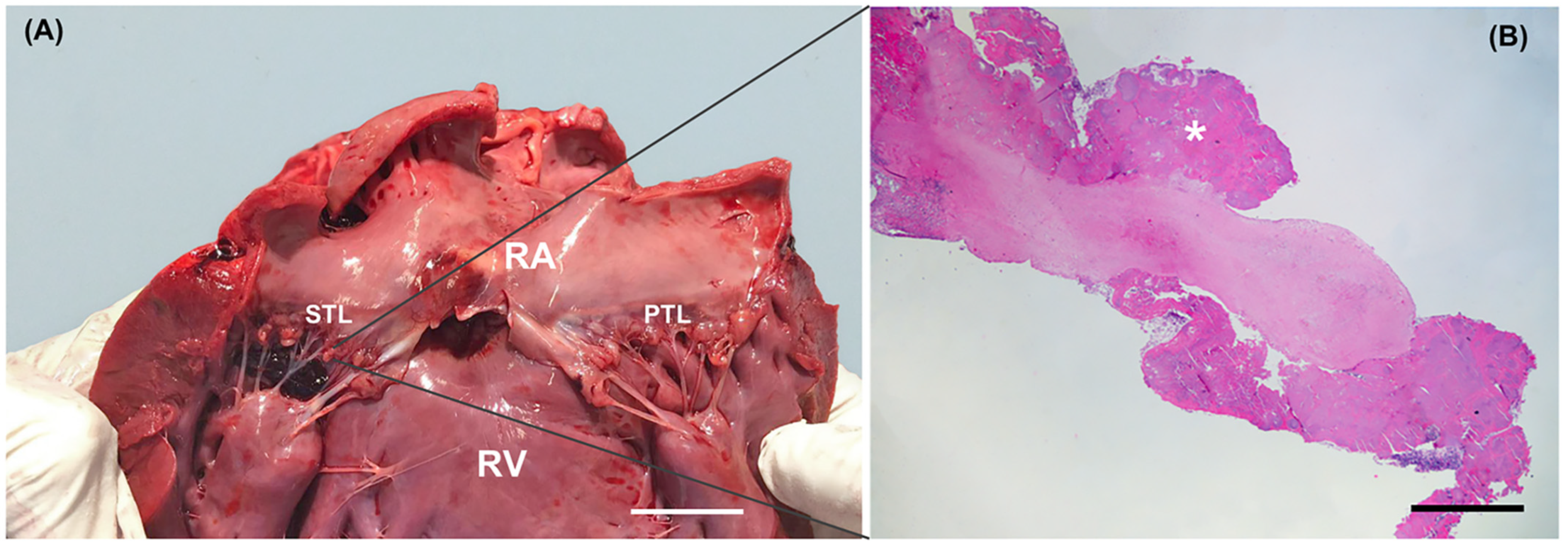
2.2. Gross Examination and Histopathology
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peddle, G.; Sleeper, M.M. Canine bacterial endocarditis: A review. J. Am. Anim. Hosp. Assoc. 2007, 43, 258–263. [Google Scholar] [CrossRef]
- Macdonald, K. Infective endocarditis in dogs: Diagnosis and therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 665–684. [Google Scholar] [CrossRef]
- Palerme, J.S.; Jones, A.E.; Ward, J.L.; Balakrishnan, N.; Linder, K.E.; Breitschwerdt, E.B.; Keene, B.W. Infective endocarditis in 13 cats. J. Vet. Cardiol. 2016, 18, 213–225. [Google Scholar] [CrossRef]
- Freedman, L.R. The pathogenesis of infective endocarditis. J. Antimicrob. Chemother. 1987, 20, 1–6. [Google Scholar] [CrossRef]
- Lepeschkin, E. On the relation between the site of valvular involvement in endocarditis and the blood pressure resting on the valve. Am. J. Med. Sci. 1952, 224, 318–322. [Google Scholar] [CrossRef]
- Rodbard, S. Blood velocity and endocarditis. Circulation 1963, 27, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Muna, W.F.; Ferrans, V.J.; Pierce, J.E.; Roberts, W.C. Discrete subaortic stenosis in Newfoundland dogs: Association of infective endocarditis. Am. J. Cardiol. 1978, 41, 746–754. [Google Scholar] [CrossRef]
- Sisson, D.; Thomas, W.P. Endocarditis of the aortic valve in the dog. J. Am. Vet. Med. Assoc. 1984, 184, 570–577. [Google Scholar]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef] [Green Version]
- Hansson, K.; Haggstrom, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Smets, P.; Daminet, S.; Wess, G. Simpson’s method of discs for measurement of echocardiographic end-diastolic and end-systolic left ventricular volumes: Breed-specific reference ranges in Boxer dogs. J. Vet. Intern. Med. 2014, 28, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Serres, F.; Chetboul, V.; Tissier, R.; Poujol, L.; Gouni, V.; Carlos Sampedrano, C.; Pouchelon, J.L. Comparison of 3 ultrasound methods for quantifying left ventricular systolic function: Correlation with disease severity and prognostic value in dogs with mitral valve disease. J. Vet. Intern. Med. 2008, 22, 566–577. [Google Scholar] [CrossRef]
- Graupner, C.; Vilacosta, I.; SanRomán, J.; Ronderos, R.; Sarriá, C.; Fernández, C.; Mújica, R.; Sanz, O.; Sanmartín, J.V.; Pinto, A.G. Periannular extension of infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Anguera, I.; Miro, J.M.; Vilacosta, I.; Almirante, B.; Anguita, M.; Muñoz, P.; San Roman, J.A.; de Alarcon, A.; Ripoll, T.; Navas, E.; et al. Aorto-cavitary Fistula in Endocarditis Working Group. Aorto-cavitary fistulous tract formation in infective endocarditis: Clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur. Heart J. 2005, 26, 288–297. [Google Scholar] [CrossRef]
- Romito, G.; Cipone, M. Infective endocarditis causing aortopulmonary fistula and intracardiac thrombosis in a dog. J. Small Anim. Pract. 2020. [Google Scholar] [CrossRef] [PubMed]
- Child, J.S. Echo-Doppler and color-flow imaging in congenital heart disease. Cardiol. Clin. 1990, 8, 289–313. [Google Scholar] [CrossRef]
- Chow, W.H.; Lee, P.K.; Cheung, K.L.; Mok, C.K. Two-dimensional and pulsed Doppler echocardiographic diagnosis of an acquired aortic right ventricular fistula. Clin. Cardiol. 1989, 12, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Frey, L.; Starck, C.; Falk, V.; Sündermann, S. Diastolic aorto-right-atrial fistulation in aortic and tricuspid valve endocarditis. Thorac. Cardiovasc. Surg. Rep. 2014, 3, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, G.J.; Calvert, C.A.; Hall, D.G.; Kraus, M. Diagnosis of right coronary artery to right atrial fistula in a dog using two-dimensional echocardiography. J. Small Anim. Pract. 1996, 37, 387–390. [Google Scholar] [CrossRef]
- Ramírez, G.A.; Espinosa de los Monteros, A.; Rodríguez, F.; Weisbrode, S.E.; Jaber, J.R.; Herráez, P. Left ventricular outflow tract-right atrial communication (Gerbode type defect) associated with bacterial endocarditis in a dog. Vet. Pathol. 2003, 40, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Peddle, G.D.; Boger, L.; Van Winkle, T.J.; Oyama, M.A. Gerbode type defect and third degree atrioventricular block in association with bacterial endocarditis in a dog. J. Vet. Cardiol. 2008, 10, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Marchesotti, F.; Tognetti, R.; Domenech, O. ECG of the Month. Atrioventricular block (AVB). J. Am. Vet. Med. Assoc. 2016, 248, 1004–1006. [Google Scholar] [CrossRef]
- Loperfido, F.; De Santis, F.; Solfanelli, N.; Pennestri, F.; Mazzari, M. Tricuspid regurgitation due to sinus of Valsalva-right heart fistula diagnosed by two-dimensional echocardiography. Chest 1984, 86, 501–503. [Google Scholar] [CrossRef]
- Nguyen, T.; Andersen, M.; Fernando, R.J.; Richardson, K. An occult aortocavitary fistula presenting as apparent tricuspid valve and aortic valve endocarditis. CASE 2018, 3, 28–34. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.A.; Chomel, B.B.; Kittleson, M.D.; Kasten, R.W.; Thomas, W.P.; Pesavento, P. A prospective study of canine infective endocarditis in northern California (1999–2001): Emergence of Bartonella as a prevalent etiologic agent. J. Vet. Intern. Med. 2004, 18, 56–64. [Google Scholar] [PubMed]
- Sykes, J.E.; Kittleson, M.D.; Chomel, B.B.; Macdonald, K.A.; Pesavento, P.A. Clinicopathologic findings and outcome in dogs with infective endocarditis: 71 cases (1992–2005). J. Am. Vet. Med. Assoc. 2006, 228, 1735–1747. [Google Scholar] [CrossRef]
- Church, W.M.; Sisson, D.D.; Oyama, M.A.; Zachary, J.F. Third degreeatrioventricular block and sudden death secondary to acutemyocarditis in a dog. J. Vet. Cardiol. 2007, 9, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Santilli, R.A.; Porteiro Vázquez, D.M.; Vezzosi, T.; Perego, M. Long-term intrinsic rhythm evaluation in dogs with atrioventricular block. J. Vet. Intern. Med. 2016, 30, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Chen, D.; Youssef, G.; Ramsay, D. Case report: Third-degree atrioventricular block secondary to septic coronary artery embolism following infective endocarditis. Eur. Heart J. Case Rep. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Turina, M.; Baboti, I.; Bussmann, W.D.; Krayenbühl, H.P. Haemodynamics of acute and chronic atrioventricular block in dogs. Cardiovasc. Res. 1969, 3, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ilebekk, A.; Miller, M.M. Efficiency of the Frank-Starling mechanism at various levels of inotropy and afterload during aortic insufficiency in the dog. Scand. J. Clin. Lab. Investig. 1978, 38, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M. Arrhythmogenic right ventricular cardiomyopathy in the boxer dog: An update. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Palermo, V.; Stafford Johnson, M.J.; Sala, E.; Brambilla, P.G.; Martin, M.W.S. Cardiomyopathy in Boxer dogs: A retrospective study of the clinical presentation, diagnostic findings and survival. J. Vet. Cardiol. 2011, 13, 45–55. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romito, G.; Diana, A.; Rigillo, A.; Morini, M.; Cipone, M. Unusual Presentation of Aortic Valve Infective Endocarditis in a Dog: Aorto-Cavitary Fistula, Tricuspid Valve Endocarditis, and Third-Degree Atrioventricular Block. Animals 2021, 11, 690. https://doi.org/10.3390/ani11030690
Romito G, Diana A, Rigillo A, Morini M, Cipone M. Unusual Presentation of Aortic Valve Infective Endocarditis in a Dog: Aorto-Cavitary Fistula, Tricuspid Valve Endocarditis, and Third-Degree Atrioventricular Block. Animals. 2021; 11(3):690. https://doi.org/10.3390/ani11030690
Chicago/Turabian StyleRomito, Giovanni, Alessia Diana, Antonella Rigillo, Maria Morini, and Mario Cipone. 2021. "Unusual Presentation of Aortic Valve Infective Endocarditis in a Dog: Aorto-Cavitary Fistula, Tricuspid Valve Endocarditis, and Third-Degree Atrioventricular Block" Animals 11, no. 3: 690. https://doi.org/10.3390/ani11030690
APA StyleRomito, G., Diana, A., Rigillo, A., Morini, M., & Cipone, M. (2021). Unusual Presentation of Aortic Valve Infective Endocarditis in a Dog: Aorto-Cavitary Fistula, Tricuspid Valve Endocarditis, and Third-Degree Atrioventricular Block. Animals, 11(3), 690. https://doi.org/10.3390/ani11030690






