Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
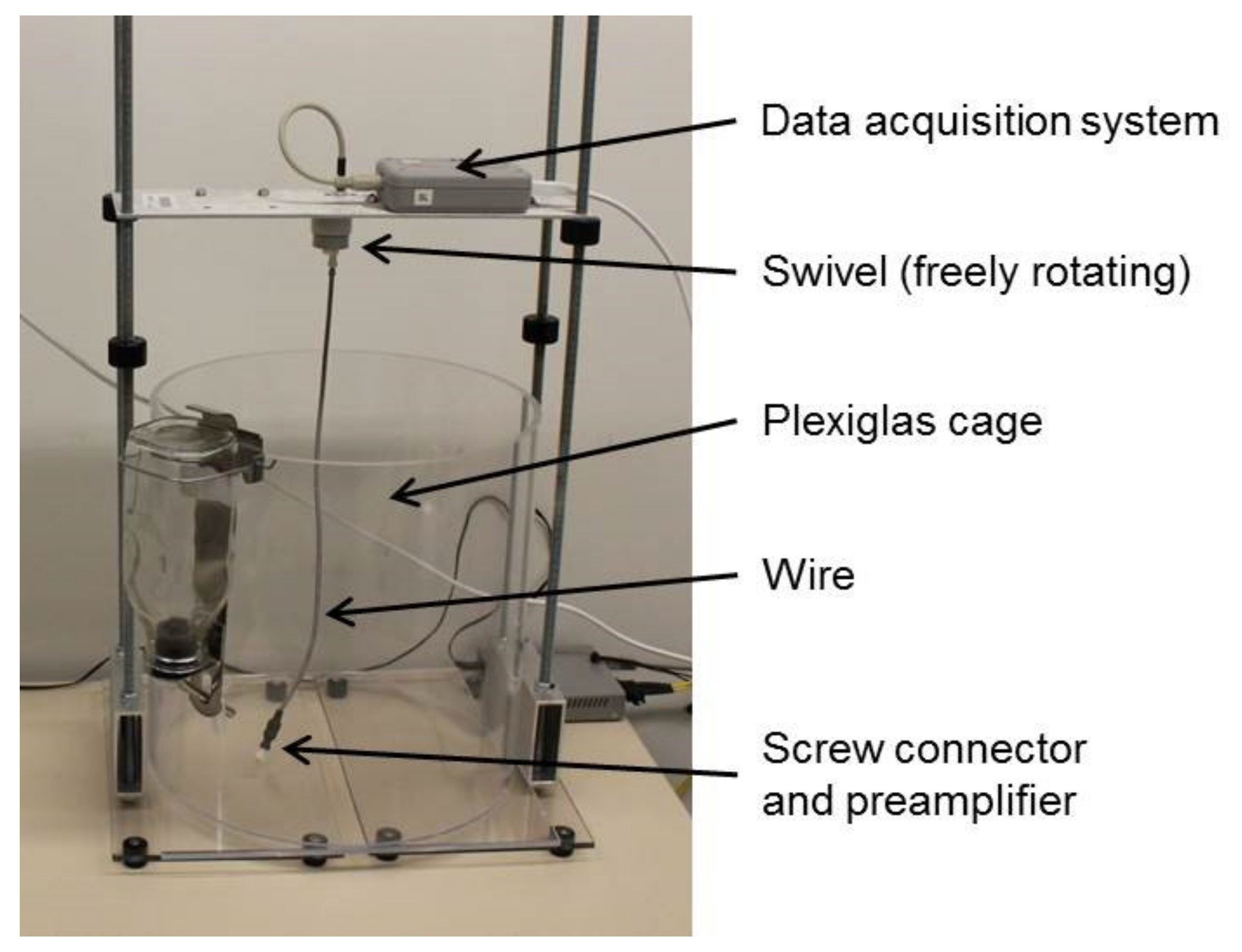
2.1. Animals and Study Design
2.2. Habituation
2.3. EEG-Electrode Implantation and Recording
2.4. Positron Emission Tomography
2.5. Sleep Deprivation (SD)
2.6. Welfare Assessment and Body Weight
2.7. Fecal Corticosterone Metabolites (FCMs)
2.8. Statistical Analysis
3. Results
3.1. Baseline (BL) Values
3.2. Concentration of Fecal Corticosterone Metabolites during Experimental Interventions
3.3. Concentration of Fecal Corticosterone Metabolites in Relation to Different Housing Conditions
3.3.1. EEG-Cage Housing (Control Group)
3.3.2. Housing in Activity Wheels and Effects of Habituation
3.4. Assessment of Welfare via Score Sheets and Correlation to Fecal Corticosterone Metabolites
3.4.1. Test Group G1
3.4.2. Control Group G2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russel, W.; Burch, R. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, L276, 33–79.
- Van Vlissingen, J.F.; Borrens, M.; Girod, A.; Lelovas, P.; Morrison, F.; Torres, Y.S. The reporting of clinical signs in laboratory animals: FELASA Working Group Report. Lab. Anim. 2015, 49, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- National Competent Authorities for the implementation of Directive 2010/63/EU on the protection of animals used for scientific purposes—Working document on a severity assessment framework. Eur. Comm. 2012.
- Fraser, D.; Ritchie, J.S.; Fraser, A.F. The term “stress” in a veterinary context. Br. Vet. J. 1975, 131, 653–662. [Google Scholar] [CrossRef]
- Adamson, T.W.; Kendall, L.V.; Goss, S.; Grayson, K.; Touma, C.; Palme, R.; Chen, J.Q.; Borowsky, A.D. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 610–616. [Google Scholar] [PubMed]
- Goldschlager, G.B.; Gillespie, V.L.; Palme, R.; Baxter, M.G. Effects of multimodal analgesia with low-dose buprenorphine and meloxicam on fecal glucocorticoid metabolites after surgery in New Zealand white rabbits (Oryctolagus cuniculus). J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 571–576. [Google Scholar] [PubMed]
- Jacobsen, K.R.; Kalliokoski, O.; Teilmann, A.C.; Hau, J.; Abelson, K.S.P. Postsurgical food and water consumption, fecal corti-costerone metabolites, and behaviour assessment as noninvasive measures of pain in vasectomized BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 69–75. [Google Scholar] [PubMed]
- Kalliokoski, O.; Jacobsen, K.R.; Darusman, H.S.; Henriksen, T.; Weimann, A.; Poulsen, H.E.; Hau, J.; Abelson, K.S.P. mice do not habituate to metabolism cage housing–a three week study of male BALB/c mice. PLoS ONE 2013, 8, e58460. [Google Scholar] [CrossRef] [Green Version]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Pfeiffenberger, U.; Yau, T.; Fink, D.; Tichy, A.; Palme, R.; Egerbacher, M.; Rülicke, T. Assessment and refinement of intra-bone marrow transplantation in mice. Lab. Anim. 2015, 49, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Björk, N.; Carlsson, H.E.; Mayo, S.; Hau, J. Impact of chronic catheterization and automated blood sampling (Ac-cusampler) on serum corticosterone and fecal immunoreactive corticosterone metabolites and immunoglobulin A in male rats. J. Endocrinol. 2004, 180, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundbom, R.; Jacobsen, K.R.; Kalliokoski, O.; Hau, J.; Abelson, K.S.P. Post-operative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 2011, 25, 335–342. [Google Scholar] [PubMed]
- Zieglowski, L.; Kümmecke, A.; Ernst, L.; Schulz, M.; Talbot, S.R.; Palme, R.; Czaplik, M.; Tolba, R.H. Severity assessment using three common behavioral or locomotor tests after laparotomy in rats: A pilot study. Lab. Anim. 2020, 54, 525–535. [Google Scholar] [CrossRef]
- Keubler, L.M.; Hoppe, N.; Potschka, H.; Talbot, S.R.; Vollmar, B.; Zechner, D.; Häger, C.; Bleich, A. Where are we heading? Challenges in evidence-based severity assessment. Lab. Anim. 2020, 54, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Bodden, C.; Siestrup, S.; Palme, R.; Kaiser, S.; Sachser, N.; Richter, S.H. Evidence-based severity assessment: Impact of repeated versus single open-field testing on welfare in C57BL/6J mice. Behav. Brain Res. 2018, 336, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice—Assessing the degree of distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef] [PubMed]
- Kumstel, S.; Wendt, E.H.U.; Eichberg, J.; Talbot, S.R.; Häger, C.; Zhang, X.; Abdelrahman, A.; Schönrogge, M.; Palme, R.; Bleich, A.; et al. Grading animal distress and side effects of therapies. Ann. N. Y. Acad. Sci. 2020, 1473, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Kröger, M.; Thümmler, J.; Tietze, L.; Palme, R.; Touma, C. Impact of three commonly used blood sampling techniques on the welfare of laboratory mice: Taking the animal’s perspective. PLoS ONE 2020, 15, e0238895. [Google Scholar] [CrossRef]
- Wright-Williams, S.L.; Courade, J.-P.; Richardson, C.A.; Roughan, J.V.; Flecknell, P.A. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in two strains of laboratory mouse. Pain 2007, 130, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Häger, C.; Keubler, L.M.; Talbot, S.R.; Biernot, S.; Weegh, N.; Buchheister, S.; Buettner, M.; Glage, S.; Bleich, A. Running in the wheel: Defining individual severity levels in mice. PLoS Biol. 2018, 16, e2006159. [Google Scholar] [CrossRef]
- Mallien, A.S.; Häger, C.; Palme, R.; Talbot, S.R.; Vogt, M.A.; Pfeiffer, N.; Brandwein, C.; Struve, B.; Inta, D.; Chourbaji, S.; et al. Systematic analysis of severity in a widely used cognitive depression model for mice. Lab. Anim. 2020, 54, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Christiansen, S.; Bouzinova, E.V.; Palme, R.; Wiborg, O. Circadian activity of the hypothalamic-pituitary-adrenal axis is differentially affected in the rat chronic mild stress model of depression. Stress 2012, 15, 647–657. [Google Scholar] [CrossRef]
- Palme, R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Lepschy, M.; Touma, C.; Hruby, R.; Palme, R. Non-invasive measurement of adrenocortical activity in male and female rats. Lab. Anim. 2007, 41, 372–387. [Google Scholar] [CrossRef] [Green Version]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef]
- Christie, M.A.; McKenna, J.T.; Connolly, N.P.; McCarley, R.W.; Strecker, R.E. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: Introduction of the rat-psychomotor vigilance task. J. Sleep Res. 2008, 17, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, T.; Elmenhorst, D.; Weisshaupt, A.; Beer, S.; Bauer, A. Reproducibility of non-invasive A1 adenosine receptor quantification in the Rat Brain Using [18F]CPFPX and positron emission tomography. Mol. Imaging Biol. 2014, 16, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; McGinty, D.; Guzman-Marin, R.; Chew, K.T.; Stewart, D.; Szymusiak, R. Activation of c-fos in GABAergic neurons in the preoptic area during sleep and in response to sleep deprivation. J. Physiol. 2004, 556, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Marín, R.; Suntsova, N.; Stewart, D.R.; Gong, H.; Szymusiak, R.; McGinty, D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J. Physiol. 2003, 549, 563–571. [Google Scholar] [CrossRef]
- Kanzler, S.; Rix, A.; Czigany, Z.; Tanaka, H.; Fukushima, K.; Kögel, B.; Pawlowsky, K.; Tolba, R.H. Recommendation for severity assessment following liver resection and liver transplantation in rats: Part I. Lab. Anim. 2016, 50, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Pinkernell, S.; Becker, K.; Lindauer, U. Severity assessment and scoring for neurosurgical models in rodents. Lab. Anim. 2016, 50, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien. Tierärztliche Mon. 2013, 10, 238–246. [Google Scholar]
- Lepschy, M.; Touma, C.; Palme, R. Faecal glucocorticoid metabolites: How to express yourself—Comparison of absolute amounts versus concentrations in samples from a study in laboratory rats. Lab. Anim. 2010, 44, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Altholtz, L.Y.; Fowler, K.A.; Badura, L.L.; Kovacs, M.S. Comparison of the stress response in rats to repeated isoflurane or CO2:O2 anesthesia used for restraint during serial blood collection via the jugular vein. J. Am. Assoc. Lab. Anim. Sci. 2006, 45, 17–22. [Google Scholar] [PubMed]
- Henke, J.; Haberstroh, J.; Sager, M.; Becker, K.; Eberspächer, E.; Bergadano, A.; Zahner, D.; Arras, M. Pain Management for Laboratory Animals—Expert Information: Committee on Anaesthesia of GV-SOLAS. 2015. [Google Scholar]
- Zegre Cannon, C.; Kissling, G.E.; Goulding, D.R.; King-Herbert, A.P.; Blankenship-Paris, T. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab. Anim. 2011, 40, 85–93. [Google Scholar] [CrossRef]
- Ciuffreda, M.C.; Tolva, V.; Casana, R.; Gnecchi, M.; Vanoli, E.; Spazzolini, C.; Roughan, J.; Calvillo, L. Rat Experimental model of myocardial ischemia/reperfusion injury: An ethical approach to set up the analgesic management of acute post-surgical pain. PLoS ONE 2014, 9, e95913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiffert, I.; Van Dijk, R.M.; Koska, I.; Di Liberto, V.; Möller, C.; Palme, R.; Hellweg, R.; Potschka, H. Toward evidence-based severity assessment in rat models with repeated seizures: III. Electrical post-status epilepticus model. Epilepsia 2019, 60, 1539–1551. [Google Scholar] [CrossRef]
- Armario, A.; Montero, J.L.; Balasch, J. Sensitivity of corticosterone and some metabolic variables to graded levels of low in-tensity stresses in adult male rats. Physiol. Bahav. 1985, 37, 559–561. [Google Scholar] [CrossRef]
- Tuli, J.S.; Smith, J.A.; Morton, D.B. Stress measurements in mice after transportation. Lab. Anim. 1995, 29, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rix, A.; Drude, N.; Mrugalla, A.; Mottaghy, F.M.; Tolba, R.H.; Kiessling, F. Performance of severity parameters to detect chemotherapy-induced pain and distress in mice. Lab. Anim. 2019. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; Van Couter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep deprivation and stress: A reciprocal relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.; Van Luijtelaar, E.L. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol. Behav. 1985, 35, 501–504. [Google Scholar] [CrossRef]
- Hairston, I.S.; Ruby, N.F.; Brooke, S.; Peyron, C.; Denning, D.P.; Heller, H.; Sapolsky, R.M. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neurosci. Lett. 2001, 315, 29–32. [Google Scholar] [CrossRef]
- Penalva, R.G.; Lancel, M.; Flachskamm, C.; Reul, J.M.; Holsboer, F.; Linthorst, A.C. Effect of sleep and sleep deprivation on serotonergic neurotransmission in the hippocampus: A combined in vivo microdialysis/EEG study in rats. Eur. J. Neurosci. 2003, 17, 1896–1906. [Google Scholar] [CrossRef]
- Sgoifo, A.; Buwalda, B.; Roos, M.; Costoli, T.; Merati, G.; Meerlo, P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology 2006, 31, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tartar, J.L.; Ward, C.P.; Cordeira, J.W.; Legare, S.L.; Blanchette, A.J.; McCarley, R.W.; Strecker, R.E. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav. Brain Res. 2009, 197, 450–453. [Google Scholar] [CrossRef] [Green Version]
- Meerlo, P.; Koehl, M.; Van Der Borght, K.; Turek, F.W. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J. Neuroendocr. 2002, 14, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. The time course of adenosine, nitric oxide (NO) and in-ducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J. Neurochem. 2011, 116, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Scaccianoce, S.; Navarra, D.; Di Sciullo, A.; Angelucci, L.; Endröczi, E. Adenosine and pituitary-adrenocortical axis activity in the rat. Neuroendocrinology 1989, 50, 464–468. [Google Scholar] [CrossRef]
- Leenaars, C.H.; Dematteis, M.; Joosten, R.N.; Eggels, L.; Sandberg, H.; Schirris, M.; Feenstra, M.G.; Van Someren, E.J. A new automated method for rat sleep deprivation with minimal confounding effects on corticosterone and locomotor activity. J. Neurosci. Methods 2011, 196, 107–117. [Google Scholar] [CrossRef]
- Gross, B.A.; Vanderheyden, W.M.; Urpa, L.M.; Davis, D.E.; Fitzpatrick, C.J.; Prabhu, K.; Poe, G.R. Stress-free automatic sleep deprivation using air puffs. J. Neurosci. Methods 2015, 251, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, L.; Lorenzini, R.N.; Cinotti, S.; Fini, M.; Giavaresi, G.; Giardino, R. Evaluation of pain and stress levels of animals used in experimental research. J. Surg. Res. 2000, 88, 114–119. [Google Scholar] [CrossRef]
- Shavit, Y.; Fish, G.; Wolf, G.; Mayburd, E.; Meerson, Y.; Yirmiya, R.; Beilin, B. The effects of perioperative pain management techniques on food consumption and body weight after laparotomy in rats. Anesthesia Analg. 2005, 101, 1112–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metab. 2013, 28, 288–296. [Google Scholar] [CrossRef]
- Harris, R.B.S.; Zhou, J.; Youngblood, B.D.; Rybkin, I.I.; Smagin, G.N.; Ryan, D.H. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am. J. Physiol. 1998, 275, R1928–R1938. [Google Scholar] [CrossRef] [PubMed]
- Kunczik, J.; Pereira, C.B.; Zieglowski, L.; Tolba, R.; Wassermann, L.; Häger, C.; Bleich, A.; Janssen, H.; Thum, T.; Czaplik, M. Remote vitals monitoring in rodents using video recordings. Biomed. Opt. Express 2019, 10, 4422–4436. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Di Perrotolo, M.S.; Sotocinal, S.G.; Flecknell, P.A. The Assessment of Post-Vasectomy Pain in Mice Using Behaviour and the Mouse Grimace Scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.S.; Wei, P.; Zhan, S.; Zhang, S.; et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Dong, Z.; Huang, W.; Zhou, C.; Zhong, W.; Hu, P.; Wen, G.; Sun, X.; Hua, H.; Cao, H.; et al. Voluntary wheel running ameliorates depression-like behaviors and brain blood oxygen level-dependent signals in chronic unpredictable mild stress mice. Behav. Brain Res. 2017, 330, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Millspaugh, J.J.; Washburn, B.E. Within-sample variation of fecal glucocorticoid measurements. Gen. Comp. Endocrinol. 2003, 132, 21–26. [Google Scholar] [CrossRef]







| Changes in FCM Concentrations and Body Weight during Post-Operative Care (Pearson Correlation Coefficients and (p-Values)) | ||
|---|---|---|
| Day | G1 (test group) | |
| Post-op 1 | −0.02 (0.97) | |
| Post-op 2 | −0.79 (0.06) | |
| Post-op 3 | −0.11 (0.81) | |
| Post-op 4 | −0.39 (0.39) | |
| Post-op 5 | 0.22 (0.63) | |
| Post-op 6 | 0.54 (0.21) | |
| Post-op 7 | 0.13 (0.77) | |
| Absolute FCM Concentrations and Assessment Scores during Different Interventions (Kendall’s Tau Rank Correlation Coefficient and (p-Values) | ||
| Day | G1 (test group) | G2 (control group) |
| Surgery | −0.36 (0.25) | |
| Post-op 1 | 0.08 (0.79) | |
| Post-op 2 | 0.11 (0.74) | |
| Post-op 3 | −0.07 (0.83) | |
| Post-op 4 | −0.23 (0.44) | |
| Post-op 5 | 0.04 (0.89) | |
| Post-op 6 | 0.24 (0.43) | |
| Post-op 7 | 0.37 (0.23) | |
| EEG 1 | 0.42 (0.16) | −0.24 (0.66) |
| EEG 2 | −0.04 (0.90) | −0.26 (0.50) |
| EEG 3 | 0.27 (0.38) | 0.23 (0.54) |
| EEG 4 | −0.40 (0.17) | 0.07 (0.85) |
| EEG 5 (PET in G1) | 0.32 (0.29) | −0.55 (0.15) |
| SD (G1)/Pseudo-SD (G2) | 0.55 (0.09) | −0.08 (0.84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroll, T.; Kornadt-Beck, N.; Oskamp, A.; Elmenhorst, D.; Touma, C.; Palme, R.; Bauer, A. Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats. Animals 2021, 11, 710. https://doi.org/10.3390/ani11030710
Kroll T, Kornadt-Beck N, Oskamp A, Elmenhorst D, Touma C, Palme R, Bauer A. Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats. Animals. 2021; 11(3):710. https://doi.org/10.3390/ani11030710
Chicago/Turabian StyleKroll, Tina, Nikola Kornadt-Beck, Angela Oskamp, David Elmenhorst, Chadi Touma, Rupert Palme, and Andreas Bauer. 2021. "Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats" Animals 11, no. 3: 710. https://doi.org/10.3390/ani11030710
APA StyleKroll, T., Kornadt-Beck, N., Oskamp, A., Elmenhorst, D., Touma, C., Palme, R., & Bauer, A. (2021). Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats. Animals, 11(3), 710. https://doi.org/10.3390/ani11030710






